Abstract
Background
Two minimally invasive approaches showed some advantages in outcomes compared to conventional approaches (CAs)—the direct anterior approach (DAA) and the supercapsular percutaneously assisted approach in THA (SuperPATH). To the best of our knowledge, DAA and SuperPATH have never been compared, neither in clinical studies, nor in a meta-analysis. To conduct a systematic review and network meta-analysis of randomized controlled trials comparing short-term outcomes of DAA and SuperPATH in total hip joint arthroplasty (THA).
Methods
A systematic literature search up to May 2020 was performed to identify randomized controlled trials (RCTs) comparing SuperPATH with CAs and DAA with CAs in THA. We measured surgical, functional, and radiological outcomes. A network meta-analysis, using frequentist methods, was performed to assess treatment effects between DAA and SuperPATH. Information was borrowed from the above-mentioned RCTs, using the CA group as a common comparator.
Results
A total of 16 RCTs involving 1392 patients met the inclusion criteria, three trials with a level I evidence, 13 trials with a level II evidence. The overall network meta-analysis showed that SuperPATH reduced operation time (fixed effect model: MD = 12.8, 95% CI 9.9 to 15.7), incision length (fixed effect model: MD = 4.3, 95% CI 4.0 to 4.5; random effect model: MD = 4.3, 95% CI 0.2 to 8.4), intraoperative blood loss (fixed effect model: MD = 58.6, 95% CI 40.4 to 76.8), and early pain intensity (VAS 1 day postoperatively with a fixed effect model: MD = 0.8, 95% CI 0.4 to 1.2). The two approaches did not differ in acetabular cup positioning angles and in functional outcome.
Conclusions
Our overall findings suggested that the short-term outcomes of THA through SuperPATH were superior to DAA. SuperPATH showed better results in decreasing operation time, incision length, intraoperative blood loss, and early pain intensity. DAA and SuperPATH were equal in functional outcome and acetabular cup positioning.
Similar content being viewed by others
Introduction
Artificial total hip arthroplasty (THA) was introduced in the twenties of the last century. THA relieves pain, corrects deformities, and improves motor function and quality of life [1]. Several approaches to the hip joint in hip replacement have been described and modified by various authors. They are divided into two main groups: conventional and minimally invasive approaches. Conventional approaches (CAs) are the following: anterior, anterolateral, lateral transgluteal, lateral transtrochanteric, posterior, posterolateral. Minimally invasive approaches are modifications of the CAs with an incision length less than 10 cm [2,3,4] and a lower tissue traumatization [5,6,7,8]. The minimally invasive approaches are divided into two groups: muscle-sparing and mini-incision approaches. However, findings in current literature did not show remarkable benefits in outcomes of minimally invasive approaches compared to CAs in hip replacement [9,10,11,12,13,14,15]. Contrary to this general picture, two minimally invasive approaches showed some advantages in outcomes compared to CAs—the direct anterior approach (DAA) and the supercapsular percutaneously assisted approach in THA (SuperPATH). DAA was originally described by the German surgeon Carl Hueter in 1881 [16]. Smith-Petersen popularized DAA with a description in English-speaking literature in 1917 [17]. Judet reported the procedure in 1985 using a traction (fracture) table (TT) [18]. SuperPATH was first described by Chow in 2011 [19]. Table 1 gives a brief comparative overview of the most important DAA and SuperPATH operation points.
There are numerous systematic reviews and meta-analyses, comparing outcomes between DAA and CAs in hip replacement [20,21,22,23,24,25,26]. In general, current literature shows better results for DAA. On the other hand, there are three meta-analyses, comparing the outcomes between SuperPATH and CAs in hip replacement [27,28,29]. They showed overall better results for SuperPATH.
To the best of our knowledge, DAA and SuperPATH have never been compared, neither in clinical studies, nor in a meta-analysis. The aim of this systematic review and network meta-analysis (NMA) was to compare the short-term outcome of THA through DAA and SuperPATH in treatment of hip diseases and fractures, including only high-quality randomized controlled trials (RCTs).
