Abstract
Background
Hip fractures in the elderly often lead to acute respiratory failure, but there is currently no tool to assess the prognosis of such patients. This study aims to develop a risk prediction model for respiratory failure in these patients.
Methods
A retrospective cross-sectional study was conducted using the Medical Information Mart for Intensive Care (MIMIC)-IV database, incorporating data from 3,266 patients with hip fractures aged over 55 years from 2008 to 2019. Data included demographic information, laboratory indicators, comorbidities, and treatment methods. Patients were divided into a training group (70%) and a validation group (30%). Least Absolute Shrinkage and Selection Operator (LASSO) regression was applied to select prognostic predictors, and a visualized nomogram model was constructed using multivariate logistic regression analysis. Model performance and clinical applicability were assessed. Statistical analyses were done using R4.2.2, with P < 0.05 deemed significant.
Results
Seven key factors, including age, height, albumin, chloride, pneumonia, acute kidney injury (AKI), and heparin use, were associated with respiratory failure risk. The model demonstrated good performance with area under the curve (AUC) values of 0.77 and 0.73 in the training and validation sets, respectively. The calibration curve showed good agreement, and decision curve analysis (DCA) indicated the model's clinical benefit.
Conclusions
This risk prediction model can effectively predict respiratory failure in hip fracture patients, assisting clinicians in identifying high-risk individuals and providing evidence-based references for treatment strategies.
Similar content being viewed by others
Introduction
Hip fracture is one of the most common traumatic fracture types in the elderly [1]. With the rapid aging of the population, hip fracture in the elderly has been paid increasing attention in the field of health care [2, 3]. The prevalence of hip fractures in the elderly population is so widespread that dedicated orthogeriatricians are even employed for the care of patients with hip fractures [4]. The prevalence of hip fractures exhibits geographical variations. Globally, it is projected that approximately 18% of women and 6% of men will experience a hip fracture. Consequently, worldwide hip fractures are anticipated to rise from 1.26 million cases in 1990 to an alarming 4.5 million by 2050 [5]. Symptoms usually include severe pain, swelling, bruising, abnormal appearance of thighs (such as shortening or eversion) [6]. Common surgical treatment methods include hip joint replacement, intramedullary nail fixation, and other techniques [7, 8].
In addition to the direct impact of the fracture, these patients may also suffer from many serious complications, such as acute respiratory failure, pulmonary embolism, pneumonia, deep vein thrombosis, urinary tract infections, and muscle loss, to name a few [9,10,11]. Acute respiratory failure not only affects the recovery of patients, but also further leads to declined quality of life, and even becomes life-threatening [12, 13]. Patients with hip fractures often need to stay in bed for long periods of time during rehabilitation, which may increase the risk of lung infection and pulmonary embolism, leading to acute respiratory failure [14, 15]. Furthermore, older people tend to have other chronic diseases, such as heart and lung diseases and diabetes, which may also increase the risk of acute respiratory failure [16, 17]. Therefore, it is very important to evaluate the risk of acute respiratory failure in elderly patients with hip fracture and carry out early intervention. Currently, there is no specific tool or model to assess and predict the risk of acute respiratory failure in these patients.
In this study, clinical data were collected from elderly patients with hip fractures. The Least Absolute Shrinkage and Selection Operator (LASSO) regression analysis was employed to identify potential predictors. A predictive model was established using logistic regression and visualized as a nomogram. The performance of this model was evaluated in both the training and validation sets to assess its robustness in predicting the occurrence of acute respiratory failure in these patients. The study was documented in line with the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) report checklist.
Methods
Data source
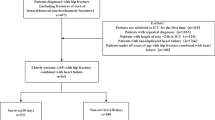
Data for this retrospective cross-sectional study were obtained from the Medical Information Mart for Intensive Care (MIMIC)-IV database [18]. It is maintained by the Computational Physiology Laboratory at Massachusetts Institute of Technology, encompassing clinical information of 58,000 patients who were admitted to the ICU of Beth Israel Deaconess Medical Center (Boston, Massachusetts, USA) between 2008 and 2019. Enrolled patients were de-identified under the Health Insurance Portability and Accountability Act (HIPAA). A team member (Yue Li) had access to the database and was responsible for data extraction (CITE Number: 55377177). The flow chart of this study was as follows (Fig. 1).
