Abstract
Background
Natural killer/T-cell lymphoma (NKTCL) is an Epstein–Barr virus (EBV)-associated, highly aggressive lymphoma. Treatment outcome remains sub-optimal, especially for advanced-stage or relapsed diseases. Programmed cell death receptor 1 (PD-1) and PD ligand 1 (PD-L1) have become promising therapeutic targets for various malignancies, but their role in the pathogenesis and their interactions with EBV in NKTCL remains to be investigated.
Methods
Expression of PD-L1 was measured in NK-92 (EBV-negative) and SNK-6 (EBV-positive) cells by western blot, quantitative real-time PCR and enzyme-linked immunosorbent assay, and flow cytometry, respectively. Latent membrane protein 1 (LMP1)-harboring lentiviral vectors were transfected into NK-92 cells to examine the correlation between LMP1 and PD-L1 expression. Proteins in the downstream pathways of LMP1 signaling were measured in NK-92 cells transfected with LMP1-harboring or negative control vectors as well as in SNK-6 cells. PD-L1 expression on tumor specimens and serum concentration of soluble PD-L1 were collected in a retrospective cohort of patients with Ann Arbor stage I~II NKTCL, and their prognostic significance were analyzed.
Results
Expression of PD-L1 was significantly higher in SNK-6 cells than in NK-92 cells, at both protein and mRNA levels. Expression of PD-L1 was remarkably upregulated in NK-92 cells transfected with LMP1-harboring lentiviral vectors compared with those transfected with negative control vectors. Proteins in the MAPK/NF-κB pathway were upregulated in LMP1-expressing NK-92 cells compared with the negative control. Selective inhibitors of those proteins induced significant downregulation of PD-L1 expression in LMP1-expressing NK-92 cells as well as in SNK-6 cells. Patients with a high concentration of serum soluble PD-L1 (≥3.4 ng/ml) or with a high percentage of PD-L1 expression in tumor specimens (≥38 %) exhibited significantly lower response rate to treatment and remarkably worse survival, compared with their counterparts. A high concentration of serum soluble PD-L1 and a high percentage of PD-L1 expression in tumor specimens were independent adverse prognostic factors among patients with stage I~II NKTCL.
Conclusions
PD-L1 expression positively correlated LMP1 expression in NKTCL, which was probably mediated by the MAPK/NF-κB pathway. PD-L1 expression in serum and tumor tissues has significant prognostic value for early-stage NKTCL.
Similar content being viewed by others
Background
Natural killer/T-cell lymphoma (NKTCL) is a distinct and aggressive clinicopathologic entity in the World Health Organization (WHO) classification of hematopoietic and lymphoid malignancies [1–3]. NKTCL is rare in North America and Europe but is more common in East Asia and South America [4]. This disease predominates in young males, and most cases originate from the nasal cavity [1, 2, 4]. No standard treatment strategy has been established due to the rarity of NKTCL. Radiotherapy (RT) has yielded curative effects for early-stage disease [5–7]. Anthracycline-based chemotherapeutic regimens have shown disappointing efficacy, probably due to the overexpression of multidrug-resistant genes [8–10]. Novel regimens containing l-asparaginase or pegaspargase have elicited promising responses [11–13]. However, the outcome of NKTCL remains sub-optimal, especially for advanced-stage or relapsed diseases [14, 15]. Therefore, it is in urgent need to identify novel therapeutic targets and corresponding agents.
Tumor immune escape is an emerging hallmark of cancer. Programmed cell death receptor 1 (PD-1) and PD ligand 1 (PD-L1) are important immune checkpoint molecules involved in T cell-mediated immune response and are key regulators of tumor immune escape [16–18]. Aberrant expression of PD-1/PD-L1 on tumor cells or tumor-infiltrating lymphocytes has conferred adverse prognostic impact in multiple solid and hematopoietic malignancies [19–23]. Blockade of the PD-1/PD-L1 interactions with monoclonal antibodies has achieved encouraging efficacy and has been approved by the US Food and Drug Administration (FDA) in many malignancies [24–27]. Expression of PD-L1 on tumor cells has been reported in patients with NKTCL, which may be a potential therapeutic target in the future [28, 29]. However, the role of PD-1/PD-L1 in the pathogenesis of NKTCL remains poorly understood.
There is a close correlation between Epstein–Barr virus (EBV) infection and NKTCL. Almost all cases of NKTCL exhibited a positive result of EBV-encoded RNA in situ hybridization in tumor samples. Additionally, pre- and post-treatment levels of circulating EBV DNA had significant prognostic implications for NKTCL patients [30–33]. In previous studies, expression of PD-1/PD-L1 could be upregulated by EBV infection in various malignancies [34–37], and blockade of PD-1/PD-L1 interactions successfully inhibited EBV-induced lymphoma growth in a mouse model [38], suggesting a possible interaction between EBV and PD-1/PD-L1 pathway in tumor immunology. Whether such an interaction exists in NKTCL, a typical EBV-associated malignancy, remains to be explored. In the present study, we aim to explore the interaction between EBV infection and PD-L1 expression in NKTCL cell lines and the clinical significance of PD-L1 expression in NKTCL patients.
