Abstract
Background
Small studies suggest an association of donor-specific anti-human leukocyte antigen (HLA) antibodies (DSAs) with primary graft failure (GF) following haploidentical stem cell transplantation, but primary graft rejection (GR) was not discriminated from primary poor graft function (PGF). In this study, we aimed to determine the association of DSAs with primary GF, including GR and PGF, in patients who underwent unmanipulated haploidentical blood and marrow transplantation.
Methods
A total of 345 subjects were prospectively recruited and randomly selected as training group (n = 173) and validation group (n = 172). Patient plasma/serum was screened. For HLA antibody positive samples with a median fluorescent intensity (MFI) >500, DSAs were further tested using a LABScreen Single Antigen Kit (One Lambda).
Results
A total of 342 patients (99.1 %) achieved sustained myeloid engraftment. The median times to neutrophil engraftment and platelet engraftment were 13 days (range, 8–28 days) and 18 days (range, 6–330 days), respectively. The cumulative incidence of primary GF was 6.4 ± 1.3 % and included GR (0.9 ± 0.5 %) and PGF (5.5 ± 1.2 %). Of the 345 cases tested, 39 (11.3 %) were DSA positive. Multivariate models showed that DSAs (MFI ≥ 10,000) were correlated to primary GR (P < 0.001) and that DSAs (MFI ≥ 2000) were strongly associated with primary PGF (P = 0.005). All patients were classified into three groups for analysis. Group A included cases that were DSA negative and those with a DSA MFI <2000 (n = 316), group B included cases with a 2000 ≤ MFI < 10,000 (n = 19), and group C included cases with a MFI ≥10,000 (n = 10). The DSAs were associated with an increased incidence of the primary GF (3.2 vs. 31.6 vs. 60 %, for groups A, B, and C, respectively, P < 0.001), transplant-related mortality (TRM) rate (17.2 vs. 14.7 vs. 33.3 %, for groups A, B, and C, respectively, P = 0.022), and inferior overall survival (OS, 77.3 vs. 85.3 vs. 44.4 %, for groups A, B, and C, respectively, P = 0.015). The primary GF was independently associated with a higher incidence of TRM (P < 0.001), inferior disease-free survival (P < 0.001), and OS (P < 0.001).
Conclusions
The findings confirmed the effect of DSAs on primary GF, including GR and PGF, and survival. Our results suggest incorporating DSAs in the algorithm for haploidentical donor selection.
Similar content being viewed by others
Background
Allogeneic stem cell transplantation (allo-SCT) is a potentially curative treatment for patients with hematologic malignancies [1–7]. However, complications, such as graft failure (GF) and relapse, remain serious problems [4, 5, 8–16]. Primary GF includes graft rejection (GR), which is defined as a failure to engraft neutrophils (absolute neutrophil count (ANC) ≤0.5 × 109/L) by day +28 for three consecutive days and the absence of donor hematopoiesis [14, 17]. It also includes poor graft function (PGF), which is the failure to achieve two or three adequate blood counts (ANC ≤0.5 × 109/L, platelet ≤20 × 109/L, or hemoglobin (Hb) ≤80 g/L) following allo-SCT in the presence of complete donor hematopoiesis [12, 14, 17]. The incidence of primary GF ranges from 2 to 15 % in patients who undergo human leukocyte antigen (HLA)-matched sibling donor transplantation, unrelated donor transplantation (MUDT), or umbilical cord blood transplantation (UCBT) [10, 13, 18–22]. In the past 10 years, HLA-mismatched/haploidentical transplants (haplo-SCTs) have been used more frequently [1–5, 23–25]; as a result, GF has become an increasing problem that contributes to high morbidity and mortality after transplantation. The incidences of GF in patients who underwent CD34-selected [5] and CD3/CD19-depleted haplo-SCTs [4] were 9 and 8 %, respectively. The rate of GF following haplo-SCTs with post-transplant cyclophosphamide was 13 % [8, 25].