Study population
Patients with hip fractures in the MIMIC-IV database were included in the study according to the International Classification of Diseases, 9th and 10th editions (ICD-9, ICD10). The ICD codes used are shown in Table 1. Data from 3266 patients with hip fractures were incorporated from the MIMIC-IV database, spanning January 2008 to December 2019. The collection of patient information and creation of the research resource were reviewed by the Institutional Review Board at the Beth Israel Deaconess Medical Center, who granted a waiver of informed consent and approved the data sharing initiative.
The inclusion criteria were as follows: ① patients aged > 55 years; ② patients diagnosed with hip fracture; ③ patients with respiratory failure that meet the diagnostic criteria for respiratory failure. Respiratory failure was defined as an arterial oxygen partial pressure (PaO2) below 60 mmHg, which may or may not be accompanied by an arterial carbon dioxide partial pressure (PaCO2) above 50 mmHg [19]. The PaO2 and PaCO2 values for each patient can be obtained from the MIMIC database.
The exclusion criteria were as follows: ① patients < 55 years old; ② patients admitted repeatedly; ③ patients with respiratory failure before admission; ④ patients who were diagnosed with diseases other than hip fractures and did not have concurrent hip fractures.
Data extraction
The data of hip fracture patients were extracted by structured query language (SQL) (PostgreSQL 15.3) on PgADmin4 (v7.1) software. The extracted data included: ① demographic information: age, gender, height, weight, blood pressure, body mass index (BMI), race, marriage, and insurance information; ② laboratory indicators: albumin, calcium, creatinine, potassium, alkaline phosphatase, phosphorus, chlorine, blood sodium, blood glucose, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), C-reactive protein (CRP), D-dimer, prothrombin time (PT), erythrocyte hemoglobin distribution width (RDW); ③ comorbidity information: dementia, obesity, pulmonary embolism, pneumonia, cirrhosis, osteoporosis, acute kidney injury (AKI), chronic obstructive pulmonary disease (COPD), cerebral infarction, sepsis, Parkinson's disease, asthma, myocardial infarction, arrhythmia, heart failure; ④ evaluation scale: Simplified Acute Physiology Score II (SAPSII), Sequential Organ Failure Assessment (SOFA) score; ⑤ treatment methods: heparin, mechanical ventilation, antibiotic treatment.
Nomogram construction
The subjects were randomly divided into a training group (70%) and a validation group (30%). The baseline data were compared between the two groups. The training set was used to build the predictive model, while the validation set was used to evaluate the performance of the model. Multiple imputation was performed for missing values < 40% [20], and outliers were converted to normal values using the capping method. In the course of this study, we employed LASSO regression, a regularized linear regression, to select prognostic predictors primarily due to its proficiency in handling high-dimensional data [21]. Lasso regression introduces an L1 penalty in the loss function, shrinking some coefficients precisely to zero, thereby excluding them from the model [22]. First, LASSO regression was used to select the best predictive feature variables, and lambda 1se was determined as the corresponding coefficient by tenfold cross-validation. Then, multivariate logistic regression analysis was used to determine the risk factors of respiratory failure in hip fracture, and a visualized nomogram model was constructed.
Nomogram validation and performance evaluation
The concordance index (C-index) and the area under the receiver operating characteristic (ROC) curve (AUC) were calculated in the training and validation groups to assess the accuracy of the nomogram model. Calibration curves were used to evaluate the calibration capability of the nomogram model. The goodness-of-fit of the logistic regression model was assessed using the Hosmer–Lemeshow test. The clinical efficacy of the nomogram model was assessed using decision curve analysis (DCA).