Methods
Cell lines and culture
The SNK-6 and NK-92 cells were routinely kept in Sun Yat-sen University Cancer Center (SYSUCC) and were incubated in a humidified incubator with 5 % CO2 at 37 °C. The SNK-6 cell line was cultured in RPMI-1640 (Gibco, USA) medium containing 2 mmol/l glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, supplemented with 1000 U/ml interleukin (IL)-2 (Sigma-Aldrich, USA) and 10 % human AB serum (Gemini Bioproducts, Woodland, CA, USA). The NK-92 cells were maintained in α-MEM (Life Technologies, Karlsruhe, Germany) containing 20 % FBS (Gibco, USA), 2 mM l-glutamate, 100 mg/ml penicillin, and 100 mg/ml streptomycin (Life Technologies) and supplemented with 10 ng/ml IL-2 (Sigma-Aldrich, USA).
Western blot analysis
Cells were washed with ice-cold PBS and were suspended in radioimmunoprecipitation assay (RIPA) lysis buffer (Biyuntian Biotech, Shanghai, China) containing 1 % phenylmethylsulfonyl. After centrifugation at 14,000 rpm for 10 min at 4 °C, the protein content of supernatant was determined using the Pierce™ BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Aliquots (20 μg protein per lane) were separated by 12 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were exposed to primary antibodies and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1: 2000, Abcam) at room temperature for 1 h, followed by labeling with horseradish peroxidase-conjugated goat anti-rabbit IgG (1: 20,000, Boster, Wuhan, China) for 40 min at room temperature. Signals were detected with enhanced chemiluminescence plus reagents (Amersham Pharmacia, Piscataway, NJ, USA). GAPDH was used as the internal control. The following primary antibodies were used: PD-L1, B-Raf, p-B-Raf, p38, p-p38, ERK, pERK (Abcam, Shanghai, China), latent membrane protein 1 (LMP1), JNK, pJNK (Santa Cruz, Shanghai, China), and p65 (Boster, Wuhan, China).
Quantitative real-time PCR (qRT-PCR) analysis
In order to quantify PD-L1 and LMP1 mRNA, total RNA was isolated from SNK-6 and NK-92 cells using TRIZOL Reagent (Invitrogen, USA) according to the instruction manual. One microgram of the total RNA was reversely transcribed into cDNA using Bestar™ qPCR RT Kit (DBI Bioscience, China). The qRT-PCR reaction was prepared in a total volume of 20 μl containing 10 μl DBI Bestar® SybrGreen qPCR Master Mix (DBI Bioscience, China), cDNA derived from 0.2 μg of input RNA, 5 pM each primer, and 7 μl double-distilled H2O. The PCR was run on Stratagene Mx3000P Real-Time PCR system (Agilent Technologies, USA). The fluorescent quantity PCR conditions were as follows: pre-denaturation at 95 °C for 2 min, followed by 40 cycles of 94 °C for 20 s, 58 °C for 20 s, and 72 °C for 30 s. Primers were as follows: PD-L1 forward 5′-GAACTACCTCTGGCACATCCT-3′, PD-L1 reverse 5′-CACATCCATCATTCTCCCTTT-3′; LMP1 forward 5′-CAACAACGGCAAGACTCCC-3′, LMP1 reverse 5′-CCTCAAAGAAGCCACCCTC-3′. Each reaction was replicated three times. The fold changes in cDNA relative to the GAPDH endogenous control were calculated using the 2−ΔΔCt method [39].
Measurement of soluble PD-L1 in cell culture supernatant
The cell mixture was centrifuged at 1500 rpm for 5 min. The supernatant was collected and determined for the concentration of soluble PD-L1 using a sandwich enzyme-linked immunosorbent assay (ELISA) kit (PDCD1LG1 ELISA kit, Cloud-Clone Corp., Wuhan, China) according to the manufacturer’s instructions.
Flow cytometry analysis
Cells were labeled with anti-PD-L1 antibody (Alexa Fluor 647; Abcam, Shanghai, China) and then were assayed by flow cytometry using the Cytomics FC 500MPL cytometer. Data were collected and analyzed with the CXP version 2.2 software (Beckman Coulter Inc.).
Construction of a LMP1-expressing NK-92 cell line
The LMP1-expressing lentivirus vector, LV5-LMP1, was constructed by the insertion of a full-length LMP1 cDNA into LV5 vector (GenePharma Co. Ltd., China) at NotI and BamHI sites. The LV5-LMP1 vector and LV5 control vector (LV5-NC) were, respectively, cotransfected with packaging vectors pGag/Pol, pRev, and pVSV-G (GenePharma Co. Ltd., China) into HEK-293T cells using Lipofectamine 2000 Transfection Reagent (Beyotime, Shanghai, China). After culturing for 72 h, the supernatants of the transfected cells were harvested. The lentiviral titers were determined by flow cytometric analysis for green fluorescence protein (GFP) expressed by viral vectors. 1 × 105/well NK-92 cells were infected with LV5-LMP1 and LV5-NC vectors, respectively, at a multiplicity of infection (MOI) of 300. After culturing for 48 h, western blot and ELISA were performed to determine the expression of proteins in NK-92 cells infected by LV5-LMP1 and LV5-NC, respectively.
