Abstract
Background
Improvement of current GVHD prophylactic therapies remains an important goal in the allo-HSCT. We have described a novel prophylaxis regimen in a single institution trial. The Chinese Bone Marrow Transplant Cooperative Group (CBMTCG) initiated a phase II multicenter study.
Methods
The study was designed as a prospective, single arm phase II open-label, multicenter clinical trial. The primary endpoint was improvement of aGVHD by 25% over historical control (40%) in Chinese patients. 508 patients were enrolled. All of the patients received cyclosporine A (CsA), methotrexate (MTX) and mycophenolate mofetil (MMF) (0.5-1.0 g daily for 30 days) as GVHD prophylaxis regimen.
Results
The primary endpoint was met with cumulative incidences of grades 2 to 4 and grades 3 to 4 aGVHD of 23.2% and 10.3%, respectively. Incidence for cGVHD was 67.4%. The non-relapse mortality (NRM) rate was 18.4% at 2 years. The probabilities of leukemia free survival (LFS) for non-advanced stage and advanced stage patients at 2 years were 69.7% and 44.8% respectively (p = 0.000). Recipient age ≥ 40 years, advanced stage and Busulfan-Fludarabine(BuFlu) conditioning regimen were identified as major risk factors for aGVHD. Recipient age ≥ 40 years, BuFlu conditioning regimens, female donor/male recipient and prior aGVHD were associated with cGVHD. Despite lower RM (relapse mortality), patients with grade 2–4 aGVHD had higher NRM and worse OS and LFS compared to patients with grade 0–1 aGVHD. In contrast, patients with cGVHD had better OS and LFS and lower RM compared to patients without cGVHD.
Conclusion
The novel GVHD regimen decreased the risk for aGVHD by 42% without improving the risk for cGVHD compared to historical controls. Development of aGVHD was associated with worse OS and LFS as well as higher NRM. In contrast, cGVHD was associated with improved OS and LFS likely attributed to a GVL effect.
Similar content being viewed by others
Introduction
Despite the use of prophylaxis regimens, Graft-versus-host disease (GVHD) remains a major cause for mortality and morbidity with allogeneic hematopoietic stem cell \transplantations (allo-HSCT). It is also the primary cause of death in 16% and 18% of deaths after HLA-match sibling and unrelated donor allo-HSCT respectively [1]. A combination consisting of a calcineurin inhibitor (CNI), cyclosporine or tacrolimus, and either methotrexate, mycophenolate mofetil (MMF), or sirolimus are considered to be the standard prophylaxis regimens. However, review of US and European literature indicates that acute GVHD (aGVHD) still occurs in 35% to 65% of BMT patients receiving human leukocyte antigen (HLA)–matched sibling transplants, and even more frequently in unrelated donor transplant recipients [2]-[6]. Analysis of Chinese transplant registries as well as relevant Chinese publications calculate the overall incidence for grade 2–4 aGVHD at approximately 40% [7]-[9]. Thus, improved prophylactic approaches are needed. Most strategies employed to reducing both aGVHD and chronic GVHD (cGVHD) (e.g. T-cell depletion) have significant drawbacks as they are offset by high rates of graft failure, malignancy relapse, infections, and Epstein-Barr virus-associated lymphoproliferative disorders [10]-[12]. For patients with hematologic malignancies, “standard of care GVHD prophylaxis” seems to have struck a reasonable balance between preventing undesirable graft-versus-host reactions and retaining desirable graft-versus-tumor effects [13]. The risk for developing GVHD depends on various factors which are determined by the patient, disease characteristics as well as by the graft, its processing, and the transplant procedure/conditioning regimen employed. Thus far, no trials have been conducted where GVHD prophylaxis has been individually stratified to the probability of GVHD occurrence or disease relapse.
We have previously described a combination prophylaxis regimen consisting of cyclosporine A (CsA), methotrexate (MTX) and a low-dose, short-course mycophenolate mofetil (MMF) (0.5 daily for 30 days) in a single institution trial for a cohort of 100 patients with hematologic malignancies who underwent HLA-matched sibling allo-HSCT. The rationale behind a regimen designed with a short course of MMF was to primarily improve aGVHD without substantially impacting the incidence of cGVHD because of the associated beneficial GVL effect. Although cGVHD is an undesired complication of BMT, we hypothesized that this strategy would lead to reduced leukemia relapse to an extent which would result in an overall survival net benefit across all patients. We did indeed report a substantial decrease in the risk for aGVHD in our initial study [14]. In order to confirm the effectiveness of this new GVHD prophylaxis regimen, the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG) initiated a prospective, open-label, multicenter clinical trial using this prophylaxis regimen in 508 patients. Furthermore, we analyzed additional risk factors for GVHD in this population consisting entirely of Chinese patients.