Statistical analysis
Kolmogorov–Smirnov (K–S) method was used to test the normality of the measurement data. The measurement data of normal distribution were described by mean ± standard deviation, and non-normal distribution data were described by median and upper and lower quartile. Enumeration data were compared by χ2 test and described as the number of cases or percentage. Logistic regression analysis could calculate the odds ratio (OR) and 95% confidence interval (CI), and the model with the minimum AIC was selected as the optimal model. Statistical analysis was performed using R4.2.2 software. The invoked packages included: mice package, glmnet package, caret package, rms package, pROC package, and rmda package. All tests were two-sided, and P < 0.05 was considered statistically significant. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Baseline information
According to the inclusion and exclusion criteria, there were 3266 hip fracture patients in the MIMIC-IV database, of whom 2942 patients did not develop respiratory failure and 324 patients developed respiratory failure. The data were randomly split in a 7:3 ratio into a training set (n = 2286) and a validation set (n = 980). The basic information is shown in Table 2. As shown in Table 2, the patients are mainly females, accounting for 70.15% of the total number (n = 2288); the proportion of cardiac dysrhythmia in comorbidity information is the highest, reaching 41.12% (n = 1342). In the continuous variables, the blood glucose, PT, and RDW values were relatively high, which may reflect the presence of hyperglycemia, coagulation dysfunction, or uneven distribution of red blood cells in the included patient population. There was no statistically significant difference in all indicators between the two groups (P > 0.05), and the baseline data were comparable.
LASSO analysis and logistic regression results
All 34 variables were included in the LASSO regression to screen for characteristic variables. When the adjustment parameter was lambda. 1se (λ = 0.005), the candidate predictors of the initial screening include the following 15 variables (Fig. 2): age, height, albumin, creatinine, chloride, AST, race, pneumonia, AKI, COPD, myocardial infarction, cardiac dysrhythmia, heart failure, heparin, and antibiotic treatment. According to the results of LASSO regression analysis, the 15 candidate predictors were included in the multivariate logistic regression model. Multivariate analysis showed that 7 variables were associated with the risk of respiratory failure in patients with hip fracture (Table 3), including age (OR: 0.98, 95%CI:0.97–0.99), height (OR: 0.94, 95%CI: 0.91–0.97), albumin (OR: 0.80, 95%CI: 0.65–0.99), chloride (OR: 0.97, 95%CI: 0.94–0.99), pneumonia (reference: without pneumonia, OR: 2.18, 95% CI: 1.53–3.12), AKI (reference: without AKI, OR: 2.89, 95% CI: 2.10–4.02), and heparin (reference: without heparin, OR: 2.54, 95%CI: 1.54–4.20).
Nomogram building and model performance evaluation
To visualize the prediction model constructed based on the results of multivariate Logistic regression analysis, a nomogram containing the 7 variables (Fig. 3) was developed, and the accuracy, calibration ability, and clinical utility of the prediction model were evaluated in the training set and the validation set. The C-index of the model in the training set and validation set was 0.77 (0.75,0.81) and 0.73 (0.75,0.81), respectively, and the AUC was 0.77 (0.74,0.80) and 0.73 (0.67,0.78), respectively. These results indicated that the prediction model has good discrimination power (Fig. 4). Additionally, the goodness-of-fit of our model was tested using the Hosmer–Lemeshow test, yielding a p-value of 0.57, suggesting a good fit of the model to the observed data. The calibration curves of the training set and the validation set had a good agreement with the diagonal (Fig. 5). The DCA curve, a measure of the clinical applicability of the predictive model, showed that the net benefit of the nomogram was higher than that of the "all treatment" or "no treatment" regimens (Fig. 6), suggesting that the model had favorable accuracy and predictive power for acute respiratory failure.
Discussion
In this study, predictors were screened through LASSO logistic analysis, and a prediction model for the risk of acute respiratory failure in elderly patients with hip fracture was constructed based on 7 variables, including age, height, albumin, chloride, pneumonia, AKI, and heparin. The model showed good performance in both the training and validation sets, the calibration curve had a good match with the diagonal, and the DCA curve showed that the nomogram had more net benefit than the "all treatment" and "no treatment" regimens.
