Abstract
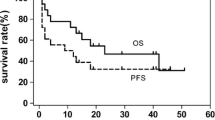
The prognosis of advanced stage natural killer/T-cell lymphoma (NKTCL) remains relatively disappointing, and the optimal treatment strategy for this disease has yet to be discovered. Seventy-three patients with Ann Arbor stage III or IV NKTCL were retrospectively reviewed. The treatment efficacies of asparaginase-containing and asparaginase-absent chemotherapy regimens were compared, and the effects of postchemotherapeutic radiotherapy were explored. The overall response rate (ORR) of the asparaginase-containing regimens was marginally higher than that of the asparaginase-absent regimens (56.5 vs 32.6 %, P = 0.057). However, no significant difference was observed in 2-year overall survival (OS) (38.3 vs 22.7 %, P = 0.418) or 2-year progression-free survival (PFS) (25.4 vs 14.9 %, P = 0.134) between the asparaginase-containing and asparaginase-absent groups. Postchemotherapeutic radiotherapy was associated with a significantly prolonged survival (2-year OS 57.5 vs 14.5 %, P < 0.001; 2-year PFS 46.3 vs 8.4 %, P < 0.001) and was an independent predictor of both OS and PFS. Radiotherapy significantly improved the prognosis among the patients who exhibited complete or partial remission after initial chemotherapy (2-year OS 81.5 vs 40.2 %, P = 0.002; 2-year PFS 65.6 vs 23.4 %, P = 0.008) but failed to provide a significant survival advantage among those who experienced stable or progressive disease after initial chemotherapy. In conclusion, the use of asparaginase did not significantly improve survival for the treatment of patients with stage III/IV NKTCL. Postchemotherapeutic radiotherapy provided additional prognostic benefits to patients who responded well to the initial chemotherapy, which requires further validation in future prospective studies using larger sample sizes.


Similar content being viewed by others
References
Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO, Liang R (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the international peripheral T-cell lymphoma project. Blood 113(17):3931–3937. doi:10.1182/blood-2008-10-185256
Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, Wang SL, Liu XF, Zhou LQ, He XH, Lu N, Yu ZH (2006) Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 24(1):181–189. doi:10.1200/JCO.2005.03.2573
Li YX, Wang H, Jin J, Wang WH, Liu QF, Song YW, Wang ZY, Qi SN, Wang SL, Liu YP, Liu XF, Yu ZH (2012) Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys 82(5):1809–1815. doi:10.1016/j.ijrobp.2010.10.040
Aviles A, Neri N, Fernandez R, Huerta-Guzman J, Nambo MJ (2013) Combined therapy in untreated patients improves outcome in nasal NK/T lymphoma: results of a clinical trial. Med Oncol 30(3):637. doi:10.1007/s12032-013-0637-1
Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, Morschhauser F, Thieblemont C, Ysebaert L, Devidas A, Petit B, de Leval L, Gaulard P, Feuillard J, Bordessoule D, Hermine O (2011) Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117(6):1834–1839. doi:10.1182/blood-2010-09-307454
Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, Suzumiya J, Kodama T, Kimura H, Hyo R, Nakamura S, Oshimi K, Suzuki R (2011) Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 29(33):4410–4416. doi:10.1200/JCO.2011.35.6287
Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, Lee DH, Huh J, Oh SY, Kwon HC, Kim HJ, Lee SI, Kim JH, Park J, Oh SJ, Kim K, Jung C, Park K, Kim WS (2006) Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 24(4):612–618. doi:10.1200/JCO.2005.04.1384
Kim TM, Lee SY, Jeon YK, Ryoo BY, Cho GJ, Hong YS, Kim HJ, Kim SY, Kim CS, Kim S, Kim JS, Sohn SK, Song HH, Lee JL, Kang YK, Yim CY, Lee WS, Yuh YJ, Kim CW, Heo DS (2008) Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol 19(8):1477–1484. doi:10.1093/annonc/mdn147
Jo JC, Yoon DH, Kim S, Lee BJ, Jang YJ, Park CS, Huh J, Lee SW, Ryu JS, Suh C (2012) Clinical features and prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol 89(2):103–110. doi:10.1111/j.1600-0609.2012.01796.x
Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, Abe M, Kinoshita T, Yoshino T, Iwatsuki K, Kagami Y, Tsuzuki T, Kurokawa M, Ito K, Kawa K, Oshimi K (2010) Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol 21(5):1032–1040. doi:10.1093/annonc/mdp418
Ji J, Liu T, Xiang B, Liu W, He C, Chen X, Li J, Chang H, Dai Y, Dong T (2014) A study of gemcitabine, l-asparaginase, ifosfamide, dexamethasone and etoposide chemotherapy for newly diagnosed stage IV, relapsed or refractory extranodal natural killer/T-cell lymphoma, nasal type. Leuk Lymphoma. doi:10.3109/10428194.2014.907894