Measurement of serum soluble PD-L1 in NKTCL patients
Serum samples were collected before treatment from 77 patients with newly diagnosed NKTCL between 2008 and 2015 at SYSUCC and from 15 healthy volunteers. Serum was collected from the whole blood by centrifuging at 4000×g and stored at −80 °C. The level of soluble PD-L1 was determined using a sandwich ELISA kit (PDCD1LG1 ELISA kit, Cloud-Clone Corp., Wuhan, China) as per the manufacturer’s protocol.
Immunohistochemical analysis of PD-L1 in biopsy specimen from NKTCL patients
Paraffin-embedded specimens were collected before treatment from the same NKTCL patients described above. Four-micrometer-thick sections were deparaffinized, rehydrated, and quenched. Immunohistochemical staining was performed using an anti-PD-L1 rabbit polyclonal antibody (1:50 dilution, Abcam, Cambridge, UK) and a two-stage immunohistochemical kit (ChemMate™ Envision Detection Kit, Peroxidase/DAB, Dako, Glostrup, Denmark) according to the manufacturer’s instructions. The number of all tumor cells and those with membrane PD-L1 staining were calculated manually under high magnification (×200) using Image Pro Plus 6.0 software (Media Cybernetics, Maryland, USA). Seven fields were calculated for each individual specimen to determine the percentage of tumor cells with membrane staining among all tumor cells. In order to minimize intra-tumor heterogeneity, two fields with the highest and lowest percentages were eliminated, and the average percentage of the remaining five fields was used to represent the level of PD-L1 expression for an individual.
Clinical data and treatment
Seventy-seven patients with previously untreated NKTCL diagnosed at SYSUCC between 2008 and 2015 were enrolled in this study. The diagnosis was based on the WHO classification of hematopoietic and lymphoid tumors, and all patients had positive results for EBV-encoded RNA (EBER) in situ hybridization (ISH) [1, 2]. The clinical characteristics and treatment modalities are summarized in Table 1. All patients had Ann Arbor stage I or II disease. The International Prognostic Index (IPI) and the natural killer/T-cell lymphoma prognostic index (NKPI) were calculated for risk stratification [40, 41]. The majority of patients were categorized into the low-risk IPI (0–1, 89.6 %) or NKPI (0–1, 72.7 %) group. All patients received induction chemotherapy followed by consolidative radiotherapy (RT) as their primary treatment. 66.2 % of the patients received GELOX (gemcitabine, l-asparaginase, and oxaliplatin) and 33.8 % received CHOP-L (cyclophosphamide, doxorubicin, vincristine, prednisone, and l-asparaginase) as induction chemotherapeutic regimen in dosages as previously reported [42]. Complete remission (CR) was achieved after primary therapy in 76.6 % of the patients. The median follow-up period for this cohort was 38.0 (9.4–79.0) months.
Statistical analysis
Continuous variables were compared using a two-tailed Student’s t test, and categorical variables were compared using the chi-square test or the Fisher’s exact test. Overall survival (OS) was measured from the date of diagnosis to the date of death or the most recent follow-up. Progression-free survival (PFS) was measured from the date of diagnosis to the date of disease progression, death, or the most recent follow-up. Survival data were calculated with the Kaplan-Meier method and compared using the log-rank test. Variables with statistical significance in univariate analysis were included in the multivariate analysis using a stepwise forward Cox regression model. Optimal cut-off values of serum and histological PD-L1 levels for predicting survival were determined using the receiver operating characteristics (ROC) curve analysis. The Spearman correlation test was used to explore the correlation between the serum and histological PD-L1 levels. Differences were considered statistically significant with a two-sided P value of <0.05. The statistical analysis was performed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA).
Results
PD-L1 expression was higher in EBV+NKTCL cell line
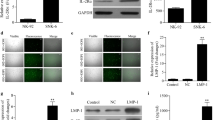
Western blot, ELISA, flow cytometry, and qRT-PCR were performed to determine protein and mRNA levels of PD-L1, respectively, in two cell lines: the human NK cell line NK-92 (EBV-negative) and the NKTCL cell line SNK-6 (EBV-positive). The protein level of PD-L1 was remarkably higher in SNK-6 cell line than that in NK-92 cells (Fig. 1a). Consistently, the relative expression level of PD-L1 mRNA in SNK-6 cells was also significantly higher than that in NK-92 cells (Fig. 1b, P < 0.05). ELISA of cell culture supernatants found a remarkably higher level of soluble PD-L1 produced by SNK-6 cells than NK-92 cells (Fig. 1c, P < 0.05). Additionally, expression of PD-L1 on cell surface was much higher in SNK-6 cells (Fig. 1e) than in NK-92 cells (Fig. 1d).