Methods
Reporting guidelines and protocol registration
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis-Protocols (PRISMA-P) guidelines [30]. The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 25 September 2020 and finally approved on 27 October 2020 (CRD42020211298) at http://www.crd.york.ac.uk/PROSPERO/.
Data sources and search strategies
We searched the following databases and checked citations of screened studies and reviews for relevant manuscripts up to May 2020.
-
PubMed
-
China National Knowledge Infrastructure (CNKI)
-
The Cochrane Library
-
Google Scholar
-
Clinical trials
We built a BOOLEAN search strategy for studies on DAA and a similar BOOLEAN search strategy for studies on SuperPATH (see appendix for both) and adapted it to the syntax of the used databases. We did not apply restrictions to publication date or language. Results of the searches were exported to a reference management software [31]. A Chinese-speaking reviewer (KL) helped with the search in CNKI.
Study screening and selection
Two independent reviewers (NR and RK) scanned titles and abstracts to select articles for further consideration. The full text of the selected articles was obtained and scanned again for inclusion by the two reviewers (NR and RK). The decision on inclusion of each study was determined by the consensus between the two reviewers. Cases of disagreement were resolved by discussion and consensus with a third reviewer (KL). Kappa coefficient was used to measure the agreement between the reviewers. A Chinese-speaking reviewer (KL) helped with the study screening and selection by translation of Chinese articles. The entire search and selection process was carried out separately for studies on DAA and studies on SuperPATH, using the same methods.
Inclusion criteria
Types of studies:
-
RCTs
Types of participants:
-
Human participants with hip disease or hip fracture
Types of interventions:
-
THA through either DAA or SuperPATH compared to CAs
Exclusion criteria
-
No outcome of interest
-
Mini-incision approaches
-
Employment of a computer navigation system
-
Hip replacement with hemiarthroplasty
Types of outcome measures
Surgical outcome:
-
The operation time (in min) was defined as period of time from the beginning of skin incision to suture. It correlates with the competence of the surgeon as well as risk of infection.
-
The incision length (in cm) was measured on graduated scale. It reflects the severity of intraoperative trauma.
-
The intraoperative blood loss (in ml) was defined as the total amount of blood from the suction device. It reflects the severity of intraoperative trauma.
-
The pain visual analog scale (VAS) is an instrument for measuring pain intensity, providing a range of scores from 0 to 10 [32, 33]. The degree of hip pain was periodically evaluated at certain time intervals after operation.
Functional outcome:
-
The Harris Hip Score (HHS) was developed for assessment of the results of hip surgery [34]. The hip joint function was periodically evaluated at time intervals after operation. The score collects points from the assessment of four aspects: pain, function, degree of deformity, and range of motion of the hip. The higher the added score, the better the results, providing a range of added scores from 0 to 100.
Radiological outcome
-
The acetabular cup anteversion angle and the inclination angle (in degrees) have ideal values for positioning: anteversion angle from 10° to 25° and inclination angle from 40° to 50° [35]. Especially, the ideal acetabular cup anteversion is of great importance, since an angle too large often leads to posterior impingement, resulting in anterior dislocation, and an angle too small leads to posterior dislocation.
Data extraction and analysis
Data extraction was performed by two reviewers (NR and RK). Cases of disagreement were resolved by discussion and consensus with a third reviewer (KL). We extracted all relevant data into a data extraction form in a standard electronic spreadsheet and the Cochrane software program Review Manager Version 5.3 [36]. We extracted the following data: first author, year of publication, number of patients, patient characteristics, risk of bias, and outcome. A Chinese speaking reviewer (KL) helped with data extraction and analysis by translation of Chinese articles.
Risk of bias and level of evidence
We examined and checked the selected studies for their risk of bias. We made an assessment using Cochrane’s Risk of Bias 2 (RoB 2) tool [37]. The level of evidence was rated for each study, in accordance with guidelines of the Centre for Evidence-Based Medicine (Oxford, UK) [38].