Patients and methods
Patient eligibility
The trial was designed as a prospective, open-label, multicenter clinical protocol and was conducted by the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG), a cooperative transplant group consisting of seven Chinese transplant centers: the Peking University Institute of Hematology, n = 264; the First Affiliated Hospital of Guangxi Medical University, n = 81; the First Affiliated Hospital of Xinjiang Medical University, n = 50; Changzheng Hospital, the Second Military Medical University, n = 10; Kunming General Hospital of Chengdu Command, n = 55; Nanfang Hospital Southern Medical University, n = 28; First Affiliated Hospital Chongqing Medical University, n = 20. Patients with hematologic malignancies in need of an allo-HSCT who had an HLA-identical sibling donor were eligible. Additional eligibility criteria included: 1) Age:15 to 65 years old; 2) Medically suitable to tolerate a myeloablative (MA) conditioning regimen; 3) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; 4) Bilirubin ≤ 2 mg/dL, 5) creatinine < 1.5 times the upper limit of normal, 6) preserved heart and lung function; 7) Negative infectious evaluation (viral, bacterial and fungal). Between August 2007 and October 2010, 508 patients were enrolled and completed treatment. The protocol was approved by the Institutional Review Board of each center, and prior treatment written informed consent was obtained from both patients and donors. All participating institutions and investigators had subscribed to the principles and conduct of the WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Patient characteristics are summarized in Table 1.
Conditioning regimen
The protocol permitted use of three myeloablative conditioning regimens at investigators discretion: 1) BuCy: Busulfan (12–16 mg/kg) and cyclophosphamide (120 mg/kg) was given to 347 patients. 2) BuFlu: Busulfan (12–16 mg/kg) combined with Fludarabine (250 mg or 150 mg/m2) was given to 134 patients and Fludarabine (250 mg) combined with Melphalan (140 mg/m2) was given to one patient. 3) TBICy: TBI (7.7-10.0Gy) and cyclophosphamide (120 mg/kg) was given to 26 patients.
Procurement of hematopoietic stem cells
The protocol permitted use of two sources of hematopoietic stem cells at investigators discretion: 1) Peripheral Blood (PB): 258 patients were grafted with human granulocyte colony-stimulating factor (rHuG-CSF)–mobilized peripheral blood stem cells (PBSC).Donors were treated with G-CSF at a dose of 5–10 ug/kg/d subcutaneously for 4 consecutive days starting 5 days before leukapheresis with a target CD34 cell number of at least 4 × 106 CD34cells per kilogram of recipient weight. The median numbers of mononuclear cells (MNC) and CD34 cells infused were 9.11 (2.93-17.6) × 108/kg and 4.25 (1.15-16.6) × 106/kg, respectively. 2) PB + BM: 250 patients were grafted with a combination PBSC and bone marrow stem cells (BMSC). PBSC were harvested after donors were treated with G-CSF at a dose of 5–10 ug/kg/d subcutaneously for 4 consecutive days and bone marrow was harvested on the following day by standard technique using general anesthesia. The ratio of CD34 cells numbers of PB to BM was 2–4:1. The median numbers of MNCs and CD34 cells infused were 7.48(3.14-12.53) × 108/kg and 2.34 (0.40-7.45) × 106/kg, respectively. This combination grafting procedure is commonly used in China and based on publications previously describing improved outcomes [15].
GVHD prophylaxis
All of the transplant recipients received CsA, MTX, and a low-dose, short-course MMF. The dosage of CsA was 2.5-3 mg/kg/d, i.v., and CsA was administered from day 1 before transplantation until recovery of bowel function. At that point, the patient was switched to oral CsA. Serum CsA concentration was monitored, and the dosage was adjusted to achieve serum concentrations ranging between 150–300 ng/ml. MMF was administered orally as doses of 0.5-1.0 g/d from day 1 before transplantation to day 30 after transplantation. The dose was assigned by weight, with patients up to and below 60 kg receiving 0.5 mg and patients above 60 kg receiving 1.0 mg. MTX was administered i.v. at doses of 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6 and 11. Fifty-nine patients omitted the 4th dose of MTX. First-line therapy of clinically significant aGVHD consisted of methylprednisolone (MP) 1 to 2 mg/kg/d. Patients whose GVHD was refractory to steroid therapy could receive secondary therapy such as tacrolimus, CD25 antibody or other therapies at investigator discretion.