Expression of PD-L1 in NK cell line NK-92 (EBV-negative) and NKTCL cell line SNK-6 (EBV-positive). The level of a PD-L1 protein detected by western blot, b PD-L1 mRNA detected by quantitative real-time PCR, c soluble PD-L1 protein in cell culture supernatant detected by ELISA, and d, e PD-L1 expression on cell surface detected by flow cytometry in NK-92 and SNK-6 cell lines, respectively. ** P < 0.05
LMP1 upregulated PD-L1 expression in NK-92 cells
To investigate whether LMP1 expression in NK-92 cells was associated with upregulated PD-L1 level, we constructed a novel LMP1-expressing NK-92 cell line using a LV5-LMP1 lentiviral vector. Good infection efficiency of both LV5-LMP1 and LV5-NC vectors for NK-92 cell lines was observed at an MOI of 300 (Fig. 2a, b). Western blot and qRT-PCR found remarkably higher levels of LMP1 protein (Fig. 2c) and mRNA (Fig. 2d, P < 0.05) in NK-92 cells transfected with LV5-LMP1 vector, compared with those transfected with LV5-NC. Accordingly, remarkable upregulation of both PD-L1 protein (Fig. 2c) and mRNA (Fig. 2e, P < 0.05) was observed in the LMP1-expressing NK-92 cells compared with the negative control. ELISA found a significantly higher concentration of soluble PD-L1 in cell supernatants produced by LMP1-expressing NK-92 compared with the negative control (Fig. 2f, P < 0.05). Additionally, expression of PD-L1 on cell surface was much higher in LMP1-expressing NK-92 cells (Fig. 2h) than in the negative control (Fig. 2g).
Expression of PD-L1 was upregulated by LMP1 in NK-92 cells. a, b Infection efficiency of LV5-LMP1 vector (a) and LV5 vector (LV5-NC, b) in NK-92 cell line at a multiplicity of infection (MOI) of 100, 200, and 300, respectively. The level of c LMP1 and PD-L1 proteins detected by western blot, d LMP1 and e PD-L1 mRNA detected by quantitative real-time PCR, f soluble PD-L1 protein in cell culture supernatant detected by ELISA, and g, h PD-L1 expression on cell surface detected by flow cytometry in LMP1-expressing NK92 (LMP1) and negative control NK92 (NC) cell lines, respectively. ** P < 0.05
LMP1 upregulated PD-L1 expression through MAPK/NF-κB pathway in NK-92 cells
To explore the possible underlying mechanisms of PD-L1 upregulation induced by LMP1, we examined the expression of several proteins in the downstream pathways of LMP1. As shown in Fig. 3a, p-Raf-B, p-p38, p-JNK, p-ERK, and p65 were upregulated in the LMP1-expressing NK-92 cells compared with the negative control. A selective inhibitor of B-Raf (SB590885) effectively inhibited the expression of p-Raf-B, p-ERK, and p65 in LMP1-expressing NK-92 cells, resulting in reduced PD-L1 expression (Fig. 3b). Additionally, PD98059 (an ERK inhibitor), SB203580 (a p38 inhibitor), and SP600125 (a JNK inhibitor) could all effectively suppress the expression of p65 as well as PD-L1 (Fig. 3c–e). Finally, pyrrolidine dithiocarbamate (PDTC) as a selective inhibitor of NF-κB could remarkably reduce the expression of PD-L1 in LMP1-expressing NK-92 cells (Fig. 3f). These data suggested that MAPK/NF-κB pathway may be responsible for the LMP1-induced PD-L1 expression in human NK-92 cells. In addition, we further repeated the experiments above in SNK-6 cells. The results demonstrated that the effects of lowered PD-L1 expression seen in LMP1-expressing NK-92 cell line by B-Raf, ERK, p38, JNK, or NF-κB inhibition could also be observed in SNK-6 cells, which confirmed the observations made in the transfected cell line (see Additional file 1: Figure S1).
LMP1 upregulated PD-L1 expression through MAPK/NF-κB pathway in NK92 cells. a The expression level of p-Raf-B, Raf-B, p-p38, p38, p-JNK, JNK, p-ERK, ERK, and p65 in LMP1-expressing NK92 (LMP1) and negative control NK92 (NC) cell lines. b The expression level of p-Raf-B, Raf-B, p-p38, p38, p-JNK, JNK, p-ERK, ERK, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 0.1 μM SB590885, a selective B-Raf inhibitor for 1 h. c The expression level of p-ERK, ERK, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 20 μM PD98059, a selective ERK inhibitor for 1 h. d The expression level of p-p38, p38, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 10 μM SB203580, a selective p38 inhibitor for 1 h. e The expression level of p-JNK, JNK, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 20 μM SP600125, a selective JNK inhibitor for 1 h. f The expression level of p65 and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 100 μM pyrrolidine dithiocarbamate (PDTC), a selective inhibitor of NF-κB for 1 h
Pretreatment histological PD-L1 expression and serum soluble PD-L1 concentration correlated with survival in early-stage NKTCL patients
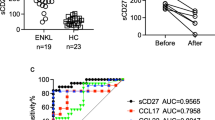
To explore the clinical significance of PD-L1 in NKTCL, we retrospectively examined the level of PD-L1 expression in paraffin-embedded tissues as well as the serum concentration of soluble PD-L1 in 77 patients with stage I–II disease. As shown in Fig. 4b, the expression level of PD-L1 in tumor tissues positively correlated with pretreatment serum concentration of soluble PD-L1 (P < 0.001). Patients with NKTCL had a significantly higher concentration of serum soluble PD-L1 than healthy individuals (Fig. 4a, P < 0.001).
