Abstract
ATP-binding cassette (ABC) transporters make up a superfamily of transmembrane proteins that play a critical role in the development of drug resistance. This phenomenon is especially important in oncology, where superfamily member ABCG2 (also called BCRP – breast cancer resistance protein) is known to interact with dozens of anti-cancer agents that are ABCG2 substrates. In addition to the well-studied and well-reviewed list of cytotoxic and targeted agents that are substrates for the ABCG2 transporter, a growing body of work links ABCG2 to multiple photodynamic therapy (PDT) agents, and there is a limited body of evidence suggesting that ABCG2 may also play a role in resistance to radiation therapy. In addition, the focus of ABC transporter research in regards to therapeutic development has begun to shift in the past few years. The shift has been away from using pump inhibitors for reversing resistance, toward the development of therapeutic agents that are poor substrates for these efflux pump proteins. This approach may result in the development of drug regimens that circumvent ABC transporter-mediated resistance entirely. Here, it is our intention to review: 1) recent discoveries that further characterize the role of ABCG2 in oncology, and 2) advances in reversing and circumventing ABC transporter-mediated resistance to anti-cancer therapies.
Similar content being viewed by others
Background
Resistance to anti-cancer therapies is one of the most studied subjects in modern biomedical research [1, 2]. Though many mechanisms of drug resistance have been identified in cancer, drug efflux mediated by xenobiotic transporters is one of the best validated. ATP-binding cassette (ABC), sub-family G, isoform 2 protein (ABCG2, also known as breast cancer resistance protein, BCRP) is a drug efflux pump and an important member of the ABC transporter superfamily. ABCG2 was identified independently by three separate groups in 1998 and 1999 [3–5]. In normal tissue, ABCG2 performs a multitude of functions. ABCG2 is expressed at very high levels in the placenta and protects the developing fetus from endo- and exotoxins [5]. ABCG2 is also found at the blood-brain barrier, where it likewise protects the brain from harmful compounds [6]. ABCG2 also regulates the homeostasis of nutrients and certain hormones. In the gastrointestinal tract, ABCG2 plays a role in nutrient absorption [7]. ABCG2 helps to concentrate vitamins and possibly hormones in breast milk [8], and may regulate testosterone levels in the prostate, as ABCG2 is expressed in normal prostate basal epithelial cells [9]. The sebaceous glands, exocrine glands located in the skin that secrete sebum to lubricate and waterproof skin and hair, also express very high levels of ABCG2 [10].
ABCG2 and cancer
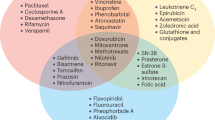
Two of the three groups that initially isolated ABCG2 did so while investigating resistance to anti-cancer agents that had developed in cell culture [3, 4]. Since ABCG2 was first identified in drug-resistant cancer cells, it was hypothesized that a variety of cytotoxic agents were substrates for ABCG2, and that resistance to these agents was the result of drug efflux by ABCG2 [3, 4]. Though not the focus of this review, we have summarized a portion of the literature in regards to ABCG2 and cytotoxic or targeted therapies (Table 1) [11–16]. Several excellent reviews cover these findings in greater detail [17–20].
Multiple in vitro studies have demonstrated that methotrexate, mitoxantrone, flavopiridol, 5-fluorouracil, as well as the camptothecin analogues topotecan, irinotecan, and SN-38 are all substrates for ABCG2, and high expression of ABCG2 correlates with decreased intracellular accumulation of these compounds and consequentially a decrease in drug potency [4, 21–27]. A number of nucleoside analogues in clinical use are also known to interact with ABCG2 [28]. In addition, gain-of-function mutations in ABCG2 (R482G and R482T) result in efflux of anthracyclines [29, 30]. A number of clinical studies have also observed correlations between high ABCG2 activity and decreased survival [31–33], and links between failure of a variety of cytotoxic and targeted therapies with ABCG2 activity [34–36]. Most recently, for example, ABCG2 has been shown to transport rucaparib [37], a PARP inhibitor under clinical investigation, as well as limit its oral bioavailability [38].
ABCG2 and photodynamic therapy
Photodynamic therapy (PDT) uses tumor-selective photosensitizers and subsequent activation by light of a specific wavelength to generate reactive oxygen species. These species, in turn, damage cancer cells and induce apoptosis and necrosis. Currently, PDT agents are approved in the U.S. for the treatment of esophageal cancer and non-small cell lung cancer. A number of PDT agents are known to be substrates of ABCG2. High ABCG2 expression decreases the intracellular accumulation and in vitro potency of the investigational PDT agents pheophorbide a [39, 40], pyropheophorbide a methyl ester [41], and chlorin e6 [41]. There is also in vitro and in vivo evidence that ABCG2 can cause resistance to a PDT agent currently under clinical investigation, 2-(1-hexyloxethyl)-2-devinyl pyropheophorbide, commonly known as HPPH [42, 43]. There is also evidence that clinically used PDT agents are substrates for ABCG2. Interest in 5-aminolevulinic acid (ALA) has been growing in recent years, as ALA is now used as a fluorescent aid during tumor resection in glioma patients [44] as well as a photosensitizer for PDT of pre-cancerous actinic keratosis [45, 46]. Robey et al. initially identified ALA as a substrate for ABCG2, and recent work by two other groups has further confirmed their findings [41, 47, 48]. Lastly, Usuda et al. reported that the potency of porfimer sodium is decreased in response to high ABCG2 activity [49]. Porfimer sodium is a photosensitizer that is approved by the FDA for the treatment of esophageal cancer and endobronchial non-small cell lung cancer lesions. Additionally, Usuda and colleagues reported that lung cancer patients with localized disease who expressed high levels of ABCG2 protein responded worse to porfimer sodium than patients with lower levels of ABCG2 [49], which further validated ABCG2 as a clinically-relevant mechanism of resistance in cancer.