Age was associated with respiratory failure after femoral fracture
Older people are more likely to have a hip fracture than younger people because they are more prone to osteoporosis. As they age, their balance declines and their risk of falls increases [23, 24]. In addition, the structure and function of the respiratory system of the elderly have changed, such as decreased lung compliance and reduced respiratory muscle strength, which affect respiratory function and oxygenation in the elderly [25]. The immune system of the elderly is also relatively weaker, which cause the elderly more susceptible to infection of respiratory pathogens [26]. It should be noted that hip fractures in the elderly often require prolonged bed rest, which can result in decreased respiratory muscle strength, increasing the risk of respiratory failure [9].
Surprisingly, our study results demonstrated that, within the population of older patients with hip fractures, those who are relatively younger exhibited a higher incidence of the disease. This phenomenon may be attributable to the following factors: ① disease base: If the total number of relatively younger old patients with hip fractures is small, but the count of patients with acute respiratory failure within this group is large, then the proportion of acute respiratory failure in this age group would be higher. Wang MT et al. found that the 10-year complication rate of hip fracture in young people was 26.50%, which was higher than that of the elderly (15.30%) [27]. ② Severity of disease: If a young person has a hip fracture, it may be due to a more serious accident, such as a high fall or a traffic accident, which may cause greater trauma to their body and increase the risk of acute respiratory failure [28].
Height is associated with respiratory failure after a femoral fracture
There was a positive correlation between height and lung volume. If the height is shortened with age or disease, such as osteoporosis and scoliosis, lung volume would decrease, which would affect respiratory function [29, 30]. Moreover, there is a negative correlation between height and obesity. A shorter height was associated with a higher risk of obesity. Obesity can increase breathing work, lower respiratory compliance, and reduce functional residual capacity, making it a risk factor for respiratory failure [31]. Studies have shown that height may be associated with bone health, which in turn is influenced by nutritional status [32]. Loss of height with advancing age is often associated with degenerative changes in bone, such as osteoporosis, as well as compression of the intervertebral disc [33]. Optimization of nutrient intake is considered to be essential to combat the physiological aging of bone, able to slow this process by maintaining bone mineral density and the integrity of bone structure [34]. Ensuring adequate dietary intake of calcium and vitamin D is a key factor in slowing the rate of bone degradation associated with aging [35]. Malnutrition is associated with decreased immune function, delayed wound healing, and difficulty in rehabilitation, and it is a risk factor for complications after respiratory failure and hip fracture [36].
Albumin levels are associated with the risk of respiratory failure after a femoral fracture
Hypoalbuminemia is a risk factor for respiratory failure because hypoalbuminemia can lead to higher pulmonary capillary permeability, increased pulmonary interstitial edema, and decreased oxygenation capacity, thereby increasing the risk of respiratory failure [37]. Hypoalbuminemia is also a risk factor for hip fracture because it can lead to decreased bone mass, damaged bone microstructure, and decreased bone strength, thereby increasing the risk of hip fracture [38]. Hypoalbuminemia may reflect a large surgical trauma, which is a risk factor for respiratory failure and complications after hip fracture because surgical trauma can lead to blood loss, infection, and hypoxia [39].
Electrolyte chloride ions are associated with the risk of respiratory failure following a femoral fracture
Hypochloremia is a risk factor for complications after hip fracture, because hypochloremia leads to increased reabsorption of sodium by renal tubules, which increases sodium levels in the blood. In order to maintain sodium homeostasis, the body will increase urine volume, resulting in complications such as dehydration, hypotension, and shock [40]. Hypochloremia may reflect the presence of other chronic diseases because it can lead to renal insufficiency, excessive use of diuretics, vomiting, or diarrhea, which can reduce chloride levels in the blood. Chronic diseases are a risk factor for complications after respiratory failure and hip fracture, because chronic diseases can lead to systemic inflammatory response, organ dysfunction, and complex drug therapy [41].