The optimal cut-off value of serum PD-L1 was 3.4 ng/ml based on the ROC analysis (see Additional file 2: Figure S2A). As shown in Table 1, clinical characteristics were almost comparable between patients with low (<3.4 ng/ml) or high (≥3.4 ng/ml) concentration of serum PD-L1, except a male predominance observed in patients with high levels of serum PD-L1 (73.1 vs. 45.1 %, P = 0.020). After primary therapy, patients with a serum PD-L1 ≥ 3.4 ng/ml achieved a significantly lower CR rate than those with a serum PD-L1 < 3.4 ng/ml (61.5 vs. 84.3 %, P = 0.026, Table 1). Univariate analysis showed a significantly inferior survival in patients with a serum PD-L1 ≥ 3.4 ng/ml compared with those with a serum PD-L1 < 3.4 ng/ml (3-year PFS: 23.5 vs. 81.4 %, P < 0.001, Fig. 5a; 3-year OS: 45.3 vs. 91.0 %, P < 0.001, Fig. 5b). Serum PD-L1 ≥ 3.4 ng/ml remained an independent adverse prognostic factor for PFS and OS in multivariate analysis (Table 2).
a Progression-free survival (PFS) for NKTCL patients with a serum soluble PD-L1 of <3.4 and ≥3.4 ng/ml. b Overall survival (OS) for NKTCL patients with a serum soluble PD-L1 of <3.4 and ≥3.4 ng/ml. c PFS for NKTCL patients with a histological PD-L1 expression of <38 and ≥38 %. c OS for NKTCL patients with a histological PD-L1 expression of <38 and ≥38 %
Representative images for PD-L1 staining in tumor tissues are shown in Additional file 3: Figure S3. The optimal cut-off value of histological PD-L1 expression in tumor tissues was 38 % based on the ROC analysis (see Additional file 2: Figure S2B). Histological PD-L1 expression was not associated with clinical characteristics (Table 1). A significantly lower rate of CR after treatment was observed in patients with PD-L1 expression of ≥38 % compared with those with PD-L1 expression of <38 % (61.5 vs. 84.3 %, P = 0.026, Table 1). Univariate analysis showed a significantly worse survival in patients with a PD-L1 expression of ≥38 % compared with those with a PD-L1 expression of <38 % (3-year PFS 25.6 vs. 82.6 %, P < 0.001, Fig. 5c; 3-year OS 46.5 vs. 90.4 %, P < 0.001, Fig. 5d). Multivariate analysis found a PD-L1 expression of ≥38 % an independent adverse prognostic factor for PFS and OS (Table 3).
Discussion
Due to the relative rarity, sub-optimal current treatment strategies, and commonly observed chemoresistance of NKTCL, it is urgently warranted to identify novel therapeutic targets. Blockade of PD-1/PD-L1 interactions has emerged as a promising immunotherapy for cancer patients [24–27]. Previous studies have revealed aberrant expressions of PD-1/PD-L1 in NKTCL cell lines and tissues as well as involvement of PD-1/PD-L1 in the downregulation of antitumor immunity, suggesting that PD-1/PD-L1 may serve as a potential candidate for immunotherapy in NKTCL [28]. In the present study, we focused on the interactions between EBV infection and PD-L1 expression in NKTCL cell lines, as well as the prognostic impact of PD-L1 expression in NKTCL patients. Our findings included the following: (1) PD-L1 expression positively correlated LMP1 expression at both protein and mRNA levels in NKTCL and NK cells; (2) PD-L1 expression was upregulated by LMP1 through the MAPK/NF-κB pathway; and (3) the levels of PD-L1 expression on tumor tissues and the pre-treatment concentration of serum soluble PD-L1 correlated with the survival in early-stage NKTCL patients treated with asparaginase-containing chemotherapy combined with RT.
EBV plays a pivotal role in the pathogenesis of several hematopoietic malignancies [43]. It has been reported that overexpression of PD-L1 are commonly observed in EBV-associated lymphomas, including classical Hodgkin’s lymphoma, EBV-positive diffuse large B-cell lymphoma, angioimmunoblastic T-cell lymphoma, and NKTCL [28, 29, 43, 44]. Recent in vitro studies have found that LMP1, an EBV-encoded antigen, was able to upregulate PD-L1 expression in EBV-associated malignancies, such as Hodgkin’s lymphoma, post-transplant lymphoproliferative disorders, and nasopharyngeal carcinoma [37, 45]. In agreement with those results, we also observed a significant upregulation of PD-L1 expression at both protein and mRNA levels induced by LMP1 expression in NKTCL. First, the PD-L1 expression was much higher in EBV-positive SNK-6 cells than in the EBV-negative NK-92 cells. Additionally, the induction of LMP1 expression in NK-92 cells with a lentiviral vector resulted in remarkable elevations of PD-L1 protein and mRNA. Our findings imply that EBV infection in NKTCL probably upregulate PD-L1 expression on tumor cells via LMP1 antigen, and therefore induce immune tolerance.