Definitions and assessments
Disease status at transplant was classified as “non-advanced stage” or “advanced stage.” The patients were categorized as “ non-advanced stage” if they were in complete remission (CR) from acute leukemia (AL) regardless of cytogenetics, chronic myelogenous leukemia (CML) in chronic phase (CP) or accelerated phase (AP), and myelodysplastic syndrome(MDS) with a blast count <20%. “Advanced stage” was defined as AL not in remission (NR) or CML in blast crisis (BC). Neutrophil engraftment was defined as the first day of an absolute neutrophil count (ANC) of 0.5 × 109/L or more for 3 consecutive days, and platelet engraftment was defined as the first day of platelets ≥ 20 × 109/L for 7 consecutive days without transfusion. Primary engraftment failure was defined as the absence of donor-derived myeloid cells at day 60 in patients surviving beyond day 28 after transplantation. Chronic GVHD was evaluated in patients who survived for greater than 100 days and had a sustained engraftment. Acute and chronic GVHD were defined according to published standard criteria [16],[17]. Relapse was defined as evidence for the presence of morphological disease in peripheral blood, marrow, or extramedullary sites. Leukemia-free survival (LFS) was defined as continuous CR at the last follow-up.
Statistical methods
For our Chinese population treated in China, historically grade 2–4 aGVHD is reported to be around 40% [7]-[9]. Our primary endpoint was to improve the grade 2–4 aGVHD incidence by 25% over historical control (reduce to 30%). Under the assumptions of 90% power and a two sided error rate of 0.05 we calculated the trial a size of 477 patients. By estimating a 5% dropout rate, we planned our sample size at 500 patients. Achieving the primary endpoint was calculated to be associated with a HR of 0.75.
Cumulative incidences were estimated for engraftment, aGVHD, cGVHD, non-relapse mortality (NRM), relapse mortality (RM) and relapse in order to evaluate competing risks. The competing risk for engraftment was death without engraftment; the competing risk for GVHD was death without GVHD and graft rejection; relapse was a competing risk for NRM; and NRM was a competing risk for relapse. The time of GVHD occurrence was defined from day 1 after graft infusion to the onset of any grade of GVHD; aGVHD was censored at day 100 after HSCT and cGVHD was censored at the last follow-up visit. The worst stage of the GVHD was assessed as the degree of the GVHD reported. The probabilities of overall survival (OS) and LFS were estimated by the Kaplan-Meier method [18]. Potential prognostic factors were evaluated in univariate analyses by the log-rank test, with a P-value of less than 0.05 being considered statistically significant. The demographics of patients and donors, the underlying disease, disease status, conditioning regimens, the source of the graft, MMF doses and other pre-transplant parameters were included in the univariate analyses. In the multivariate analysis, all of the factors found to influence the outcomes in the univariate analysis with a P < 0.1 were included into a Cox proportional hazard model using a forward: conditional method (SAS version 8.2, SAS Institute, Cary, NC, and S Plus 2000, Mathsoft, Seattle, WA). Data cut off for survival follow-up was October 31, 2011. The median follow-up time was 22.8 months.
Results
Engraftment
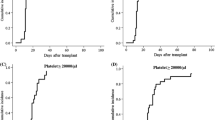
503 (99.0%) patients achieved sustained myeloid engraftment. The median time to reaching an ANC above 0.5 × 109 cells/L was 14 (7–24) days. During the follow-up period, 496 patients (97.6%) exhibited platelet engraftment, and the median time to reach a platelet count above 20 × 109 cells/ L was 13 (6–124) days.
Graft-versus-host disease
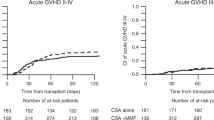
At 100 days after transplantation, the cumulative incidence was 23.2% (95% CI, 21.2%-25.2%) for grade 2 to 4 aGVHD, and 10.3% (95% CI, 9.8%-11.8%) for grade 3 to 4 aGVHD (Figure 1-A). The cumulative incidence was 67.4% (95% CI, 64.9%-69.9%) for total cGVHD and 45.1% (95% CI, 42.0%-48.2%) for extensive cGVHD at 2 years after transplantation (Figure 1-B).