Donor-specific antibodies (DSAs) refer to anti-HLA antibodies that specifically correspond to a mismatched antigen of the donor [9, 20–22, 26–28]. The role of DSAs in solid organ transplantation is well established [29]. In allo-SCTs [20, 21, 30], DSAs have been associated with primary GF after either MUDT or UCBT. In haplo-SCT, Ciurea et al. [22] showed that 75 % of pretransplant DSA-positive patients with a median fluorescent intensity (MFI) >1500 failed to engraft, compared with 5 % of DSA-negative patients (P = 0.008). In another study, the authors found that three of five patients with high levels of DSA (MFI > 10,000) had GF. [9] Although the association of DSAs with primary GF after haplo-SCT has been observed [9, 22, 31], there are some limitations of previous studies: (1) most studies were retrospective [22, 31], except one [9]; (2) they included small numbers of patients [9, 22, 31]; (3) primary GR was not discriminated from primary PGF [9, 22, 31]; and (4) there were no training and validation groups [9, 22, 31].
In our center, we established an unmanipulated haploidentical blood and marrow transplant (HBMT) protocol that can achieve outcomes comparable with HLA-identical sibling or unrelated donor transplantation. The incidence of primary GR was approximately 1 % in patients undergoing unmanipulated HBMT. [1, 32] However, we found that primary PGF, with an incidence of approximately 4–5 %, was a severe complication with a higher incidence of mortality after unmanipulated HBMT (unpublished data). Therefore, we prospectively investigated the influence of DSAs on primary GF, including GR and PGF, after unmanipulated HBMT in a training group of 173 patients and validated the results in an independent cohort of 172 cases.
Results
Patient characteristics
The median age of the patients was 26 years (range, 2–58 years). All patients were treated with a myeloablative conditioning regimen. The median infused total nucleated cell dose (TNC) and CD34+ cell dose were 8.34 × 108/kg (range, 1.78–23.69 × 108/kg) and 2.59 × 106/kg (range, 0.39–16.82 × 106/kg), respectively. Other demographics are listed in Table 1. The characteristics of the patients in the training group and validation set were similar.
Transplant outcomes
A total of 342 patients (99.1 %) achieved sustained myeloid engraftment. The median times to neutrophil engraftment and platelet engraftment were 13 days (range, 8–28 days) and 18 days (range, 6–330 days), respectively. The cumulative incidence of primary GF was 6.4 ± 1.3 % and included GR (n = 3, 0.9 ± 0.5 %) and PGF (n = 19, 5.5 ± 1.2 %). At 100 days after transplant, the cumulative incidence of grade 2 to 4 acute graft-versus-host disease (GVHD) was 42.7 ± 3.1 %. After a median follow-up of 384 days (range, 25–784 days), the cumulative incidence of chronic GVHD was 43.3 ± 3.1 %. The 2-year probabilities of relapse, transplant-related mortality (TRM), disease-free survival (DFS), and overall survival (OS) were 8.8 ± 1.8 %, 18.4 ± 2.8 %, 75.1 ± 2.9 %, and 76.2 ± 3.0 %, respectively.
Anti-HLA antibodies and DSAs
Of the 345 cases tested, 87 (25.2 %) were anti-HLA antibody positive, including 44 males and 43 females. Of the positive cases, 39 (11.3 %) were DSA positive. Among the 39 cases, 31 had antibodies against HLA class I antigens, 15 had antibodies against HLA class II, and 7 against classes I and II. The MFI was 4726 (range, 504–19,948). Among 144 female cases, the patients with a pregnancy history had a higher anti-HLA antibody positive rate (100 vs. 17.2 %, P < 0.001) and a higher DSA positive rate (59.1 vs. 8.2 %, P < 0.001) than those without.
Association of DSAs on primary GR after transplantation
In this study, we defined a MFI ≥ 10,000 of DSAs as a cutoff value for primary GR using receiver operating characteristic curves in all the 345 patients who underwent unmanipulated HBMT due to the low incidence of primary GR [1, 2, 32]. The incidence of primary GR with a MFI ≥10,000 was higher than those with a MFI <10,000 (20 vs. 0.3 %, P = 0.002) in all the 345 patients. The numbers of patients with primary GR in training and validation sets were two cases and one case, respectively. The higher incidences of primary GR in patients with a MFI ≥ 10,000 than those with a MFI < 10,000 were also observed in the training group (16.7 vs. 0.6 %, P = 0.041) and the validation group (25.0 vs. 0 %, P = 0.023). Univariate analysis showed that factors, including age (P = 0.002), disease status (P = 0.018), donor-recipient relationship (P = 0.057), anti-HLA antibodies (P < 0.001), and DSAs (P < 0.001), were correlated with primary GR after unmanipulated HBMT. Multivariate analysis demonstrated that the presence of DSAs (MFI ≥ 10,000) was associated with primary GR (hazard ratio (HR) 71.556, 95 % confidence interval (CI) 6.488–789.129; P < 0.001). The onset of primary GR was associated with increased TRM (HR 18.893, 95 % CI 5.538–64.452; P < 0.001), inferior DFS (HR 9.883, 95 % CI 3.005–32.502; P < 0.001), and OS (HR 11.747, 95 % CI 3.546–38.916; P < 0.001).