ABCG2 and cancer stem cells
ABCG2 has also been implicated in another realm of cancer that is somewhat separate from treatment response: the cancer stem cell (CSC) phenotype. CSCs are a subset of cancer cells that share properties with “normal” stem cells: self-renewal and the ability to differentiate into multiple types of cells (reviewed in [50]). CSCs have been hypothesized to play a role in tumorigenesis, resistance, recurrence, metastasis, and tumor heterogeneity [50–52]. CSCs are often isolated or identified by detection of cell surface markers, including CD44, CD24, CD133, and others [50, 53], and also by detection of aldehyde dehydrogenase activity [54]. However, another method of isolating CSCs has been through the identification of a subpopulation that is able to efflux chemotherapeutics or, more commonly, the dye Hoechst 33342, an ABCG2 substrate [55].
The Hoechst 33342 efflux assay was developed by Dr. Margaret Goodell and colleagues who were attempting to use the DNA binding dye to measure DNA content in cycling bone marrow cells [55]. After treating murine bone marrow cells with Hoechst 33342, the group excited the cells with an ultraviolet laser and recorded emission at two wavelengths using a 450/20 nm band pass filter (the standard filter for evaluating DNA content using Hoechst 33342) and a 675 nm long pass edge filter [55]. Simultaneously displaying emission at both wavelengths allowed the team to identify a population of cells that was removed from the main body which they hypothesized was the result of dye efflux mediated by molecular efflux pumps [55]. This “side population”, as they called it, contained cells that were enriched for hematopoietic stem cell markers and were better able to repopulate the bone marrow of mice after radiation [55]. Several groups were able to later identify ABCG2 as a key contributor to Hoechst 33342 efflux and an important side population marker [56–59]. Since then, the Hoechst 33342 efflux assay has been used to successfully isolate a number of normal stem-like populations, such as retinal stem cells [60] and primitive neural cells [61], as well as CSC-like populations in lung cancer cell lines [62], head and neck cancer cell lines [63], hepatocellular carcinoma cell lines [64], a glioma cell line [65], primary neuroblastoma cultures [66], ovarian cancer cell lines [67], and in ascites cells from patients with ovarian cancer [67].
ABCG2 is often associated with CSCs because of the presence of ABCG2-positive cells in the side population identified by dye efflux assays. However, several groups have concluded that ABCG2 is not a defining feature of all stem-like populations. For example, ABCG2 deficiency does not prevent normal hematopoietic development in mice [56], and CSC populations isolated using cell surface markers do not always express ABCG2 [68]. Additionally, ABCG2-negative cancer cells are able to form tumors in breast, prostate, colon, and glioma xenograft models at the same rate as ABCG2-positive cancer cells [69]. However, a number of reports have identified two areas of interest where ABCG2 may be functionally relevant in regards to CSCs and therapeutic response: in castration-resistant prostate cancer, and in radiation resistant cancer cells.
ABCG2 and hormone-refractory cancers
Some groups have hypothesized that, although not all CSCs are ABCG2-positive, ABCG2-positive cancer cells with at least some stem-like qualities become more relevant during the development of treatment resistance. In addition to resistance to treatment modalities discussed earlier, this would also allow for resistance to hormone-based therapies, as ABCG2 has been shown to efflux a number of androgenic and estrogenic hormone conjugates [70–73]. This phenomenon has been studied most extensively in the prostate cancer, where ABCG2 is highly expressed in a small population of stem-like cancer cells and is able to regulate the efflux of androgen [72]. Overexpression of ABCG2 has also been shown to promote a stem-like phenotype in prostate cells [74]. These cells are believed to be less responsive to androgen, as they do not express detectable levels of the androgen receptor. Further, Gu et al. demonstrated that androgen receptor-negative prostate CSCs can repopulate tumors in animals following serial implantation [75]. Based on this evidence, it has been hypothesized that this population of androgen-independent cells may be the progenitor cells that are responsible for the development of castration-resistance disease: as androgen deprivation therapy induces apoptosis in androgen-dependent cells (reviewed in [76]), the tumor could potentially be repopulated by CSCs that are not reliant on androgen.