The underlying mechanisms of PD-1/PD-L1 activation vary between different types of cancers [45–47]. Activation of the NF-κB pathway has been observed in NKTCL and is involved in proliferation, invasiveness, metastasis, and chemoresistance [48, 49]. LMP1 has been reported to contribute to the aberrant activation of the NF-κB pathway in NKTCL [50, 51]. In addition, previous studies using genome-wide miRNA expression profiling and exome sequencing revealed upregulation of the MAPK signaling pathway in NKTCL, but its biological significance remains to be understood [52, 53]. In the present study, the results showed a correlation between LMP1 and upregulation of PD-L1 expression in NK-92 cells, and the MAPK and NF-κB pathways were potentially involved. In line with our findings, Fang et al. reported that LMP1 upregulated PD-L1 through STAT3, MAPKs/AP-1, and NF-κB pathways in nasopharyngeal carcinoma [45]. The results mutually show that MAPK and NF-κB pathways may be potentially responsible for the LMP1-induced upregulation of PD-L1 expression in EBV-driven malignancies.
In a retrospective cohort of 30 NKTCL patients reported by Han et al., PD-L1 was aberrantly expressed in nasal NKTCL specimens compared with the rhinitis specimens and PD-L1 expression closely correlated with some clinical and histopathological parameters [28]. However, the prognostic impact of PD-L1 expression in NKTCL patients was not reported in this study. Another finding of our study is that the levels of PD-L1 expression on tumor tissues and the concentration of serum soluble PD-L1 correlated with survival in early-stage NKTCL patients. For early-stage (stage I~II) NKTCL, RT has been well established as the primary treatment [5–7], and chemotherapy may yield additional benefits for high-risk individuals [54]. Chemotherapies containing asparaginase (such as GELOX regimen) have produced superior response than anthracycline-based regimens (such as CHOP or EPOCH regimen) [8–13]. In our previous study, patients with early-stage NKTCL achieved a 3-year OS of 87.0 % after receiving GELOX chemotherapy plus RT, which was significantly better than that in patients receiving CHOP (54.0 %) or EPOCH (54.0 %) plus RT [42]. In the present study, early-stage NKTCL patients with a high concentration of serum soluble PD-L1 or a high percentage of PD-L1 expression on tumor tissues exhibited dismal survivals (3-year OS 45.3 and 46.5 %, respectively) even if they were uniformly treated with asparaginase-containing chemotherapy plus RT. Therefore, overexpression of PD-L1 confers a negative effect on survival for early-stage NKTCL and novel agents or treatment strategies are warranted for this particular subgroup of patients. Monoclonal antibodies blocking the PD-1/PD-L1 interactions have exhibited promising response in several types of lymphoma [25, 55]. There is an ongoing phase II study evaluating the efficacy and safety of pembrolizumab (a PD-1 antibody) in patients with relapsed/refractory T-cell lymphomas including NKTCL (NCT02535247), and its results may help offering another treatment choice for this relatively rare malignancy.
Several limitations exist in this retrospective study. The dynamic alterations of serum PD-L1 concentration after treatment and during follow-up, as well as their value in predicting relapse or prognosis, were not analyzed. Additionally, we did not analyze the prognostic impact of PD-L1 among patients with advanced-stage NKTCL due to the heterogeneous treatment delivered to those patients. Furthermore, we were unable to examine the levels of EBV mRNA and LMP1 expression in tumor samples due to the limited availability of paraffin-embedded tissues, which may provide useful information to understand the correlation between PD-L1 expression and EBV infection. Another issue that needs to be addressed is that the criteria of determining PD-L1 positivity varied among different studies. In Han’s study, the product of staining intensity and percentage of positive tumor cells was used to classify positive cases. However, in the present and some previous studies, only the percentage of cells with PD-L1 staining was used due to the heterogeneity of staining intensity, subjectivity of visional grading, and clinical feasibility [56–58]. A uniform and widely accepted standard to determine PD-L1 positivity is required for studies in the future.
Conclusions
The present study revealed a positive correlation between LMP1 and PD-L1 expression, which was probably mediated by the MAPK/NF-κB pathway in NKTCL. It also showed a significant prognostic value of PD-L1 expression level on tumor tissues and serum soluble PD-L1 concentration in early-stage NKTCL. Further studies are warranted to validate our findings in a prospective cohort and to explore the therapeutic value of PD-1/PD-L1 in NKTCL.
References
Chan JK, Quintanilla-Martinez L, Ferry JA, Peh S-C. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 285–8.
Chan JK, Jaffe ES, Ralfkiaer E. Extranodal NK/T-cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2001. p. 204–7.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–7.