Association of DSAs with primary PGF after unmanipulated HBMT
We further investigated the effects of DSAs on primary PGF after unmanipulated HBMT. In the training set (n = 173), a cutoff value of a DSA MFI ≥2000 was identified to predict the onset of primary PGF. The patients with a MFI ≥2000 experienced a significantly higher incidence of primary PGF than those with a MFI <2000 [27.3 % (3/11) vs. 1.9 % (3/162), P = 0.003]. Multivariate models showed that the presence of DSAs was strongly associated with primary PGF (HR 10.575, 95 % CI 2.029–55.117; P = 0.005). The same threshold of DSAs was applied in the independent validation set of the 172 patients. In the validation group, the incidence of primary PGF was higher in patient with a MFI ≥2000 compared with those with a MFI <2000 [33.3 % (6/18) vs. 4.5 % (7/154), P = 0.001]. Multivariate analysis further confirmed the presence of DSAs was independently associated with the onset of primary PGF (HR 3.949, 95 % CI 1.501–10.389; P = 0.005) after unmanipulated HBMT. After identifying primary GR as a competing risk for primary PGF, the correlation of DSAs with primary PGF was also demonstrated in the training group and the validation group (data not shown).
In the training set, multivariate analysis showed that the onset of primary PGF was independently associated with a higher incidence of TRM (HR 7.114, 95 % CI 2.054–24.639; P = 0.002), inferior DFS (HR 3.356, 95 % CI 1.047–10.759; P = 0.042), and OS (HR 3.687, 95 % CI 1.129–12.039; P = 0.031). These independent associations of primary PGF with a higher incidence of TRM (HR 5.031, 95 % CI 1.993–12.704; P = 0.001), inferior DFS (HR 3.011, 95 % CI: 1.247–7.617; P = 0.014), and OS (HR 3.530, 95 % CI 1.445–8.626; P = 0.006) were confirmed in the validation group.
Effects of DSA on primary graft failure and transplant outcomes
After separately analyzing the association of DSAs with either primary GR or primary PGF, we further investigated the association of DSAs with primary GF, including both GR and PGF, in all the 345 patients. These patients were classified into three groups, group A included cases that were DSA negative or had a DSA MFI <2000 (n = 316), group B included cases with a 2000 ≤ MFI < 10,000 (n = 19), and group C included those with a MFI ≥10,000 (n = 10). The cumulative incidence of neutrophil engraftment of patients in group A was 100 %, which was significantly higher than the cumulative incidence of group C (80.0 ± 12.6 %, P = 0.005) and comparable with group B (94.7 ± 5.1 %, P = 0.169) (Fig. 1a). The cumulative incidence of platelet engraftment of patients in group A was 97.1 ± 1.30 %, which was significantly higher than the incidences of group B (93.9 ± 5.9 %, P = 0.030) and group C (77.5 ± 18.1 %, P = 0.004) (Fig. 1b). Multivariate analysis showed that the presence of DSAs was strongly associated with platelet engraftment and primary graft failure, but not neutrophil engraftment (Table 2).
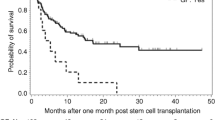
Pretransplant DSA and cumulative incidence of neutrophil (a) and platelet (b) engraftment. All patients were classified into three groups, group A includes cases with DSA negative and those with a DSA MFI <2000 (n = 316, solid line), group B includes cases with 2000 ≤ MFI < 10,000 (n = 19, dotted line), and group C includes those with a MFI ≥ 10,000 (n = 10, dashed line)
The incidences of primary GF, including GR and PGF, in groups A, B, and C were 3.2 % (10/316), 31.6 % (6/19), and 60 % (6/10), respectively (P < 0.001). The cumulative incidences of the TRM rate were 17.2 %, 14.7 %, and 33.3 %, for patients in groups A, B, and C, respectively (Fig. 2a, P = 0.022). The overall survival rates were 77.3, 85.3, and 44.4 % for patients in groups A, B, and C, respectively (Fig. 2b, P = 0.015). Multivariate analysis showed that the presence of DSAs was strongly associated with primary GF (Table 2). The onset of primary GF was also independently associated with a higher incidence of TRM and inferior DFS and OS (Table 2 and Fig. 3a, b). As shown in Table 3, the causes of death in patients with primary GF were mainly infections and hemorrhage, which occurred significantly more often than those without GF (P < 0.001). There were no effects of DSAs on GVHD and relapse (Table 2).