Pneumonia is associated with respiratory failure following a femoral fracture
Pneumonia is a common cause of respiratory failure because it can lead to ventilation/blood flow imbalance, diffusion disturbance, decreased lung compliance, and decreased respiratory muscle strength, resulting in hypoxemia and/or hypercapnia [42]. Pneumonia is a common postoperative complication, especially in patients undergoing hip replacement or internal fixation for hip fractures [43]. The incidence of pneumonia is related to factors such as operation time, age, complications, and preoperative status. At the same time, a hip fracture can also lead to bed rest, limited activity, and analgesic drug use, thus affecting respiratory function [44].
AKI is associated with respiratory failure after femoral fracture
If patients develop AKI after a hip fracture, they may be at higher risk of developing respiratory failure. Postoperative acute renal injury will lead to deterioration of respiratory function, because it will lead to water retention, pulmonary edema, and decreased oxygenation capacity, thereby resulting in hypoxic respiratory failure [45]. Postoperative acute renal injury will also cause acidosis, potassium ion disorder, and calcium ion disorder, thus affecting neuromuscular function and respiratory drive, leading to hypercapnia respiratory failure [46].
Heparin therapy is associated with respiratory failure after fetal fracture
Heparin is a commonly used postoperative prophylactic drug, which can prevent postoperative deep vein thrombosis by inhibiting thrombin activity [47]. Heparin therapy may be beneficial for respiratory failure because it can prevent and treat respiratory microcirculation disorders and pulmonary edema caused by pulmonary embolism, infection, and trauma [48].
Surprisingly, our results indicated that the incidence of the disease increased in the elderly who received heparin therapy, which may be related to the following factors: ① severity of the disease: Heparin is commonly used to prevent or treat thrombosis. Patients treated with heparin may be severely ill or have a higher risk of thrombosis, which makes them more susceptible to acute respiratory failure. ② Complications: The use of heparin may cause complications such as bleeding and heparin-induced thrombocytopenia [49]. These complications may increase the burden on the patient, thereby increasing the risk of acute respiratory failure. ③ Therapeutic response: Some patients may be resistant or intolerant to heparin [50].
Generalizability and economic and clinical implications of the study
Considering the generalizability and economic implications of our study, it is crucial to note that the research was based on the MIMIC-IV database, primarily representing a large tertiary care center population in the USA. Therefore, the results might not directly apply to other types of hospitals or diverse healthcare systems. Nevertheless, the identified predictors in our model, such as age, height, albumin levels, are universally accepted and commonly measured factors in clinical practice, suggesting a broad potential application of our model.
Simultaneously, our model's economic implications should not be overlooked. The variables utilized in our model are routinely collected in clinical settings, thus requiring no additional cost for data collection. Moreover, by accurately identifying patients at high risk of respiratory failure, our model can guide timely interventions, which may lead to improved patient outcomes, shorter hospital stays, and reduced healthcare costs. Despite these advantages, further validation of this model in diverse patient populations and different clinical settings is still recommended to ensure its reliability and broad applicability.
Limitations of the study
Nevertheless, our study had some limitations. ① Although the sample size was large, this was a single-center retrospective cross-sectional study with inevitable bias; ② while our model showed promising results with the current dataset, its applicability to diverse patient groups, especially across different regions and ethnicities, requires further validation; ③ hip fractures were not categorized by type, potentially introducing bias; ④ our model relied on available clinical variables from the database, omitting potentially relevant factors like genetics and lifestyle; ⑤ there was a lack of external validation, notably from domestic datasets. Prospective, multi-center studies will be pivotal in refining the model. Future iterations should expand the sample size, test across different populations, and incorporate broader influencing factors; ⑥ our choice to handle missing data with a 40% threshold, while preserving sample size, may introduce biases. Although our missing data were random, future studies should adopt stricter thresholds and enhanced data cleaning techniques; ⑦ the age inclusion criterion set in our study, at 55 years and above, diverges from the World Health Organization (WHO)'s definition of old age beginning at 60 years. A threshold of 55 years was selected to maintain a larger sample size of patients with acute respiratory failure, ensuring robustness in the analyses. However, this discrepancy might influence the generalizability of the findings to a broader elderly population defined by the WHO standards.