Bi XW, Li YX, Fang H, Jin J, Wang WH, Wang SL, et al. High-dose and extended-field intensity modulated radiation therapy for early-stage NK/T-cell lymphoma of Waldeyer’s ring: dosimetric analysis and clinical outcome. Int J Radiat Oncol Biol Phys. 2013;87:1086–93.
Li YX, Wang H, Jin J, Wang WH, Liu QF, Song YW, et al. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1809–15.
Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–9.
Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol. 2008;83:795–9.
Kim BS, Kim DW, Im SA, Kim CW, Kim TY, Yoon SS, et al. Effective second-line chemotherapy for extranodal NK/T-cell lymphoma consisting of etoposide, ifosfamide, methotrexate, and prednisolone. Ann Oncol. 2009;20:121–8.
Wang L, Xia ZJ, Huang HQ, Lu Y, Zhang YJ. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in the treatment of stage IE/IIE extranodal natural killer/T cell lymphoma, nasal type: 13-year follow-up in 135 patients. Int J Hematol. 2012;96:617–23.
Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834–9.
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973–80.
Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–6.
Nie M, Bi XW, Zhang WW, Sun P, Xia Y, Liu PP, et al. Consolidative treatment after salvage chemotherapy improves prognosis in patients with relapsed extranodal natural killer/T-cell lymphoma. Sci Rep. 2016;6:23996.
Bi XW, Jiang WQ, Zhang WW, Huang JJ, Xia Y, Wang Y, et al. Treatment outcome of patients with advanced stage natural killer/T-cell lymphoma: elucidating the effects of asparaginase and postchemotherapeutic radiotherapy. Ann Hematol. 2015;94:1175–84.
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–75.
Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38.
Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–66.
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9.
Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. New Engl J Med. 2015;373:1803–13.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New Engl J Med. 2015;372:311–9.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. New Engl J Med. 2015;372:320–30.
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30.
Han L, Liu F, Li R, Li Z, Chen X, Zhou Z, et al. Role of programmed death ligands in effective T-cell interactions in extranodal natural killer/T-cell lymphoma. Oncol Lett. 2014;8:1461–9.
Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–73.
Lim SH, Hyun SH, Kim HS, Lee JY, Yoo KH, Jung KS, et al. Prognostic relevance of pretransplant Deauville score on PET-CT and presence of EBV DNA in patients who underwent autologous stem cell transplantation for ENKTL. Bone Marrow Transplant. 2016;51:807–12.
Kwong YL, Pang AW, Leung AY, Chim CS, Tse E. Quantification of circulating Epstein-Barr virus DNA in NK/T-cell lymphoma treated with the SMILE protocol: diagnostic and prognostic significance. Leukemia. 2014;28:865–70.
Kim HS, Kim KH, Kim KH, Chang MH, Ji SH, Lim DH, et al. Whole blood Epstein-Barr virus DNA load as a diagnostic and prognostic surrogate: extranodal natural killer/T-cell lymphoma. Leuk Lymphoma. 2009;50:757–63.
Lei KI, Chan LY, Chan WY, Johnson PJ, Lo YM. Diagnostic and prognostic implications of circulating cell-free Epstein-Barr virus DNA in natural killer/T-cell lymphoma. Clin Cancer Res. 2002;8:29–34.
Laurent C, Fabiani B, Do C, Tchernonog E, Cartron G, Gravelle P, et al. Immune-checkpoint expression in Epstein-Barr virus positive and negative plasmablastic lymphoma: a clinical and pathological study in 82 patients. Haematologica. 2016;101:976–84.
Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7:32925–32.
Fang W, Hong S, Chen N, He X, Zhan J, Qin T, et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget. 2015;6:33019–32.
Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O'Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–8.
Ma SD, Xu X, Jones R, Delecluse HJ, Zumwalde NA, Sharma A, et al. PD-1/CTLA-4 blockade inhibits Epstein-Barr virus-induced lymphoma growth in a cord blood humanized-mouse model. PLoS Pathog. 2016. doi:10.1371/journal.ppat.1005642.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402–8.
Shipp MA, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, Cabanillas F, et al. A predictive model for aggressive non-Hodgkin’s lymphoma: the international non-Hodgkin’s lymphoma prognostic factors project. New Engl J Med. 1993;329:987–94.
Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612–8.
Wang L, Wang WD, Xia ZJ, Zhang YJ, Xiang J, Lu Y. Combination of gemcitabine, L-asparaginase, and oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment of patients with stage IE/IIE extranodal natural killer/T cell lymphoma: a retrospective study in a cohort of 227 patients with long-term follow-up. Med Oncol. 2014;31:860.
Gru AA, Haverkos BH, Freud AG, Hastings J, Nowacki NB, Barrionuevo C, et al. The Epstein-Barr virus (EBV) in T cell and NK cell lymphomas: time for a reassessment. Curr Hematol Malig Rep. 2015;10:456–67.
Paydas S, Bagir E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94:1545–52.
Fang W, Zhang J, Hong S, Zhan J, Chen N, Qin T, et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5:12189–202.
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8.
Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–63.
Kim K, Ryu K, Ko Y, Park C. Effects of nuclear factor-kappaB inhibitors and its implication on natural killer T-cell lymphoma cells. Br J Haematol. 2005;131:59–66.