ABCG2 and radiation therapy
Studies have noted that a number of ABC transporters are often upregulated in cells that are resistant to radiation therapy [77–79]. Conventional wisdom was that these pumps were not actively radioprotective, but were instead indicative of the stem-like phenotype common in radiation resistance populations of cells. However, at least one group had proposed a functional role for ABC transporters in radiation resistance as early as 2007, positing that efflux pump-mediated glutathione modulation could play a role in radiation resistance [80]. Though that specific hypothesis has remained largely unstudied, a small number of reports have emerged that may suggest a functional role for ABC transporters, including ABCG2, in radiation resistance. In 2009, Ning et al. published a report detailing a population of stem-like cells in the bladder cancer line T24. They reported that this stem-like population, isolated by Hoechst 33342 efflux and expressing higher ABCG2 mRNA than unsorted T24 cells, was resistant to radiation treatment [81]. Intriguingly, administration of verapamil, an inhibitor of ABC transporters as well as an inhibitor of calcium and potassium transporters, sensitized these cells to radiation treatment [81]. More recently, Ingram and colleagues reported high ABCG2 expression in medulloblastoma cells that survived exposure to long-term low-dose radiation as well as short-term high-dose radiation [82]. They observed a reversal of radioresistance following treatment with R-verapamil, which has weaker anti-ion channel activity compared to S-verapamil, yet retains its ability to inhibit ABC transporters. Curiously, they did not see reversal of radioresistance following treatment with specific inhibitors of ABCG2, such as Ko143 [82]. The authors interpreted this as evidence for redundancy of ABC transporters in regards to transporter-mediated radiation resistance, though they did not rule out residual anti-ion channel activity [82]. In our view, more studies are needed before a functional role for ABC transporters in radioresistance can be conclusively established.
Therapeutic strategies to overcome ABCG2-mediated resistance
Inhibition of ABCG2 activity
Since the discovery of ABCG2, researchers have been investigating methods to reverse or circumvent ABCG2-induced resistance. One attractive possibility was to inhibit ABCG2 activity, thereby halting drug efflux. This strategy initially showed promise in vitro where potent inhibitors of ABCG2, such as fumitremorgin C, were able to restore the potency of ABCG2 substrates [83, 84]. However, attempts to translate this strategy to the clinic have been unsuccessful so far. The first problem to arise was the toxicity of first-generation ABCG2 inhibitors in animal models. Fumitremorgin C, for example, causes severe neurotoxicity [85]. Other groups have reported that less toxic inhibitors of ABCG2, such as tariquidar, may not result in clinically beneficial increases in drug accumulation [86]. Interestingly, results from studies using tyrosine kinase inhibitors that also inhibit ABCG2 activity are more encouraging.
Many tyrosine kinase inhibitors that are substrates for ABCG2 are also able to function as competitive inhibitors of ABCG2-mediated efflux of cytotoxics, as the two drug classes share a common binding site [87, 88]. Like fumitremorgin C, several tyrosine kinase inhibitors, including sunitinib, nilotinib, sorafenib, and imatinib, are able to sensitize cells with high ABCG2 expression to cytotoxics which are ABCG2 substrates, including topotecan and SN-38 [89, 90]. Mazard et al. were able to demonstrate that sorafenib enhanced the intracellular accumulation of SN-38 and that combination treatment of sorafenib with irinotecan improved survival of mice implanted with irinotecan-resistant tumors [91]. However, clinical trials using tyrosine kinase inhibitors as ABCG2 inhibitors or in combination with cytotoxic agents that are ABCG2 substrates have shown mixed outcomes. A phase III trial of sunitinib in combination with the FOLFIRI regimen was discontinued due to increased adverse events, including increased drug toxicity-related deaths, in the sunitinib-treated arm with no benefit to overall survival [92]. However, a phase I/II trial of sorafenib in combination with irinotecan in patients who had previously failed irinotecan-containing regimens was concluded in 2014 with promising results [93].
Inhibition of ABCG2 expression as an emerging therapeutic option
Another proposed strategy in overcoming ABCG2-mediated resistance to chemotherapeutics was to inhibit ABCG2 protein expression. The concept of inhibiting ABC transporter expression is not new; Hiroyuki Kobayashi and colleagues first reported on hammerhead ribozymes that were able to target ABCG2 mRNA in 1994 [94]. More recently, RNA interference (RNAi) has been used to knock down ABCG2 expression in cell culture and restore therapeutic benefit to anti-cancer agents that are ABCG2 substrates [95–98]. While there was some doubt for a number of years about the practicality of RNAi translating to clinical use [99, 100], the reports in the last five years of successful cleavage of targeted sequences in humans using nanoparticle delivery of siRNA particles [101, 102] opens the door for renewed interest in the potential application of RNAi as a means of disrupting multi-drug resistance mediated by ABC transporters.