Transplant-related mortality (a) and overall survival (b). All patients were classified into three groups, group A includes cases with DSA negative and those with a DSA MFI <2000 (n = 316, solid line), group B includes cases with 2000 ≤ MFI < 10,000 (n = 19, dotted line), and group C includes those with a MFI ≥10,000 (n = 10, dashed line)
Discussion
We confirmed the association of DSAs with primary GF, as previously reported [9, 22], in this prospective study with randomly assigned training and validation sets. This finding along with the results reported by others [9, 10, 15, 16, 20–22, 28, 30] suggests that the presence of DSAs may contribute to the pathophysiology of GF not only in MUDT and UCBT but also in haplo-SCT with T cell depletion or T cell replete. Most importantly, for the first time, we found a correlation between the presence of DSAs and primary PGF, indicating that DSAs may be involved in the pathogenesis of this complication. The finding that primary GF, including both GR and PGF, can result in inferior OS provides evidence that the presence of DSAs must be considered when choosing a haploidentical donor and should be incorporated in the donor selection algorithm [2, 33].
Our previous reports of a low incidence of primary GR [32, 34] and the association of DSAs with primary GR led us to this investigation of the effects of DSAs on primary PGF in patients receiving our haploidentical transplant protocol. [1, 2, 32, 34] Importantly, we identified for the first time that a MFI ≥2000 was the DSA threshold for primary PGF after haplo-SCT. Our results demonstrated that the presence of DSAs was strongly associated with the onset of primary PGF, in both the training and validation sets. Moreover, we found that primary PGF was an independent variable, which led to inferior survival. Therefore, except for CD34(+)-selected stem cell boost and other methods [13, 17], targeting DSAs may provide a novel method to treat PGF, although the DSA MFI threshold for primary PGF needs to be confirmed in other haploidentical transplant modalities.
The definition of a threshold for DSAs, according to MFI, is a premise for analyzing the association of DSAs with primary GF. In CBTs, Takanashi et al. [30] considered a MFI >1000 to be DSA positive. In a case-control study conducted by Ciurea et al. [20], a MFI ≥500 was considered positive. In haplo-SCT, MFI values >1500 or 5000 were defined as DSA positive by Ciurea et al. [22] and Yoshihara et al. [9], respectively. In our study, we identify a MFI ≥10,000 and MFI ≥2000 as the cutoff values for primary GR and primary PGF, respectively. The differences in the reported thresholds of DSAs between other studies [9, 20–22] and this report may be related to different transplant protocols and different methods for DSA detection [9, 10, 15, 16, 20–22, 28, 30], although these studies demonstrated that the antibody titer is important for the effects of DSAs on primary GF. In addition, we observed that high and low antibody titers of DSAs led to GR and PGF, respectively. Both GR and PGF contributed to inferior survival, although the survival was reduced in GR compared with PGF (Fig. 3). Therefore, our results suggest that high and low MFIs of DSAs should be dealt with differently.
After investigating the association of DSAs with GR and PGF, respectively, we further investigated this association of DSAs with primary GF by classifying all the 345 patients into three groups according to the cutoff value of the DSA MFI. We found that patients with a DSA MFI ≥10,000 experienced a significantly lower cumulative incidence of platelet engraftment, but not neutrophil engraftment, after multivariate analysis. This finding is in agreement with a previous study [9]. The result of the lack of an effect of DSAs on neutrophil engraftment may be related to the routine use of G-CSF in our transplant protocol. [1, 32] Moreover, the effect of DSAs on primary PGF was also demonstrated in the overall cohort. As demonstrated by Cutler et al. [21] in CBTs, we showed that pretransplant DSAs were associated with a higher TRM rate and inferior survival, although a multicenter study with a larger sample of cases is needed to confirm these effects in multivariate analysis. Our study results support the logical theory that the presence of DSAs results in primary GF and may contribute to inferior survival.