Conclusion
In summary, the nomogram drawn in this study has good discrimination, calibration ability, and clinical utility, which can help clinicians better understand the risk of acute respiratory failure in elderly patients with hip fracture and then tailor medical intervention measures. The model can not only provide a reference for disease risk stratification, but also help to develop prognostic treatment strategies and follow-up strategies for survival.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Abbreviations
- MIMIC:
-
Medical information mart for intensive care
- HIPAA:
-
Health Insurance Portability and Accountability Act
- PT:
-
Prothrombin time
- RDW:
-
Erythrocyte hemoglobin distribution width
- COPD:
-
Chronic obstructive pulmonary disease
- SAPSII:
-
Simplified Acute Physiology Score II
- K-S:
-
Kolmogorov–Smirnov
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- HIT:
-
Heparin-induced thrombocytopenia
- ROC:
-
Receiver operating characteristic
- C-index:
-
Concordance index
- DCA:
-
Decision curve analysis
- LASSO:
-
Least Absolute Shrinkage and Selection Operator
- AKI:
-
Acute kidney injury
- AUC:
-
Area under the curve
- TRIPOD:
-
Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis
- ICD:
-
International Classification of Disease
- PaO2 :
-
Oxygen partial pressure
- PaCO2 :
-
Carbon dioxide partial pressure
- SOFA:
-
Sequential Organ Failure Assessment
- SQL:
-
Structured query language
- BMI:
-
Body mass index
- CK:
-
Creatine kinase
- CRP:
-
C-reactive protein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- Lambda.1se:
-
Lambda.1se refers to the value of the regularization parameter lambda at which the cross-validated error is within one standard error of the minimum error observed in the regularization path of a LASSO model. This criterion is often used as a more conservative approach to selecting the model, aiming to improve model generalizability by trading off some bias for lower variance
References
Zhao Z, Fan W, Wang L, Chu Q. The paradoxical association of lipids with survival and walking ability of hip fractures in geriatric patients after surgery: a 1-year follow-up study. Int J Gen Med. 2023;16:3907–19.
Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–809.
Kang HY, Yang KH, Kim YN, Moon SH, Choi WJ, Kang DR, et al. Incidence and mortality of hip fracture among the elderly population in South Korea: a population-based study using the national health insurance claims data. BMC Public Health. 2010;10:230.
Mukherjee K, Brooks SE, Barraco RD, Como JJ, Hwang F, Robinson BRH, et al. Elderly adults with isolated hip fractures- orthogeriatric care versus standard care: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2020;88(2):266–78.
Veronese N, Maggi S. Epidemiology and social costs of hip fracture. Injury. 2018;49(8):1458–60.
LeBlanc KE, Muncie HL Jr, LeBlanc LL. Hip fracture: diagnosis, treatment, and secondary prevention. Am Fam Physician. 2014;89(12):945–51.
Marsillo E, Pintore A, Asparago G, Oliva F, Maffulli N. Cephalomedullary nailing for reverse oblique intertrochanteric fractures 31A3 (AO/OTA). Orthop Rev. 2022;14(6):38560.
Gargano G, Poeta N, Oliva F, Migliorini F, Maffulli N. Zimmer Natural Nail and ELOS nails in pertrochanteric fractures. J Orthop Surg Res. 2021;16(1):509.
Chen J, Tian Z, Zhang H, Shi L, Bao W, Huang T, et al. Risks of postoperative respiratory failure in elderly patients after hip surgery: a retrospective study. J Orthop Surg Res. 2022;17(1):140.
Maffulli N, Aicale R. Proximal femoral fractures in the elderly: a few things to know, and some to forget. Medicina. 2022;58(10):1314.