Alternatively, several groups have proposed pharmacologic inhibition of ABCG2 expression as a potential tool to combat drug resistance. Again, tyrosine kinase inhibitors have been used to demonstrate proof of principle in vitro. Nakanishi et al. demonstrated that tyrosine kinase inhibitors that target the PI3K-Akt pathway, such as LY294002, are able to downregulate ABCG2 expression, thereby sensitizing cells to therapeutic agents that are ABCG2 substrates [103]. Other groups have investigated the use of phosphodiesterase-5 inhibitors for the same purpose. One phosphodiesterase-5 inhibitor, sildenafil, has shown promise as an inhibitor of multiple ABC transporters at clinically achievable levels [104, 105]. One report describes the reversal of methotrexate, mitoxantrone, and paclitaxel resistance due to sildenafil-mediated downregulation of ABCG2 and P-gp in breast cancer cells [106]. Xanthines, such as caffeine and theophylline, are also being investigated for the same purpose and have shown promising results in vitro [107]. Unfortunately, no trials have yet investigated the potential benefit of this strategy in a clinical setting.
Circumventing ABCG2-mediated resistance by using agents that are poor substrates
A final and more recent proposed strategy to deal with ABC transporter-induced resistance to clinically used agents had been the development of agents that are poor substrates for efflux pumps. This strategy is having an immediate impact in the clinic. In 2010, the FDA approved the use of a novel semi-synthetic taxane derivative, cabazitaxel, in patients with castration-resistant prostate cancer who had previously failed docetaxel-based regimens [108]. Cabazitaxel was originally selected for clinical testing based in part on its poor affinity for the drug efflux pump P-glycoprotein 1 (P-gp, also known as multidrug resistance protein 1 and ABCB1) [108]. In a randomized open-label phase III trial in patients whose disease had progressed following docetaxel, cabazitaxel plus oral prednisone was superior to mitoxantrone plus oral prednisone in terms of both overall survival and progression-free survival [109]. Cabazitaxel serves as an encouraging “proof of concept” that suggests that this strategy of circumventing resistance using agents that are poor substrates for drug efflux pumps could be expanded to other ABC transporters, including ABCG2, to address the increasingly complex issue of drug failure.
Several research groups have attempted to identify anti-cancer agents that are poor substrates for ABCG2. As early as 2004, researchers in Japan were attempting to identify derivatives of camptothecin that were poor substrates of ABCG2 in the hopes of addressing irinotecan and topotecan resistance [110]. Other groups in the United States and Europe followed suit, though only a small handful of compounds have since been identified [111–113], and none have yet progressed to clinical trials. Our group recently reported on a novel semi-synthetic analogue of camptothecin, FL118 (10,11-methylenedioxy-20(S)-camptothecin), that has strong anti-cancer activity both in vitro and in vivo [114–116]. FL118 is better able to control tumor growth compared to both irinotecan and topotecan in a number of xenograft models, and has picomolar EC50 values for growth inhibition in a number of cell culture models [114, 117]. More recently, we have also reported that FL118 is a poor substrate for both ABCG2 [118] and P-gp [119]. High expression of ABCG2 and/or P-gp did not result in FL118 resistance in cell culture, and FL118 was able to inhibit tumor growth better than irinotecan in xenograft models that expressed high levels of ABCG2 [118]. Subsequently, we also observed that FL118 could eradicate xenografts that had acquired resistance to either irinotecan or topotecan [119]. This suggests that FL118 may be able to restore therapeutic efficacy in patients who had originally benefitted from irinotecan or topotecan but who later stopped responding due to acquired resistance mediated by drug efflux pumps.
Lastly, researchers who are developing the next generation of PDT agents have been particularly attracted to the notion of designing agents that are not susceptible to efflux by ABC transporters. Although a number of PDT agents are known substrates of ABCG2, including clinically used compounds, several groups had previously noted that affinity for ABCG2 was not a universally shared characteristic of common PDT drug classes [39, 41]. More recently, one group from Norway reported on amphiphilic sulfonated photosensitizers that were not effluxed out of breast cancer cells with high ABCG2 expression [120]. Specifically, they investigated sulfonated members of PDT agents in different classes, including porphines, chlorins, and phtalocyanines, and found that none of them were effluxed by ABCG2. Furthermore, another group was able to demonstrate that the potency of certain sugar-conjugated analogues of the clinically investigated HPPH were not affected by ABCG2 [42]. Taken together, these studies suggest that modifications to already-proven classes of PDT agents may produce compounds that are both efficacious and insensitive to ABCG2 activity.
Conclusions
ABCG2 has been linked to treatment failure and decreased survival in a number of different cancers. In addition to the well-known effects of ABCG2 on cytotoxics and targeted agents, ABCG2 is also increasingly linked with failure of PDT, while the role of ABCG2 in resistance to radiation therapy remains to be further investigated. ABCG2 has also been linked to cancer cells that exhibit stem-like properties. As a result, intensive research efforts have been expended in the search for options to reverse or circumvent ABCG2-mediated resistance. In the past, most research was dedicated to identifying pharmacologic agents that would inhibit ABCG2 activity, with the hypothesis that concomitant treatment with ABCG2 inhibitors and conventional chemotherapy would increase treatment efficacy. In recent years, however, we begin to see a transition toward other strategies. Though none of these ABCG2-specific efforts have yet resulted in improvements in patient care, the broader concept of circumventing drug efflux pump-mediated resistance has led to the development and approval of cabazitaxel for prostate cancer. Encouragingly, a number of anti-cancer agents that are not substrates for ABC transporters are currently in preclinical development. FL118, for example, is a unique camptothecin analogue that is not effluxed by either ABCG2 or P-gp, and is able to overcome resistance to irinotecan and topotecan in cancer xenograft models. A number of other molecules are also currently in preclinical development, also with the goal of providing treatment options to patients who had initially responded to conventional therapy options but who had developed resistance due to increased efflux pump activity.