Previous studies demonstrated that DSAs may kill donor cells through antibody-dependent cell-mediated cytotoxicity (ADCC) [26], indicating that an immune-mediated mechanism may contribute to the pathogenesis of primary GF. In the present study, the findings that a high MFI of DSAs led to GR and a low MFI resulted in PGF suggest that not only the onset GR but also PGF may involve immune-mediated mechanisms. In renal transplantation, DSAs may result in allograft injury though endothelial cell apoptosis [35, 36]. In systemic sclerosis, the ADCC effect via the Fas pathway can lead to bone marrow (BM) endothelial cell apoptosis [37]. Based on previous reports [35, 36] and the results of this study, it is conceivable that higher titers of DSAs directed against antigens expressed by all full donor cells may lead to necrosis, resulting in primary GR. While, lower titers of DSAs directed against antigens of donor cells may cause apoptosis and the onset of primary PGF. [14, 15] Our study suggests that abnormalities in the BM microenvironment, especially endothelial progenitor cells (EPCs), may cause PGF. [14] Therefore, the effects of DSAs on the BM microenvironment, especially EPCs, during the development of primary GF should be further investigated.
Conclusions
The findings of this study confirmed the effects of DSAs on primary GR. Impressively, we, for the first time, demonstrated that the presence of DSAs might contribute to the pathogenesis of primary PGF after unmanipulated HBMT. Due to the involvement of DSAs in primary GF, including GR and PGF, and inferior survival, the proportions of DSAs can be used in haploidentical transplant settings to decide who is the best donor [2, 33].
Methods
Study cohort
Patients who underwent unmanipulated HBMT were eligible for this study and prospectively enrolled. All cases were treated according to our institutional transplant protocol, as previously described in detail [1, 32, 34]. A total of 345 subjects were recruited between May 2012 and March 2014. These cases were randomly selected as part of the training group (n = 173) or validation group (n = 172). The protocol was approved by the Institutional Review Board of Peking University and signed informed consent was obtained from all the subjects. This study was conducted in accordance with the Declaration of Helsinki.
Transplant protocol
Institutional protocols for unmanipulated HBMT have been previously described [32, 34]. In brief, the conditioning therapy was consisted of cytarabine (4 g/m2/day, on days −10 to −9), busulfan (3.2 mg/kg/day, intravenously on days −8 to −6), cyclophosphamide (1.8 g/m2/day, on days −5 to −4), 1-(2-chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Me-CCNU, 250 mg/m2, once on day −3), and ATG (2.5 mg/kg/ day, rabbit; Sang Stat, Lyon, France, on days −5 to −2) [38]. All transplant recipients received mixture allografts of G-CSF-mobilized bone marrow and peripheral blood stem cell harvests. Cyclosporine A, mycophenolate mofetil, and short-term methotrexate were used for prophylaxis of GVHD [39]. Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) monitoring and prevention and donor lymphocyte infusion were performed according to previous studies [34, 40, 41].
Methodology to detect anti-HLA antibodies
Patient plasma/serum was screened for class I (i.e., HLA-A,-B,-C) and class II (i.e., HLA-DR) HLA antibodies with a LABScreen Mixed Kit (One Lambda, Canoga Park, CA, USA). The samples (7 uL) were incubated with mixed HLA class I- and class II-coated microspheres for 30 min in the dark under gentle agitation. The specimens were then washed before being incubated with anti-human immunoglobulin G-conjugated fluorescein isothiocyanate as described above for the first incubation. Next, the samples were analyzed with a Luminex 200 flow analyzer (Luminex, Austin, TX, USA), and the data were analyzed with the HLA Fusion 3.2 software (One Lambda). The MFI of anti-HLA antibodies was obtained from the output file generated by the flow analyzer and adjusted for the background signal using the formula: sample beads − negative control beads. The samples with a MFI >500 were further tested for the specificity of the antibody, using a LABScreen Single Antigen Kit (One Lambda). The MFI was adjusted for the background signal using the formula described above. The patients and donors underwent HLA allele typing of at least the A, B, and DRB1 loci routinely.