Chen YP, Kuo YJ, Hung SW, Wen TW, Chien PC, Chiang MH, et al. Loss of skeletal muscle mass can be predicted by sarcopenia and reflects poor functional recovery at one year after surgery for geriatric hip fractures. Injury. 2021;52(11):3446–52.
Davidson C, Banham S, Elliott M, Kennedy D, Gelder C, Glossop A, et al. British Thoracic Society/Intensive Care Society Guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. BMJ Open Respir Res. 2016;3(1):e000133.
Palakshappa JA, Krall JTW, Belfield LT, Files DC. Long-term outcomes in acute respiratory distress syndrome: epidemiology, mechanisms, and patient evaluation. Crit Care Clin. 2021;37(4):895–911.
Ackermann L, Schwenk ES, Lev Y, Weitz H. Update on medical management of acute hip fracture. Cleve Clin J Med. 2021;88(4):237–47.
Teasell R, Dittmer DK. Complications of immobilization and bed rest. Part 2: Other complications. Can Fam Phys. 1993;39:1440–2.
Ray P, Birolleau S, Lefort Y, Becquemin MH, Beigelman C, Isnard R, et al. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Crit Care. 2006;10(3):R82.
Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2012;125(1):79–86.
Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10(1):1.
Fujishima S. Guideline-based management of acute respiratory failure and acute respiratory distress syndrome. J Intensive Care. 2023;11(1):10.
Chowdhry AK, Gondi V, Pugh SL. Missing data in clinical studies. Int J Radiat Oncol Biol Phys. 2021;110(5):1267–71.
Emmert-Streib F, Dehmer M. High-dimensional LASSO-based computational regression models: regularization, shrinkage, and selection. Mach Learn Knowl Extr. 2019;1(1):359–83.
Demir-Kavuk O, Kamada M, Akutsu T, Knapp EW. Prediction using step-wise L1, L2 regularization and feature selection for small data sets with large number of features. BMC Bioinform. 2011;12:412.
Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366(9480):129–35.
Jo S, Lee SH, Lee HJ. The correlation between the fracture types and the complications after internal fixation of the femoral neck fractures. Hip Pelvis. 2016;28(1):35–42.
Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–60.
Delerme S, Ray P. Acute respiratory failure in the elderly: diagnosis and prognosis. Age Ageing. 2008;37(3):251–7.
Wang MT, Yao SH, Wong P, Trinh A, Ebeling PR, Tran T, et al. Hip fractures in young adults: a retrospective cross-sectional study of characteristics, injury mechanism, risk factors, complications and follow-up. Arch Osteoporos. 2017;12(1):46.
Grant M, Mehdian-Staffell R, Webb M, Scott S. Hip fractures in the young polytrauma patient: a review and the latest data from the UK. Eur J Orthop Surg Traumatol. 2021;31(5):817–23.
Kjensli A, Ryg M, Falch JA, Armbrecht G, Diep LM, Eriksen EF, et al. Does body height reduction influence interpretation of lung function in COPD patients? Eur Respir J. 2010;36(3):540–8.
Quanjer PH, Hall GL, Stanojevic S, Cole TJ, Stocks J. Age- and height-based prediction bias in spirometry reference equations. Eur Respir J. 2012;40(1):190–7.
Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–67.
Kupisz-Urbanska M, Marcinowska-Suchowierska E. Malnutrition in older adults-effect on falls and fractures: a narrative review. Nutrients. 2022;14(15):3123.
Nakano M, Nakamura Y, Suzuki T, Kobayashi T, Takahashi J, Shiraki M. Implications of historical height loss for prevalent vertebral fracture, spinal osteoarthritis, and gastroesophageal reflux disease. Sci Rep. 2020;10(1):19036.
Rizzoli R, Biver E, Brennan-Speranza TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9(9):606–21.
Lee AM, Sawyer RK, Moore AJ, Morris HA, O’Loughlin PD, Anderson PH. Adequate dietary vitamin D and calcium are both required to reduce bone turnover and increased bone mineral volume. J Steroid Biochem Mol Biol. 2014;144:159–62.