Kim HJ, Bang SM, Lee J, Kwon HC, Suh C, Kim HJ, Lee JH, Ryoo BY, Park YH, Kwon JM, Oh SY, Lee HR, Kim K, Jung CW, Park K, Kim WS (2006) High-dose chemotherapy with autologous stem cell transplantation in extranodal NK/T-cell lymphoma: a retrospective comparison with non-transplantation cases. Bone Marrow Transplant 37(9):819–824. doi:10.1038/sj.bmt.1705349
Lee J, Au WY, Park MJ, Suzumiya J, Nakamura S, Kameoka J, Sakai C, Oshimi K, Kwong YL, Liang R, Yiu H, Wong KH, Cheng HC, Ryoo BY, Suh C, Ko YH, Kim K, Lee JW, Kim WS, Suzuki R (2008) Autologous hematopoietic stem cell transplantation in extranodal natural killer/T cell lymphoma: a multinational, multicenter, matched controlled study. Biol Blood Marrow Transplant 14(12):1356–1364. doi:10.1016/j.bbmt.2008.09.014
Chan JK, Quintanilla-Martinez L, Ferry JA, Peh S-C (2008) Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (eds) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC, Lyon, pp 285–288
Chan JK, Jaffe ES, Ralfkiaer E (2001) Extranodal NK/T-cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) WHO classification of tumours of haematopoietic and lymphoid tissues, 3rd edn. IARC, Lyon, pp 204–207
Shipp MA, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, Cabanillas F, Canellos GP, Coiffier B, Connors JM, Cowan RA, Crowther D, Dahlberg S, Engelhard M, Fisher RI, Gisselbrecht C, Horning SJ, Lepage E, Lister TA, Meerwaldt JH, Montserrat E, Nissen NI, Oken MM, Peterson BA, Tondini C, Velasquez WS, Yead BY (1993) A predictive model for aggressive non-Hodgkin's lymphoma: the International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 329(14):987–994. doi:10.1056/NEJM199309303291402
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17(4):1244–1253
Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, Egashira M, Schuster SM, Oshimi K (2005) Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol 130(6):860–868. doi:10.1111/j.1365-2141.2005.05694.x
Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y (2008) Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol 83(10):795–799. doi:10.1002/ajh.21256
Wang L, Wang WD, Xia ZJ, Zhang YJ, Xiang J, Lu Y (2014) Combination of gemcitabine, L-asparaginase, and oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment of patients with stage IE/IIE extranodal natural killer/T cell lymphoma: a retrospective study in a cohort of 227 patients with long-term follow-up. Med Oncol 31(3):860. doi:10.1007/s12032-014-0860-4
Kim SJ, Yang DH, Kim JS, Kwak JY, Eom HS, Hong DS, Won JH, Lee JH, Yoon DH, Cho J, Nam TK, Lee SW, Ahn YC, Suh C, Kim WS (2014) Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol 93(11):1895–1901. doi:10.1007/s00277-014-2137-6
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, Leung AY, Chim CS (2012) SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 120(15):2973–2980. doi:10.1182/blood-2012-05-431460
Kim M, Kim TM, Kim KH, Keam B, Lee SH, Kim DW, Lee JS, Jeon YK, Kim CW, Heo DS (2014) Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus L-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol. doi:10.1007/s00277-014-2228-4
Chauchet A, Michallet AS, Berger F, Bedgedjian I, Deconinck E, Sebban C, Antal D, Orfeuvre H, Corront B, Petrella T, Hacini M, Bouteloup M, Salles G, Coiffier B (2012) Complete remission after first-line radio-chemotherapy as predictor of survival in extranodal NK/T cell lymphoma. J Hematol Oncol 5:27. doi:10.1186/1756-8722-5-27
Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, Keijser A, Faivre-Finn C, Senan S (2014) Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. doi:10.1016/S0140-6736(14)61085-0
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81071950, 81301903).
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
This is a retrospective study, and all patients provided informed consent for the collection of medical information at their first medical visit. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xi-Wen Bi and Wen-Qi Jiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bi, XW., Jiang, WQ., Zhang, WW. et al. Treatment outcome of patients with advanced stage natural killer/T-cell lymphoma: elucidating the effects of asparaginase and postchemotherapeutic radiotherapy. Ann Hematol 94, 1175–1184 (2015). https://doi.org/10.1007/s00277-015-2336-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2336-9