Definitions and evaluation
Neutrophil engraftment was defined as achieving an ANC of 0.5 × 109/L or greater for three consecutive days, and platelet recovery was defined as achieving a platelet count of 20 × 109/L or greater, without platelet transfusions, for seven days. A Hb level of at least 80 g/L without transfusion support is the accepted threshold for red cell engraftment [12]. Full donor chimerism was defined as ≥95 % leukocytes of donor origin in peripheral blood or marrow samples, measured according to our previous report. [14] Mixed chimerism was defined as more than 5 % but less than 95 % leukocytes of donor origin.
Primary GF included GR and PGF. As described in the introduction, GR is the failure to engraft neutrophils (ANC ≤0.5 × 109/L) by day +28 for three consecutive days and the absence of donor hematopoiesis. Because delayed red cell engraftment may happen for many months post-transplant and is more difficult to evaluate in an unarguable manner, in the present study, primary PGF was defined as the presence of three cytopenic counts (ANC ≤0.5 × 109/L, platelet ≤20 × 109/L, or hemoglobin (Hb) ≤80 g/L) beyond day +28 with a transfusion requirement associated with hypoplastic-aplastic BM, in the presence of complete donor chimerism. The patients with evidence of severe GVHD or hematologic relapse were excluded [14].
The diagnosis and grading of acute and chronic GVHD was assigned by the transplant center using standard criteria [42, 43]. Transplant-related mortality (TRM), relapse, DFS, and overall survival (OS) was defined according to our previous studies [2, 32, 34].
Statistical analysis
The patient baseline characteristics were reported descriptively. The Fisher exact test or Wilcoxon rank sum test was used for two-group comparisons. Death without engraftment was considered a competing risk for engraftment, primary GR, and primary PGF, while primary GR was considered a competing risk for primary PGF. The surviving patients were censored at their date of last known follow-up. The log-rank test was used for comparisons of Kaplan-Meier curves, and a Gray test was used for comparisons of cumulative incidence curves. Potential prognostic factors for OS, DFS, relapse, TRM, and engraftment were examined in proportional hazards models. To explore whether the DSA intensity, measured as MFI, predicted primary GF, including GR and PGF, an analysis of receiver operator characteristics was performed. Cox regression models were developed to test the impact of variables on primary GF. Unless otherwise specified, P values are based on two-sided hypothesis tests. Alpha was set at 0.05. We used SPSS 16.0 (Mathsoft, Seattle, WA, USA) for most analyses.
References
Chang YJ, Huang XJ. Haploidentical hematopoietic stem cell transplantation with unmanipulated granulocyte colony stimulating factor mobilized marrow and blood grafts. Curr Opin Hematol. 2012;19(6):454–61.
Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124(6):843–50.
Reisner Y, Hagin D, Martelli MF. Haploidentical hematopoietic transplantation: current status and future perspectives. Blood. 2011;118(23):6006–17.
Federmann B, Bornhauser M, Meisner C, Kordelas L, Beelen DW, Stuhler G, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica. 2012;97(10):1523–31.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–54.
Wang L, Xiao H, Zhang X, Wang C, Huang H. The role of telomeres and telomerase in hematologic malignancies and hematopoietic stem cell transplantation. J Hematol Oncol. 2014;7:61.
Baron F, Zachee P, Maertens J, Kerre T, Ory A, Seidel L, et al. Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian Hematological Society. J Hematol Oncol. 2015;8(1):4.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–50.
Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508–15.
Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704–8.
Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–7.
Shah VO, Civin CI, Loken MR. Flow cytometric analysis of human bone marrow. IV. Differential quantitative expression of T-200 common leukocyte antigen during normal hemopoiesis. J Immunol. 1988;140(6):1861–7.
Locatelli F, Lucarelli B, Merli P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opin Pharmacother. 2014;15(1):23–36.
Kong Y, Chang YJ, Wang YZ, Chen YH, Han W, Wang Y, et al. Association of an impaired bone marrow microenvironment with secondary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(10):1465–73.
Nordlander A, Mattsson J, Sundberg B, Sumitran-Holgersson S. Novel antibodies to the donor stem cell population CD34+/VEGFR-2+ are associated with rejection after hematopoietic stem cell transplantation. Transplantation. 2008;86(5):686–96.