Abbreviations
- ABC:
-
ATP-binding cassette
- ABCG2:
-
ATP-binding cassette, subfamily G, isoform 2 protein
- ALA:
-
5-aminolevulinic acid
- BCRP:
-
Breast cancer resistance protein
- CSC:
-
Cancer stem cell
- HPPH:
-
2-(1-hexyloxethyl)-2-devinyl pyropheophorbide
- PDT:
-
Photodynamic therapy
- P-gp:
-
P-glycoprotein
- RNAi:
-
RNA interference
References
Niero EL, Rocha-Sales B, Lauand C, Cortez BA, de Souza MM, Rezende-Teixeira P, et al. The multiple facets of drug resistance: one history, different approaches. J Exp Clin Cancer Res. 2014;33:37.
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–26.
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci. 1998;95(26):15665–70.
Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular Cloning of cDNAs Which Are Highly Overexpressed in Mitoxantrone-resistant Cells Demonstration of Homology to ABC Transport Genes. Cancer Res. 1999;59(1):8–13.
Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58(23):5337–9.
Eisenblätter T, Hüwel S, Galla H-J. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood–brain barrier. Brain Res. 2003;971(2):221–31.
Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol. 2005;70(5):695–9.
Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11(2):127–9.
Pascal LE, Oudes AJ, Petersen TW, Goo YA, Walashek LS, True LD, et al. Molecular and cellular characterization of ABCG2 in the prostate. BMC Urol. 2007;7(1):6.
Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, et al. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006;235(1):84–92.
Shiozawa K, Oka M, Soda H, Yoshikawa M, Ikegami Y, Tsurutani J, et al. Reversal of breast cancer resistance protein (BCRP/ABCG2)‐mediated drug resistance by novobiocin, a coumermycin antibiotic. Int J Cancer. 2004;108(1):146–51.
Allen JD, Van Dort SC, Buitelaar M, van Tellingen O, Schinkel AH. Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediates etoposide resistance and transport, but etoposide oral availability is limited primarily by P-glycoprotein. Cancer Res. 2003;63(6):1339–44.
Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65(7):2577–82.
Hegedűs C, Özvegy‐Laczka C, Apati A, Magocsi M, Nemet K, Őrfi L, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti‐cancer effects and pharmacological properties. Br J Pharmacol. 2009;158(4):1153–64.
Elkind NB, Szentpétery Z, Apáti Á, Özvegy-Laczka C, Várady G, Ujhelly O, et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res. 2005;65(5):1770–7.
Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, et al. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1−/−/Mdr1a/1b−/−(triple-knockout) and wild-type mice. Mol Cancer Ther. 2008;7(8):2280–7.
Stacy AE, Jansson PJ, Richardson DR. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol Pharmacol. 2013;84(5):655–69.
Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26(1):39–57.
Krishnamurthy P, Schuetz J. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410.
Mo W, Zhang J-T. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012;3(1):1–27.
Nakatomi K, Yoshikawa M, Oka M, Ikegami Y, Hayasaka S, Sano K, et al. Transport of 7-ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells. Biochem Biophys Res Commun. 2001;288(4):827–32.
Scheffer GL, Maliepaard M, Pijnenborg AC, van Gastelen MA, de Jong MC, Schroeijers AB, et al. Breast cancer resistance protein is localized at the plasma membrane in mitoxantrone-and topotecan-resistant cell lines. Cancer Res. 2000;60(10):2589–93.
Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22(47):7340–58.
Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res. 2003;63(17):5538–43.
Robey RW, Medina-Pérez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7(1):145–52.
Yi H, Cho H-J, Cho S-M, Jo K, Park J-A, Lee S-H, et al. Effect of 5-FU and MTX on the expression of drug-resistance related cancer stem cell markers in non-small cell lung cancer cells. Korean J Physiol Pharmacol. 2012;16(1):11–6.
Maliepaard M, van Gastelen MA, Tohgo A, Hausheer FH, van Waardenburg RC, de Jong LA, et al. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res. 2001;7(4):935–41.
de Wolf C, Jansen R, Yamaguchi H, de Haas M, van de Wetering K, Wijnholds J, et al. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008;7(9):3092–102.
Özvegy-Laczka C, Köblös G, Sarkadi B, Váradi A. Single amino acid (482) variants of the ABCG2 multidrug transporter: major differences in transport capacity and substrate recognition. Biochim Biophys Acta Biomembr. 2005;1668(1):53–63.
Robey R, Honjo Y, Morisaki K, Nadjem T, Runge S, Risbood M, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89(10):1971–8.
Benderra Z, Faussat A-M, Sayada L, Perrot J-Y, Chaoui D, Marie J-P, et al. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10(23):7896–902.