Analysis of risk factors for aGVHD
The risk factors for aGVHD determined by univariate analysis are listed in Table 2. By univariate analysis, patient and donor age ≥ 40 years old, advanced stage as well as BuFlu conditioning regimen were associated with grade 2 to 4 aGVHD incidence. Advanced stage, BuFlu conditioning regimen were associated with the risk of grade 3 to 4 aGVHD. Donor-recipient sex match, donor-recipient blood type match, MMF dose and CML were not associated with grade 2 to 4 or grade 3 to 4 aGVHD incidences. Because in our study all donors were siblings, the age range for patients and donors remained relatively narrow (r = 0.797, p = 0.000). For recipients younger than 40 years of age, no impact of donor age on the incidence or severity of aGVHD was observed. Older patients (≥40 years) had higher odds to experience grade 2–4 aGVHD than the younger patients (<40 years) (30.0% vs 18.5%, p = 0.01).Patients transplanted at advanced stage had significantly higher odds of developing grade 2–4 aGVHD (30.8% vs 21.7%, p = 0.045) and grade 3–4 aGVHD (21.8% vs 8.5%, p = 0.000) when compared to patients who were transplanted at non-advanced stage. By multivariate analysis, patient age ≥ 40 years and BuFlu conditioning regimen were associated with grade 2 to 4 aGVHD incidence. Advanced stage and BuFlu conditioning regimen were associated with the risk of grade 3 to 4 aGVHD (Table 3).
Analysis of risk factors for cGVHD
Risk factors for cGVHD determined by univariate analysis are listed in Table 4. By univariate analysis, patient age ≥ 40 years old, female donor/male recipient, BuFlu regimen for conditioning and prior aGVHD were the risk factors for both cGVHD and extensive cGVHD. Univariate analysis did not identify donor age, advanced stage, donor-recipient blood type match or CML to be risk factors for cGVHD and extensive cGVHD. Patients ≥ 40 years old were found to have higher odds for developing cGVHD and extensive cGVHD than younger patients (<40 years) (75.9% vs 62.3%, p = 0.016) and (55.9% vs 38.6%, p = 0.007).No significant differences for cGVHD and extensive cGVHD were found for patients transplanted at non-advanced stage or advanced stage (67.3% vs 62.5%, p = 0.374) and (45.6% vs 33.0%, p = 0.888). Multivariate analysis confirmed female donor/male recipient, BuFlu regimen for conditioning and prior aGVHD as risk factor for cGVHD and extensive cGVHD (Table 5).
Survival, relapse and long-term follow-up
As of Apr 30, 2011, 377 patients were alive following transplantation, with a median survival time of 22.8 m (range: 6-52 m). Probabilities for OS and LFS in all patients at 2 years were 68.0% (95% CI =63.3%-75.8%) and 66.0% (95% CI =63.3%-75.8%) respectively. Probabilities for OS in patients transplanted at non-advanced stage and advanced stage at 2 years were 71.4% (95% CI =65.9%-77.0%) and 41.8% (95% CI =20.9%-62.6%) (p = 0.000), respectively. The probabilities of LFS in non-advanced stage and advanced stage patients at 2 years were 69.7% (95% CI =64.7%-74.8%) and 44.8% (95% CI =30.7%-58.9%)(p = 0.000), respectively.
Sixty-nine patients relapsed after transplantation, reaching a cumulative relapse of 17.7% (95% CI, 15.6%-19.8%) at 2 years. The NRM rate was 4.3% (95% CI = 3.4% - 5.2%) and 18.4% (95% CI = 16.3%-20.5%) at 100 days and 2 years, respectively. 53 out of 131 patients died of leukemia relapse. Seventy-eight patients died from other causes than relapse. The most frequent cause of death was infection, specifically, pneumonia. Severe GVHD was determined as cause of death in nineteen patients.
Comparison of outcomes in patients between with and without acute and/or chronic GVHD
In order to analyze the impact of GVHD on the overall clinical outcomes of our patients, we compared outcomes for patients with and without acute and/or chronic GVHD (Table 6). Although the patients with grade 2–4 aGVHD had lower RM, they had higher NRM and worse OS and LFS when compared with the patients grade 0–1 aGVHD. In contrast, patients with cGVHD had improved OS and LFS and lower RM compared to patients without cGVHD (Table 6).