Yoshihara S, Taniguchi K, Ogawa H, Saji H. The role of HLA antibodies in allogeneic SCT: is the ‘type-and-screen’ strategy necessary not only for blood type but also for HLA? Bone Marrow Transplant. 2012;47(12):1499–506.
Klyuchnikov E, El-Cheikh J, Sputtek A, Lioznov M, Calmels B, Furst S, et al. CD34(+)-selected stem cell boost without further conditioning for poor graft function after allogeneic stem cell transplantation in patients with hematological malignancies. Biol Blood Marrow Transplant. 2014;20(3):382–6.
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351(22):2265–75.
Kato M, Matsumoto K, Suzuki R, Yabe H, Inoue M, Kigasawa H, et al. Salvage allogeneic hematopoietic SCT for primary graft failure in children. Bone Marrow Transplant. 2013;48(9):1173–8.
Ciurea SO, Thall PF, Wang X, Wang SA, Hu Y, Cano P, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118(22):5957–64.
Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691–7.
Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88(8):1019–24.
Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121(5):849–57.
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124(17):2735–43.
Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120(22):4285–91.
Barge AJ, Johnson G, Witherspoon R, Torok-Storb B. Antibody-mediated marrow failure after allogeneic bone marrow transplantation. Blood. 1989;74(5):1477–80.
Taylor PA, Ehrhardt MJ, Roforth MM, Swedin JM, Panoskaltsis-Mortari A, Serody JS, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109(3):1307–15.
Ruggeri A, Rocha V, Masson E, Labopin M, Cunha R, Absi L, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Societe Francophone d’Histocompatibilite et d’Immunogenetique (SFHI) and Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) analysis. Haematologica. 2013;98(7):1154–60.
Terasaki PI, Ozawa M, Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7(2):408–15.
Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–46.
Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19(4):647–52.
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119(5):978–85.
Ciurea SO, Champlin RE. Donor selection in T cell-replete haploidentical hematopoietic stem cell transplantation: knowns, unknowns, and controversies. Biol Blood Marrow Transplant. 2013;19(2):180–4.
Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant. 2014;49(3):426–33.
Cailhier JF, Laplante P, Hebert MJ. Endothelial apoptosis and chronic transplant vasculopathy: recent results, novel mechanisms. Am J Transplant. 2006;6(2):247–53.
Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: implications for treatment. Am J Transplant. 2008;8(7):1367–73.
Del Papa N, Quirici N, Soligo D, Scavullo C, Cortiana M, Borsotti C, et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54(8):2605–15.
Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol. 2013;6:15.
Lai YR, Chen YH, Hu DM, Jiang M, Liu QF, Liu L, et al. Multicenter phase II study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG). J Hematol Oncol. 2014;7:59.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor lymphocyte infusion for the treatment of leukemia relapse after HLA-mismatched/haploidentical T-cell-replete hematopoietic stem cell transplantation. Haematologica. 2007;92(3):414–7.
Lin R, Liu Q. Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2013;6:94.
Ramsay NK, Kersey JH, Robison LL, McGlave PB, Woods WG, Krivit W, et al. A randomized study of the prevention of acute graft-versus-host disease. N Engl J Med. 1982;306(7):392–7.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56.
Acknowledgements
This work was supported (in part) by the National High Technology Research and Development Program of China (Program 863) (Grant No. 2013AA020401), the Milstein Medical Asian American Partnership Foundation, The Key Program of National Natural Science Foundation of China (Grant No. 81230013), and the Scientific Research Foundation for Capital Medicine Development (Grant No. 2011-4022-08). We would like to thank San Francisco Edit (www.sfedit.net) for their assistance in editing this manuscript. We thank every faculty member who has participated in these studies.
This study has been presented in part as an oral presentation at the 41st Annual Meeting of the EBMT (23 March 2015) in Istanbul, Turkey.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HXJ designed the research. CYJ and ZXY performed the experiments. HXJ, CYJ, and ZXY analyzed the data. XLP, ZXH, WY, HW, CH, WFR, MXD, ZYY, HMR, ZXS, KY, and LKY provided technical and clinical expertise. CYJ and ZXY wrote the manuscript. HXJ edited the manuscript. All authors have read and approved the final manuscript.
Ying-Jun Chang and Xiang-Yu Zhao contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Chang, YJ., Zhao, XY., Xu, LP. et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol 8, 84 (2015). https://doi.org/10.1186/s13045-015-0182-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-015-0182-9