Liu X, Wang B, Ma X, Guo Y. NF-kappaB activation through the alternative pathway correlates with chemoresistance and poor survival in extranodal NK/T-cell lymphoma, nasal type. Jpn J Clin Oncol. 2009;39:418–24.
Sun L, Zhao Y, Shi H, Ma C, Wei L. LMP-1 induces survivin expression to inhibit cell apoptosis through the NF-kappaB and PI3K/Akt signaling pathways in nasal NK/T-cell lymphoma. Oncol Rep. 2015;33:2253–60.
Ng SB, Selvarajan V, Huang G, Zhou J, Feldman AL, Law M, et al. Activated oncogenic pathways and therapeutic targets in extranodal nasal-type NK/T cell lymphoma revealed by gene expression profiling. J Pathol. 2011;223:496–510.
Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY, Zhang ZG, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet. 2015;47:1061–6.
Ng SB, Yan J, Huang G, Selvarajan V, Tay JL, Lin B, et al. Dysregulated microRNAs affect pathways and targets of biologic relevance in nasal-type natural killer/T-cell lymphoma. Blood. 2011;118:4919–29.
Yang Y, Zhu Y, Cao JZ, Zhang YJ, Xu LM, Yuan ZY, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015;126:1424–32.
Matsuki E, Younes A. Checkpoint inhibitors and other immune therapies for Hodgkin and non-Hodgkin lymphoma. Curr Treat Options Oncol. 2016;17:31.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012. doi:10.1126/scitranslmed.3003689.
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med. 2012;366:2443–54.
Acknowledgements
We thank all the treating physicians for allowing us to enroll their patients and thank all the patients for allowing us to analyze their data.
Funding
This study was funded by National Natural Science Foundation of China [contract/grant number: 81400159], the Outstanding Young Talents Project of Sun Yat-sen University Cancer Center [contract/grant number: 04190101#], and the Clinical Medical Scientist Project of Sun-Yat sen University Cancer Center [contract/grant number: 09020101#].
Availability of data and materials
XWB, HW, and LW had full access to all the data in the study (available upon data specific request). Although all our data is de-identified, we opt not to share the data and materials in public due to further study on this subject. However, we will share the data in request by other researchers if necessary. All of the methods including the software programs or reagents used in this study are on the market, which are accessible by other researchers.
Authors’ contributions
XWB and LW designed the research and/or analyzed the data. HW, WWZ, JHW, WJL, ZJX, HQH, WQJ, and YJZ provided the clinical data. XWB, HW, LW, and WWZ wrote the manuscript. All authors read and approved the final manuscript. XWB, HW, and WWZ were co-first authors.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of SYSUCC and complied with country-specific regulatory requirements. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Patients provide informed consent authorizing the use of their personal information for research purposes.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Figure S1.
PD-L1 was upregulated in SNK-6 cells through MAPK/NF-κB pathway. (A) The expression level of p-Raf-B, Raf-B, p-p38, p38, p-JNK, JNK, p-ERK, ERK, p65, and PD-L1 in SNK-6 cells or SNK-6 cells treated with 0.1 μM SB590885, a selective B-Raf inhibitor for 1 h. (B) The expression level of p-ERK, ERK, p65, and PD-L1 in SNK-6 cells or SNK-6 cells treated with 20 μM PD98059, a selective ERK inhibitor for 1 h. (C) The expression level of p-p38, p38, p65, and PD-L1 in SNK-6 cells or SNK-6 cells treated with 10 μM SB203580, a selective p38 inhibitor for 1 h. (D) The expression level of p-JNK, JNK, p65, and PD-L1 in SNK-6 cells or SNK-6 cells treated with 20 μM SP600125, a selective JNK inhibitor for 1 h. (E) The expression level of p65 and PD-L1 in SNK-6 cells or SNK-6 cells treated with 100 μM pyrrolidine dithiocarbamate (PDTC), a selective inhibitor of NF-κB for 1 h. (TIF 1305 kb)
Additional file 2: Figure S2.
The receiver operating curve (ROC) of (A) pretreatment serum concentration of PD-L1 and (B) histological PD-L1 expression in tumor tissues. The optimal cut-off value for pretreatment serum concentration of PD-L1 to predict mortality is 3.4 ng/ml (area under curve = 0.799, sensitivity = 88.9 %, specificity = 72.9 %). The optimal cut-off value for histological PD-L1 expression in tumor tissues to predict mortality is 38 % (area under curve = 0.760, sensitivity = 77.8 %, specificity = 79.7 %). (TIF 873 kb)
Additional file 3: Figure S3.
Immunohistochemical analysis of PD-L1 expression in tumor tissues from patients with natural killer/T-cell lymphoma. Representative images of (A) strong and (B) weak cell membrane staining (brown) of PD-L1 are shown (×200 magnification). (TIF 7689 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bi, Xw., Wang, H., Zhang, Ww. et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol 9, 109 (2016). https://doi.org/10.1186/s13045-016-0341-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-016-0341-7