Discussion
Although GVHD has been recognized more than fifty years as a complication of allo-HSCT, current prophylactic therapies remain insufficient and a high medical need to improve outcomes remains [19]-[21]. Thus far, no significant progress has been made in developing novel aGVHD regimens and most approaches are not improved over historical results. Although Devine et al. reported in their study grade 2–4 aGVHD in only 22.4% and extensive cGVHD of 6.8%, this was offset by only 58% 3 years DFS in patients transplanted in AML-CR1. Likely, this was related to graft T cell depletion (TCD) which was part of the GVHD prophylaxis in this study [22]. Compared to historic transplant results derived from China, European and US centers, this multicenter trial demonstrates a substantial decrease of aGVHD in HLA-matched sibling allo-HSCT without increasing disease relapse or adversely impacting survival in standard risk patients, Our outcomes rather compare to results published by Tanimoto TE, et al. from Japan ( Table 7) [2]-[5],[23]-[25].
Several studies have identified risk factors for GVHD over the past 3 decades [21],[26]-[33]. Gale et al. analyzed data of 2036 recipients of HLA-identical sibling transplants between 1978 and 1985 within the IBMTR. They found donor/ recipient sex-match, patient age ≥40 years and lack of GVHD prophylaxis to be associated with moderate to severe GVHD [26]. Hahn et al. analyzed IBMTR data of 1,960 adults after sibling HLA-identical myeloablative transplant performed between 1995 and 2002. They reported risk factors for grade 2 to 4 acute GVHD to be age 40 and older, use of total body irradiation (TBI), grafting with mobilized blood cells , CML versus AML/ALL, white/Black versus Asian/Hispanic race (recipient), Karnofsky performance score less than 90 versus 90 to 100 ,and recipient/donor cytomegalovirus-seronegative status [21]. Another study by Flowers et al. analyzed 2941 adult and pediatric patients with both related and unrelated HLA –matched allo-HSCT performed between 1992 and 2005 in Seattle [33]. Risk factors for developing grade 2 to 4 acute GVHD were unrelated donors, use of TBI, lack of ATG utilization, female donor/male recipient and the underlying diagnosis of CML. Concurring with most other previous reports we determined in our study the main risk factors for both aGVHD and cGVHD to be age ≥ 40 years [21],[26] . In contrast to most other reports, we found that neither donor age, nor donor/recipient sex match, blood type match or CML enhanced the likelihood for aGVHD, however, donor/recipient sex match was associated with an increased risk of cGVHD. Whether donor age impacts the risk of GVHD in our patient population needs to be further studied. Kollman et al. reported that donor age was associated with aGVHD and cGVHD in unrelated donor HSCT [34]. Because all donors were siblings, the age range for patients and donors in our study remained in a relatively narrow range, and therefore our sample size was insufficient for certain subset analysis. However, we did not observe in recipients younger than 40 years of age any impact of the donor age on either aGVHD or cGVHD. Our data suggest that the BuFlu conditioning regimen significantly increases the incidence rates for both aGVHD and cGVHD when compared with the BuCy regimen. Such association has previously not been reported in other studies and therefore these results need to be interpreted with caution. In fact, Chae et al. reported the opposite, that the BuFlu regimen decreased both incidence rates of aGVHD as well as cGVHD when compared with BuCy regimen [35]. On the other hand, Lee [36] and Liu [37] found no significant differences for the incidence rates of aGVHD and cGVHD between BuCy and BuFlu conditioning regimens in two randomized trials.
We observed cumulative incidences of cGVHD and extensive cGVHD at 2 years at 67.4% and 45.1%, respectively. Compared to some historic studies (Table 7), at least this aspect of our study could be interpreted as lack of improvement over current standards. The cumulative incidences for cGVHD and extensive cGVHD were slightly lower with 53.3% and 28.2% reported in our prior study [14]. We explain this relatively high incidence of cGVHD in our study with the fact that MMF was only given for 30 days. However, this short course was deliberately chosen to maintain some cGVHD in an effort to maximize the GVL effect [38]. Another explanation for this finding might be that all of our patients’ grafts were PBSCT based and consisted either of PBSCT alone or PBCST + BM. Therefore higher rates of cGVHD are expected compared to studies using pure BMT grafts [15]. For instance, Wang Y et al. reported the incidence for cGVHD at 50% for haploBMT [39]. There is no study providing conclusive evidence regarding any differences for the incidence of GVHD between Chinese and Caucasian patients. However, a key factor determining development of cGVHD is the duration of immunosuppression. The design of our regimen prescribes only a short course (30 days). Although the regimen is effective in preventing aGVHD, the remaining immunosuppression after MMF discontinuation may not have been sufficient enough and thus resulted in the observed cGVHD increase.