Statistical analysis
Indirect comparison: network meta-analysis
A NMA, using frequentist methods [39], was performed to assess treatment effects between DAA and SuperPATH. First, a direct comparison was applied to calculate the results for either DAA or SuperPATH and CAs. Mean differences (MDs) with 95% confidence intervals (CIs) were estimated through fixed and random effects models for all outcomes. Study weighting was performed by inverse variance [40]. Then, information was borrowed from the above-mentioned direct comparisons, using the CA group as a common comparator and reference node within the network. Thereby, effect estimates were obtained in which the difference between the estimations was equivalent to the network estimate. Furthermore, we calculated prediction intervals to estimate where to expect the findings of future NMA on this topic. The network estimates were presented in forest plots. The calculations were done in the R language and environment for statistical computation. From within R, we used the meta [41] and netmeta [42] package. We followed the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions as basis for the methodology and presentation of the data [43]. All surgical approaches were mapped in a network plot (Fig. 1).
Assessment of heterogeneity
We assessed clinical and statistical heterogeneity. We did not pool study data that were clinically too diverse. Heterogeneity was assessed using a test on Cochrane’s Q statistic, which followed a distribution with k-degrees of freedom (p value < 0.10 is indicative of heterogeneity), and a Higgins’ test I2 (low heterogeneity, < 25%; moderate heterogeneity, 25–75%; and high heterogeneity, > 75%) [44]. The number of degrees of freedom k (χ2k) was equal to the number of studies minus the number of designs. For the distribution of Q of a single pooled estimate, k equals one, whereas k equals two for the network estimate. Results were presented regardless of the detection of heterogeneity in order to maintain the informative value within the forest plots.
Results
Study identification and selection
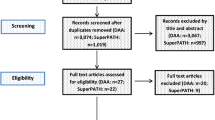
A description of the study selection process is given in a PRISMA flow diagram (Fig. 2). The PRISMA checklist is given as a Supplemental file.
DAA
After removing 324 duplicates, a total of 2924 studies were identified in our initial literature search. Thirty-seven studies were assessed for eligibility after first screening procedure by title and abstract (κ = 0.95) with disagreement between the reviewers concerning 2 studies. Of these studies, 29 were excluded after second screening procedure by full-paper analysis (κ = 1.0), leaving a total of 8 studies on DAA for inclusion in final meta-analysis.
SuperPATH
After removing 153 duplicates, a total of 1337 studies were identified in our initial literature search. Fifteen studies were assessed for eligibility after first screening procedure by title and abstract (κ = 1.0) with total agreement by the reviewers. Of these studies, 7 were excluded after second screening procedure by full-paper analysis (κ = 1.0), leaving a total of 8 studies on SuperPATH for inclusion in final meta-analysis.
Characteristics of the RCTs
The main characteristics of the 16 RCTs on DAA and SuperPATH with overall 1392 included patients are presented in Table 2. The main preoperative diagnoses were osteoarthritis, femoral neck fracture, and avascular necrosis of the femoral head.
DAA
The 8 studies, comparing DAA with CAs, were published between 2009 and 2018, altogether involving 898 patients (with 902 operated hip joints). Of the included patients, 390 were operated through DAA and 508 through CAs. The sample size of the studies on DAA ranged from 46 to 169 patients. All studies on DAA were published in English language. Of the 8 studies, 3 included conventional THA through posterolateral approach [45, 51, 52], 5 through lateral transgluteal approach [46,47,48,49,50].
SuperPATH
The 8 studies, comparing SuperPATH with CAs, were published between 2016 and 2019, altogether involving 494 patients (with 517 operated hip joints). Of the included patients, 232 were operated through SuperPATH and 262 through CAs. The sample size of the studies on SuperPATH ranged from 4 to 154 patients. Two studies were published in English language, [54, 57] and 6 studies were published in Chinese with an English abstract [53, 55, 56, 58,59,60]. Of the 8 studies, 4 included conventional THA through posterolateral approach [54, 55, 59, 60], 1 through posterior approach [57], 1 through lateral transgluteal approach [58]. In 2 studies, the surgical approach was conventional, but not further specified [53, 56].