Benderra Z, Faussat AM, Sayada L, Perrot J-Y, Tang R, Chaoui D, et al. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11(21):7764–72.
Uggla B, Ståhl E, Wågsäter D, Paul C, Karlsson MG, Sirsjö A, et al. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res. 2005;29(2):141–6.
Kim YH, Ishii G, Goto K, Ota S, Kubota K, Murata Y, et al. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer. 2009;65(1):105–11.
Ota S, Ishii G, Goto K, Kubota K, Kim YH, Kojika M, et al. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64(1):98–104.
Kim Y-K, Lee S-S, Jeong S-H, Ahn J-S, Yang D-H, Lee J-J, et al. OCT-1, ABCB1, and ABCG2 Expression in Imatinib-Resistant Chronic Myeloid Leukemia Treated with Dasatinib or Nilotinib. Chonnam Med J. 2014;50(3):102–11.
Parrish KE, Cen L, Murray J, Calligaris D, Kizilbash S, Mittapalli RK, et al. Efficacy of PARP inhibitor rucaparib in orthotopic glioblastoma xenografts is limited by ineffective drug penetration into the central nervous system. Mol Cancer Ther. 2015;13(12):2735–43.
Durmus S, Sparidans RW, van Esch A, Wagenaar E, Beijnen JH, Schinkel AH. Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) Restrict Oral Availability and Brain Accumulation of the PARP Inhibitor Rucaparib (AG-014699). Pharm Res. 2015;32(1):37–46.
Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, et al. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64(4):1242–6.
Kim JH, Park JM, Roh YJ, Kim I-W, Hasan T, Choi M-G. Enhanced efficacy of photodynamic therapy by inhibiting ABCG2 in colon cancers. BMC Cancer. 2015;15(1):504.
Robey RW, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol Ther. 2005;4(2):195–202.
Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, et al. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res. 2007;13(8):2463–70.
Morgan J, Jackson JD, Zheng X, Pandey SK, Pandey RK. Substrate affinity of photosensitizers derived from chlorophyll-a: the ABCG2 transporter affects the phototoxic response of side population stem cell-like cancer cells to photodynamic therapy. Mol Pharm. 2010;7(5):1789–804.
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401.
Jeffes EW, McCullough JL, Weinstein GD, Fergin PE, Nelson JS, Shull TF, et al. Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid: a pilot dose-ranging study. Arch Dermatol. 1997;133(6):727–32.
Piacquadio DJ, Chen DM, Farber HF, Fowler Jr JF, Glazer SD, Goodman JJ, et al. Photodynamic Therapy With Aminolevulinic Acid Topical Solution andVisible Blue Light in the Treatment of Multiple Actinic Keratoses of the Faceand Scalp: Investigator-Blinded, Phase 3, Multicenter Trials. Arch Dermatol. 2004;140(1):41–6.
Barron GA, Moseley H, Woods JA. Differential sensitivity in cell lines to photodynamic therapy in combination with ABCG2 inhibition. J Photochem Photobiol B Biol. 2013;126:87–96.
Hagiya Y, Endo Y, Yonemura Y, Takahashi K, Ishizuka M, Abe F, et al. Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagn Photodyn Ther. 2012;9(3):204–14.
Usuda J, Tsunoda Y, Ichinose S, Ishizumi T, Ohtani K, Maehara S, et al. Breast cancer resistant protein (BCRP) is a molecular determinant of the outcome of photodynamic therapy (PDT) for centrally located early lung cancer. Lung Cancer. 2010;67(2):198–204.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–68.
Tomao F, Papa A, Rossi L, Strudel M, Vici P, Lo Russo G, et al. Emerging role of cancer stem cells in the biology and treatment of ovarian cancer: basic knowledge and therapeutic possibilities for an innovative approach. J Exp Clin Cancer Res. 2013;32:48.
Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–51.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8.
Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci. 1999;96(16):9118–23.
Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–806.
Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci. 2002;99(19):12339–44.
Zhou S, Schuetz JD, Bunting KD, Colapietro A-M, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–34.
Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99(2):507–12.
Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8(1):22–8.
Bhattacharya S, Jackson JD, Das AV, Thoreson WB, Kuszynski C, James J, et al. Direct identification and enrichment of retinal stem cells/progenitors by Hoechst dye efflux assay. Invest Ophthalmol Vis Sci. 2003;44(6):2764–73.
Hulspas R, Quesenberry PJ. Characterization of neurosphere cell phenotypes by flow cytometry. Cytometry. 2000;40(3):245–50.
Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827–33.
Wang J, Guo L-P, Chen L-Z, Zeng Y-X, Lu SH. Identification of cancer stem cell–like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67(8):3716–24.
Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell–like properties. Hepatology. 2006;44(1):240–51.
Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101(3):781–6.
Hirschmann-Jax C, Foster A, Wulf G, Nuchtern J, Jax T, Gobel U, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101(39):14228–33.
Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci. 2006;103(30):11154–9.
Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 2004;298(1):144–54.
Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207–19.
Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278(25):22644–9.
Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64(3):610–8.
Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistance protein–mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res. 2005;65(15):6640–50.
Janvilisri T, Venter H, Shahi S, Reuter G, Balakrishnan L, van Veen HW. Sterol transport by the human breast cancer resistance protein (ABCG2) expressed in Lactococcus lactis. J Biol Chem. 2003;278(23):20645–51.
Gangavarapu KJ, Azabdaftari G, Morrison CD, Miller A, Foster BA, Huss WJ. Aldehyde dehydrogenase and ATP binding cassette transporter G2 (ABCG2) functional assays isolate different populations of prostate stem cells where ABCG2 function selects for cells with increased stem cell activity. Stem Cell Res Ther. 2013;4(5):132.
Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67(10):4807–15.
Floyd MSJ, Teahan S, Fitzpatrick J, Watson R. Differential mechanisms of bicalutamide-induced apoptosis in prostate cell lines. Prostate Cancer Prostatic Dis. 2008;12(1):25–33.
Hill BT, Moran E, Etiévant C, Perrin D, Masterson A, Larkin A, et al. Low-dose twice-daily fractionated X-irradiation of ovarian tumor cells in vitro generates drug-resistant cells overexpressing two multidrug resistance-associated proteins, P-glycoprotein and MRP1. Anti-Cancer Drugs. 2000;11(3):193–200.
Henness S, Davey MW, Harvie RM, Davey RA. Fractionated irradiation of H69 small-cell lung cancer cells causes stable radiation and drug resistance with increased MRP1, MRP2, and topoisomerase IIα expression. Int J Radiat Oncol Biol Phys. 2002;54(3):895–902.
Bottke D, Koychev D, Busse A, Heufelder K, Wiegel T, Thiel E, et al. Fractionated irradiation can induce functionally relevant multidrug resistance gene and protein expression in human tumor cell lines. Radiat Res. 2008;170(1):41–8.
Leitner HM, Kachadourian R, Day BJ. Harnessing drug resistance: using ABC transporter proteins to target cancer cells. Biochem Pharmacol. 2007;74(12):1677–85.
Ning Z, Huang Y, Lin T, Zhou Y, Jiang C, Xu K, et al. Subpopulations of stem-like cells in side population cells from the human bladder transitional cell cancer cell line T24. J Int Med Res. 2009;37(3):621–30.
Ingram WJ, Crowther LM, Little EB, Freeman R, Harliwong I, Veleva D, et al. ABC transporter activity linked to radiation resistance and molecular subtype in pediatric medulloblastoma. Exp Hematol Oncol. 2013;2:26.
Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, et al. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;58(24):5850–8.
Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and Specific Inhibition of the Breast Cancer Resistance Protein Multidrug Transporter in Vitro and in Mouse Intestine by a Novel Analogue of Fumitremorgin C. Mol Cancer Ther. 2002;1(6):417–25.
Lewis C, Anderson J, Smith J. Health-related aspects of the genus Aspergillus. Aspergillus: Springer; 1994. p. 219–61.
Gardner ER, Smith NF, Figg WD, Sparreboom A. Influence of the dual ABCB1 and ABCG2 inhibitor tariquidar on the disposition of oral imatinib in mice. J Exp Clin Cancer Res. 2009;28(1):99.
Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37(2):359–65.
Eadie L, Hughes T, White D. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin Pharmacol Ther. 2014;95(3):294–306.
Dohse M, Scharenberg C, Shukla S, Robey RW, Volkmann T, Deeken JF, et al. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010;38(8):1371–80.
Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, et al. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64(7):2333–7.
Mazard T, Causse A, Simony J, Leconet W, Vezzio-Vie N, Torro A, et al. Sorafenib overcomes irinotecan resistance in colorectal cancer by inhibiting the ABCG2 drug-efflux pump. Mol Cancer Ther. 2013;12(10):2121–34.
Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol. 2013;31(10):1341–7.
Samalin E, Bouché O, Thézenas S, Francois E, Adenis A, Bennouna J, et al. Sorafenib and irinotecan (NEXIRI) as second-or later-line treatment for patients with metastatic colorectal cancer and KRAS-mutated tumours: a multicentre Phase I/II trial. Br J Cancer. 2014;110(5):1148–54.
Kobayashi H, Dorai T, Holland JF, Ohnuma T. Reversal of drug sensitivity in multidrug-resistant tumor cells by an MDR1 (PGY1) ribozyme. Cancer Res. 1994;54(5):1271–5.
Zhang W, Li J, Allen SM, Weiskircher EA, Huang Y, George RA, et al. Silencing the breast cancer resistance protein expression and function in caco-2 cells using lentiviral vector-based short hairpin RNA. Drug Metab Dispos. 2009;37(4):737–44.
Priebsch A, Rompe F, Tönnies H, Kowalski P, Surowiak P, Stege A, et al. Complete reversal of ABCG2-depending atypical multidrug resistance by RNA interference in human carcinoma cells. Oligonucleotides. 2006;16(3):263–74.