Although we found in our study grade 2–4 aGVHD to be associated with lower rates of relapse mortality, this also resulted in worse OS and LFS mainly due to increased NRM. In contrast, we found that cGVHD was associated with better OS and LFS outcomes which we explain with concurrently decreased rates of relapse mortality (Table 6). Nevertheless, these data have to be interpreted with caution because 30.7% of our patients had a diagnosis of CML, a disease known to be more susceptible to GVL manipulations and the follow up has been only 2 years. Moreover, other studies showed worse survival for both cGVHD and aGVHD, despite a favorable association of cGVHD on disease relapse (AML and MDS) [40]. Clearly, additional data need to be generated to fine-tune GVHD regimens in order to maximize the therapeutic benefit and find the optimal balance between aGVHD, cGVHD and GVL.
Conclusion
In summary, although due to the study design a direct comparison of the low dose short course MMF containing regimen to CsA/MTX alone for GVHD prophylaxis could not be performed, we demonstrated a substantial decrease for the risk of aGVHD development. In contrast, the incidence for cGVHD could not be improved when compared to historical results in Chinese patients. Our results also suggest that Chinese patients may have slight variations in risk factors for developing GVHD.
Abbreviations
- allo-HCST:
-
Allogeneic hematopoietic cell transplantation
- GVHD:
-
Graft-versus-host disease
- aGVHD:
-
Acute GVHD
- cGVHD:
-
Chronic GVHD
- CsA:
-
Cyclosporine
- MTX:
-
Methotrexate
- MMF:
-
Mycophenolate mofetil
- HLA:
-
Human leukocyte antigen
- LFS:
-
Leukemia free survival
- OS:
-
Overall survival
- CR:
-
Complete remission
- TBI:
-
Total body irradiation
- G-CSF:
-
Granulocyte colony-stimulating factor
- MNC:
-
Mononuclear cells
- NRM:
-
Non-relapse mortality
- RM:
-
Relapse mortality
References
Pasquini MC, Wang Z: Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2012. Available at: ., [http://www.cibmtr.org]
Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, Kashyap A, Flowers ME, Lilleby K, Chauncey TR, Storb R, Appelbaum FR: Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001, 344 (3): 175-181. 10.1056/NEJM200101183440303.
Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, Shore TB, Walker IR, Browett P, Messner HA, Panzarella T, Lipton JH: Canadian Bone Marrow Transplant Group. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002, 100 (5): 1525-1531. 10.1182/blood-2002-01-0048.
Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, Russell N, Apperley JF, Gorin NC, Szer J, Bradstock K, Buzyn A, Clark P, Borkett K, Gratwohl A: European Group for Blood and Marrow Transplantation. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002, 100 (3): 761-767. 10.1182/blood-2001-12-0304.
Tanimoto TE, Yamaguchi T, Tanaka Y, Saito A, Tajima K, Karasuno T, Kasai M, Kishi K, Mori T, Maseki N, Morishima S, Miyakoshi S, Kasai M, Ohno Y, Kim SW, Numata A, Kami M, Takaue Y, Mori S, Harada M: Comparative analysis of clinical outcomes after allogeneic bone marrow transplantation versus peripheral blood stem cell transplantation from a related donor in Japanese patients. Br J Haematol. 2004, 125 (4): 480-493. 10.1111/j.1365-2141.2004.04943.x.
Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005, 23 (22): 5074-5087. 10.1200/JCO.2005.09.020.
Liu QF, Sun J, Zhang Y, Liu XL, Xu D, Xu B, Feng R, Meng FY, Zhou SY: Hematopoietic stem cell transplantation for patients with chronic myelogenous leukemia. Ai Zheng. 2004, 23 (4): 426-429.
He Y, Feng SZ, Wang M, Wei JL, Qin TJ, Zhou Z, Zhai WJ, Qiu LG, Han MZ: HLA-identical sibling allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia in first chronic phase. Analysis of 51 cases. Zhonghua Xue Ye Xue Za Zhi. 2005, 26 (7): 389-392.
Liu D, Guo N, Zhang Y: Allogeneic bone marrow transplantation for chronic myeloid leukemia: 118 cases analysis. Zhonghua Xue Ye Xue Za Zhi. 1999, 20 (8): 424-426.
Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, Champlin RE, Dicke KA, Goldman JM, Good RA, Herzig RH, Hong R, Masaoka T, Rimm AA, Ringdh O, Speck B, Weiner RS, Bortin MM: T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991, 78 (8): 2120-2130.
Urbano-Ispizua A, Rozman C, Martínez C, Marín P, Briones J, Rovira M, Féliz P, Viguria MC, Merino A, Sierra J, Mazzara R, Carreras E, Montserrat E: Rapid engraftment without significant graft-versus-host disease after allogeneic transplantation of CD34+ selected cells from peripheral blood. Blood. 1997, 89 (11): 3967-3973.
Wagner JE, Thompson JS, Carter SL, Kernan NA: Unrelated donor marrow transplantation trial. effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell depletion trial): a multi-centre, randomised phase II-III trial. Lancet. 2005, 366 (9487): 733-741. 10.1016/S0140-6736(05)66996-6.
Storb R, Antin JH, Cutler C: Should methotrexate plus calcineurin inhibitors be considered standard of care for prophylaxis of acute graft-versus-host disease?. Biol Blood Marrow Transplant. 2010, 16 (1 Suppl): S18-27. 10.1016/j.bbmt.2009.10.016.
Lai Y, Ma J, Schwarzenberger P, Li W, Cai Z, Zhou J, Peng Z, Yang J, Luo L, Luo J, Deng D, Li Q, Zhou Y, Liang J: Combination of CsA, MTX and low-dose, short-course mycophenolate mofetil for GVHD prophylaxis. Bone Marrow Transplant. 2009, 43 (1): 61-67. 10.1038/bmt.2008.265.
Zhao XS, Chen Y, Zhao XY, Liu DH, Xu LP, Wang Y, Han W, Chen YH, Chen H, Zhang XH, Liu KY, Huang XJ: Improved outcomes using G-CSF-mobilized blood and bone marrow grafts as the source of stem cells compared with G-PB after HLA-identical sibling transplantation in patients with acute leukemia. Clin Transplant. 2013, 27 (6): 844-851. 10.1111/ctr.12225.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED: Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974, 18 (4): 295-304. 10.1097/00007890-197410000-00001.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J: Thomas ED.1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995, 15 (6): 825-828.
Gooley TA, Leisenring W, Crowley J, Storer BE: Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999, 18 (6): 695-706. 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O.
Billingham RE: The biology of graft-versus-host reactions. Harvey Lect. 1966–1967, 62: 21-78.
Mastaglio S, Stanghellini MT, Bordignon C, Bondanza A, Ciceri F, Bonini C: Progress and prospects: graft-versus-host disease. Gene Ther. 2010, 17 (11): 1309-1317. 10.1038/gt.2010.83.
Hahn T, McCarthy PL, Zhang MJ, Wang D, Arora M, Frangoul H, Gale RP, Hale GA, Horan J, Isola L, Maziarz RT, van Rood JJ, Gupta V, Halter J, Reddy V, Tiberghien P, Litzow M, Anasetti C, Pavletic S, Ringdén O: Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008, 26 (35): 5728-5734. 10.1200/JCO.2008.17.6545.
Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, Lazarus HM, Linker C, Stadtmauer EA, Alyea EP, Keever-Taylor CA, O’Reilly RJ: Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011, 17 (9): 1343-1351. 10.1016/j.bbmt.2011.02.002.
Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, Snyder D, Pullarkat V, Kogut N, Rosenthal J, Smith E, Karanes C, O’Donnell M, Krishnan AY, Senitzer D, Forman SJ: A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010, 115 (5): 1098-1105. 10.1182/blood-2009-03-207563.
Yang K, Liu QF, Fan ZP, Sun J, Xu D, Wei YQ, Zhang Y, Meng FY: A comparison of the therapeutic effects between related donor and unrelated donor allogeneic hematopoietic stem cell transplantation in treatment of leukemia. Zhonghua Nei Ke Za Zhi. 2007, 46 (2): 135-139. [Chinese]
Ringdén O, Labopin M, Gorin NC, Volin L, Torelli GF, Attal M, Jouet JP, Milpied N, Socié G, Cordonnier C, Michallet M, Atienza AI, Hermine O, Mohty M: Acute Leukaemia working party of the European Group for Blood and Marrow Transplantation. Growth factor-associated graft-versus-host disease and mortality 10 years after allogeneic bone marrow transplantation. Br J Haematol. 2012, 157 (2): 220-229. 10.1111/j.1365-2141.2012.09034.x.