Risk of bias and level of evidence
The quality of the included studies was assessed by the Cochrane Collaboration’s tool for risk of bias. Table 3 shows the summarized risk of bias assessment. Three out of 16 studies were blinded RCTs with a level I evidence [48, 52, 54]; the other 13 studies were non-blinded RCTs with a level II evidence [45,46,47, 49,50,51, 53, 55,56,57,58,59,60].
Clinical and statistical heterogeneity
No relevant differences were found between the patients in the experimental (either SuperPATH or DAA) and control group (CAs) in clinical characteristics for gender, age and BMI (Table 2). The statistical heterogeneity of all measured outcomes is shown in Figs. 3, 4, 5, and 6.
Outcomes
Surgical outcomes
Operation time
In indirect comparison between DAA and SuperPATH, data on 379 patients were pooled from 11 RCTs (I2 = 95%, p < 0.01, Fig. 3, Table 4). The operation time of DAA was 12.8 min longer than the operation time of SuperPATH, using a fixed effect model (MD = 12.8, 95% CI 9.9 to 15.7). There was no difference in operation time, using a random effect model (MD = 7.0, 95% CI − 8.6 to 22.5).
Incision length
In indirect comparison between DAA and SuperPATH, data on 371 patients were pooled from 10 RCTs (I2 = 99%, p < 0.01, Fig. 3, Table 4). The incision length of DAA was 4.3 cm longer than the incision length of SuperPATH, using a fixed effect model (MD = 4.3, 95% CI 4.0 to 4.5). The incision length of DAA was 4.3 cm longer than the incision length of SuperPATH, using a random effect model (MD = 4.3, 95% CI 0.2 to 8.4).
Intraoperative blood loss
In indirect comparison between DAA and SuperPATH, data on 330 patients were pooled from 10 RCTs (I2 = 99%, p < 0.01, Fig. 3, Table 4). The intraoperative blood loss of DAA was 58.6 ml higher than the intraoperative blood loss of SuperPATH, using a fixed effect model (MD = 58.6, 95% CI 40.4 to 76.8). There was no difference in intraoperative blood loss, using a random effect model (MD = 32.5, 95% CI − 146.7 to 211.6).
Pain VAS 1 day postoperatively
In indirect comparison between DAA and SuperPATH, data on 224 patients were pooled from 6 RCTs (I2 = 84%, p < 0.01, Fig. 4, Table 4). The pain VAS 1 day postoperatively of DAA was 0.8 points higher than the pain VAS 1 day postoperatively of SuperPATH, using a fixed effect model (MD = 0.8, 95% CI 0.4 to 1.2). There was no difference in pain VAS 1 day postoperatively, using a random effect model (MD = 0.1, 95% CI − 1.3 to 1.5).
Pain VAS 3 days postoperatively
In indirect comparison between DAA and SuperPATH, data on 181 patients were pooled from 5 RCTs (I2 = 75%, p < 0.01, Fig. 4, Table 4). There was no difference in pain VAS 3 days postoperatively, using a fixed effect model (MD = 0.4, 95% CI − 0.2 to 1.0). There was no difference in pain VAS 3 days postoperatively, using a random effect model (MD = − 0.1, 95% CI − 1.5 to 1.4).
Functional outcome: Harris Hip Score
HHS 3 months postoperatively
In indirect comparison between DAA and SuperPATH, data on 461 patients were pooled from 12 RCTs (I2 = 92%, p < 0.01, Fig. 5, Table 4). There was no difference in HHS 3 months postoperatively of DAA, using a fixed effect model (MD = 1.8, 95% CI − 0.1 to 3.6) and a random effect model (MD = 1.6, 95% CI − 2.9 to 6.0).
HHS 6 months postoperatively
In indirect comparison between DAA and SuperPATH, data on 325 patients were pooled from 8 RCTs (I2 = 0%, p = 0.92, Fig. 5, Table 4). There was no difference in HHS 6 months postoperatively, using a fixed effect model (MD = 0.2, 95% CI − 1.9 to 2.4) and a random effect model (MD = 0.2, 95% CI − 1.9 to 2.4).