Ee PR, He X, Ross DD, Beck WT. Modulation of breast cancer resistance protein (BCRP/ABCG2) gene expression using RNA interference. Mol Cancer Ther. 2004;3(12):1577–84.
Li W, Zhou G, Song X, Chi W, Ren R, Wang X. Modulation of BCRP mediated atypical multidrug resistance phenotype by RNA interference. Neoplasma. 2004;52(3):219–24.
Whitehead KA, Dahlman JE, Langer RS, Anderson DG. Silencing or stimulation? siRNA delivery and the immune system. Annu Rev Chem Biomol Eng. 2011;2:77–96.
Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–33.
Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–70.
Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–17.
Nakanishi T, Shiozawa K, Hassel BA, Ross DD. Complex interaction of BCRP/ABCG2 and imatinib in BCR-ABL–expressing cells: BCRP-mediated resistance to imatinib is attenuated by imatinib-induced reduction of BCRP expression. Blood. 2006;108(2):678–84.
Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275(39):30069–74.
Chen Z-S, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-β-D-glucuronide by multidrug resistance protein 4 resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276(36):33747–54.
Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim I-W, et al. Sildenafil reverses ABCB1-and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71(8):3029–41.
Ding R, Shi J, Pabon K, Scotto KW. Xanthines down-regulate the drug transporter ABCG2 and reverse multidrug resistance. Mol Pharmacol. 2012;81(3):328–37.
Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9(9):677–8.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54.
Yoshikawa M, Ikegami Y, Hayasaka S, Ishii K, Ito A, Sano K, et al. Novel camptothecin analogues that circumvent ABCG2‐associated drug resistance in human tumor cells. Int J Cancer. 2004;110(6):921–7.
Endo M, Miwa M, Ura M, Tanimura H, Taniguchi K, Miyazaki Y, et al. A water soluble prodrug of a novel camptothecin analog is efficacious against breast cancer resistance protein-expressing tumor xenografts. Cancer Chemother Pharmacol. 2010;65(2):363–71.
Duan J-X, Cai X, Meng F, Sun JD, Liu Q, Jung D, et al. 14-Aminocamptothecins: Their synthesis, preclinical activity, and potential use for cancer treatment. J Med Chem. 2011;54(6):1715–23.
Takagi K, Dexheimer TS, Redon C, Sordet O, Agama K, Lavielle G, et al. Novel E-ring camptothecin keto analogues (S38809 and S39625) are stable, potent, and selective topoisomerase I inhibitors without being substrates of drug efflux transporters. Mol Cancer Ther. 2007;6(12):3229–38.
Ling X, Cao S, Cheng Q, Keefe JT, Rustum YM, Li F. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS One. 2012;7(9):e45571.
Ling X, Xu C, Fan C, Zhong K, Li F, Wang X. FL118 Induces p53-Dependent Senescence in Colorectal Cancer Cells by Promoting Degradation of MdmX. Cancer Res. 2014;74(24):7487–97.
Zhao J, Ling X, Cao S, Liu X, Wan S, Jiang T, et al. Antitumor Activity of FL118, a Survivin, Mcl-1, XIAP, and cIAP2 Selective Inhibitor, Is Highly Dependent on Its Primary Structure and Steric Configuration. Mol Pharm. 2014;11(2):457–67.
Ling X, Li F. An intravenous (iv) route-compatible formulation of FL118, a survivin, Mcl-1, XIAP, and cIAP2 selective inhibitor, improves FL118 antitumor efficacy and therapeutic index (TI). Am J Transl Res. 2013;5(2):139–54.
Westover D, Ling X, Lam H, Welch J, Jin C, Gongora C, et al. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol Cancer. 2015;14(1):92.
Ling X, Liu X, Zhong K, Smith N, Prey J, Li F. FL118, a novel camptothecin analogue, overcomes irinotecan and topotecan resistance in human xenograft models. Am J Transl Res. 2015;7(10):1765–81.
Selbo PK, Weyergang A, Eng MS, Bostad M, Mælandsmo GM, Høgset A, et al. Strongly amphiphilic photosensitizers are not substrates of the cancer stem cell marker ABCG2 and provides specific and efficient light-triggered drug delivery of an EGFR-targeted cytotoxic drug. J Control Release. 2012;159(2):197–203.
Acknowledgements
We would like to thank Drs. Wendy Huss, Kalyan Gangavarapu, and Ms. Neha Sabnis for constructive discussion, and Madam Katherine Plante for her editorial check of this manuscript before submission.
Funding
This work was sponsored in part by grants from the U.S. Department of Defense (W81XWH-12-1-0305) and NIH/NCI (CA180764) to Fengzhi Li, and by a University at Buffalo Arthur A. Schomburg Fellowship Award to David Westover.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
FL118 will be further developed in Canget BioTekpharma LLC, a Roswell Park Cancer Institute spinoff company. FL is an initial investor in Canget BioTekpharma.
Authors’ contributions
FL and DW conceived of this review, wrote and edited the manuscript, and created the accompanying table. Both authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Westover, D., Li, F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J Exp Clin Cancer Res 34, 159 (2015). https://doi.org/10.1186/s13046-015-0275-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-015-0275-x