Gale RP, Bortin MM, van Bekkum DW, Biggs JC, Dicke KA, Gluckman E, Good RA, Hoffmann RG, Kay HE, Kersey JH, Marmont A, Masaokal T, Rimml AA, van Rood JJ, Zwaan FE: Risk factors for acute graft-versus-host disease. Br J Haematol. 1987, 67 (4): 397-406. 10.1111/j.1365-2141.1987.tb06160.x.
Weisdorf D, Hakke R, Blazar B, Miller W, McGlave P, Ramsay N, Kersey J, Filipovich A: Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991, 51 (6): 1197-1203. 10.1097/00007890-199106000-00010.
Nash RA, Pepe MS, Storb R, Longton G, Pettinger M, Anasetti C, Appelbaum FR, Bowden RA, Deeg HJ, Doney K, Martin PJ, Sullivan KM, Sanders J, Witherspoon RP: Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992, 80 (7): 1838-1845.
Eisner MD, August CS: Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone Marrow Transplant. 1995, 15 (5): 663-668.
Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, Thomas ED, Hansen JA: Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990, 29 (2): 79-91. 10.1016/0198-8859(90)90071-V.
Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, Sanders JE, Stewart P, Buckner CD, Storb R, Thomas ED, Hansen JA: Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985, 313 (13): 765-771. 10.1056/NEJM198509263131301.
Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, Fernandez-Vina M, Flomenberg N, Horowitz M, Hurley CK, Noreen H, Oudshoorn M, Petersdorf E, Setterholm M, Spellman S, Weisdorf D, Williams TM, Anasetti C: High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007, 110 (13): 4576-4583. 10.1182/blood-2007-06-097386.
Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, Pereira SE, Nash RA, Mielcarek M, Fero ML, Warren EH, Sanders JE, Storb RF, Appelbaum FR, Storer BE, Martin PJ: Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011, 117 (11): 3214-3219. 10.1182/blood-2010-08-302109.
Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, Hegland J, Kamani N, Kernan NA, King R, Ratanatharathorn V, Weisdorf D, Confer DL: Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001, 98 (7): 2043-2051. 10.1182/blood.V98.7.2043.
Chae YS, Sohn SK, Kim JG, Cho YY, Moon JH, Shin HJ, Chung JS, Cho GJ, Yang DH, Lee JJ, Kim YK, Kim HJ: New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation: comparison with BuCy2. Bone Marrow Transplant. 2007, 40 (6): 541-547. 10.1038/sj.bmt.1705770.
Lee JH, Joo YD, Kim H, Ryoo HM, Kim MK, Lee GW, Lee JH, Lee WS, Park JH, Bae SH, Hyun MS, Kim DY, Kim SD, Min YJ, Lee KH: Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013, 31 (6): 701-709. 10.1200/JCO.2011.40.2362.
Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, Zhang Y, Huang F, Zhou H, Fan Z, Tu S, Li Y, Guo X, Yu G, Liu Q: Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol. 2013, 6: 15-10.1186/1756-8722-6-15.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B, Truitt RL, Zwaan FE, Bortin MM: Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990, 75 (3): 555-562.
Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, Chen H, Han W, Chen YH, Wang FR, Wang JZ, Chen Y, Yan CH, Huo MR, Li D, Huang XJ: Who is the best donor for a related HLA- haplotype-mismatched transplant? Blood. 2014 Jun 10. pii: blood-2014-03-563130. [Epub ahead of print].
Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M: Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012, 18 (11): 1727-1733. 10.1016/j.bbmt.2012.06.014.
Acknowledgements
We thank Yang Xiaobo,MD,Ph.D(Guangxi Medical University) , who has participated in this study. We thank every faculty member who has participated in this study.
Supported by grants from the National Natural Science Foundation of China (Grant No.30971292), the Natural Science Foundation of Beijing (Grant No. 7122193), the National High Technology Research and Development Program of China (Program 863) (Grant No. 2011AA020105), the Clinical Subject’s Key Project of the Ministry of Health and the National Natural Science Foundation of China (Grant No. 30725038).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YRL and YHC designed the research, interpreted the data and wrote the manuscript; DMH, MJ, QL, LL, JH, PS, QCL, ZMZ and KYL performed the study and contributed to writing the manuscript; XJH is the principal investigator, designed the research, interpreted the data, and wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lai, Yr., Chen, Yh., Hu, Dm. et al. Multicenter phase ii study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG). J Hematol Oncol 7, 59 (2014). https://doi.org/10.1186/s13045-014-0059-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-014-0059-3