Gil-Romero V, Puertas-Molina L, Lleixà-Méndez N, Ibarra-Rubio M. Prevalence and factors associated with malnutrition in hospitalized patients with proximal femur fracture: experience at Hospital Universitari Mutua Terrassa. Enferm Clin (Engl Ed). 2022;32(4):234–8.
Thongprayoon C, Cheungpasitporn W, Chewcharat A, Mao MA, Thirunavukkarasu S, Kashani KB. Risk of acute respiratory failure among hospitalized patients with various admission serum albumin levels: a cohort study. Medicine. 2020;99(9):e19352.
Higashikawa T, Shigemoto K, Goshima K, Horii T, Usuda D, Morita T, et al. Mortality and the risk factors in elderly female patients with femoral neck and trochanteric fractures. J Clin Med Res. 2020;12(10):668–73.
Zalavras C, Velmahos GC, Chan L, Demetriades D, Patzakis MJ. Risk factors for respiratory failure following femoral fractures: the role of multiple intramedullary nailing. Injury. 2005;36(6):751–7.
Zandijk AJL, van Norel MR, Julius FEC, Sepehrvand N, Pannu N, McAlister FA, et al. Chloride in heart failure: the neglected electrolyte. JACC Heart Fail. 2021;9(12):904–15.
Labaki WW, Han MK. Chronic respiratory diseases: a global view. Lancet Respir Med. 2020;8(6):531–3.
Bohl DD, Sershon RA, Saltzman BM, Darrith B, Della Valle CJ. Incidence, risk factors, and clinical implications of pneumonia after surgery for geriatric hip fracture. J Arthroplasty. 2018;33(5):1552-6.e1.
Zhang X, Shen ZL, Duan XZ, Zhou QR, Fan JF, Shen J, et al. Postoperative pneumonia in geriatric patients with a hip fracture: incidence, risk factors and a predictive nomogram. Geriatr Orthop Surg Rehabil. 2022;13:21514593221083824.
Han SB, Kim SB, Shin KH. Risk factors for postoperative pneumonia in patients undergoing hip fracture surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2022;23(1):553.
Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis. 2008;15(3):284–96.
Park BD, Faubel S. Acute kidney injury and acute respiratory distress syndrome. Crit Care Clin. 2021;37(4):835–49.
Tydén J, Herwald H, Sjöberg F, Johansson J. Increased plasma levels of heparin-binding protein on admission to intensive care are associated with respiratory and circulatory failure. PLoS One. 2016;11(3):e0152035.
Erelel M, Kaskal M, Akbal-Dagistan O, Issever H, Dagistanli AS, Balkanci H, et al. Early effects of low molecular weight heparin therapy with soft-mist inhaler for covid-19-induced hypoxemia: a phase IIb trial. Pharmaceutics. 2021;13(11):1768.
Liu Y, Lu H, Xu H, Fu Z, Zhang D, Jiang B. Heparin-induced thrombocytopenia and acute colonic pseudo-obstruction after therapeutic anticoagulation in a very elderly patient with hip fracture: a case report. BMC Musculoskelet Disord. 2020;21(1):85.
Levy JH, Connors JM. Heparin resistance—clinical perspectives and management strategies. N Engl J Med. 2021;385(9):826–32.
Acknowledgements
Not applicable.
Funding
This study was supported by Youth Innovation Team Program of Shaanxi Education Department (22JP018).
Author information
Authors and Affiliations
Contributions
YL collected the data, analyzed the data, and drafted the manuscript. BD conceived of the study, participated in its design and coordination, and helped to draft the manuscript. BD was responsible for the whole project, reviewed the manuscript, designed the study, and supervised the study. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Dong, B. Development and validation of risk prediction nomograms for acute respiratory failure in elderly patients with hip fracture. J Orthop Surg Res 18, 899 (2023). https://doi.org/10.1186/s13018-023-04395-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04395-z