HHS 12 months postoperatively
In indirect comparison between DAA and SuperPATH, data on 256 patients were pooled from 7 RCTs (I2 = 0%, p = 0.89, Fig. 5, Table 4). There was no difference in HHS 12 months postoperatively of DAA, using a fixed effect model (MD = 0.1, 95% CI − 1.8 to 1.9) and a random effect model (MD = 0.1, 95% CI − 1.8 to 1.9).
Radiological outcome
Acetabular cup anteversion angle
In indirect comparison between DAA and SuperPATH, data on 183 patients were pooled from 6 RCTs (I2 = 6%, p = 0.37, Fig. 6, Table 4). The acetabular cup anteversion angle of DAA was 3.7° lower than the acetabular cup anteversion angle of SuperPATH, using a fixed effect model (MD = − 3.7, 95% CI − 4.6 to − 2.7). The in acetabular cup anteversion angle of DAA was 3.7° lower than the acetabular cup anteversion angle of SuperPATH, using a random effect model (MD = − 3.7, 95% CI − 4.7 to − 2.7).
Acetabular cup inclination angle
In indirect comparison between DAA and SuperPATH, data on 295 patients were pooled from 8 RCTs (I2 = 73%, p < 0.01, Fig. 6, Table 4). There was no difference in acetabular cup inclination angle, using a fixed effect model (MD = − 0.5, 95% CI − 2.2 to 1.1) and a random effect model (MD = 0.6, 95% CI − 2.5 to 3.8).
Discussion
Main and new findings
Sixteen randomized controlled trials with 1392 patients were included in this NMA. The studies on DAA consisted of 898 patients; the studies on SuperPATH consisted of 494 patients. In our NMA, the DAA group consisted of 390 patients, the SuperPATH group of 232 patients, and the CAs group as a common comparator of a total of 770 patients. In general, our NMA indicated that THA through SuperPATH was superior to THA through DAA regarding the investigated outcomes. SuperPATH showed better results on decreasing operation time, incision length, intraoperative blood loss, and early postoperative pain intensity in THA. DAA and SuperPATH were equal in short-term postoperative functional outcome after THA. Furthermore, both approaches showed sufficient results in acetabular cup positioning. Three studies out of 16 were blinded RCTs with a level I evidence [48, 52, 54]; the other 13 studies were non-blinded RCTs with a level II evidence [45,46,47, 49,50,51, 53, 55,56,57,58,59,60].
The value of this NMA comes from the inclusion of high-quality RCTs and the employment of high-quality statistical methods. We calculated the results with both a fixed and a random effect model, offering a higher informative value. Our NMA is the first attempt to systematically and quantitatively review the literature comparing DAA with SuperPATH. To the best of our knowledge, these approaches to the hip joint have never been compared, neither in clinical studies, nor in a meta-analysis.
DAA vs. SuperPATH
Our indirect comparison between DAA and SuperPATH included 16 RCTs and 1392 patients. The DAA group consisted of 390 patients, the SuperPATH group consisted of 232 patients.There was no difference in operation time, using a random effect model. DAA showed a 12.8 min longer operation time than SuperPATH, using a fixed effect model. This is an important advantage of SuperPATH since prolonged operative times (> 90 min) are associated with increased rates of superficial infections [61]. A 2019 analysis of 89,802 cases of THA by Surace showed that prolonged operation time was associated with perioperative complications [62]. Additionally, the authors suggested an optimal operation time of approximately 80 min with a lower risk of perioperative complications. The mean operation time of the studies included in our NMA ranged from 71 to 121 min for DAA and from 52 to 115 min for SuperPATH. Both approaches are known to have a prolonged learning curve for operating surgeons [63, 64]. SuperPATH may have potential for even shorter operation time, since it is a relatively new approach. In Table 5, our results were compared with the operation time of DAA and SuperPATH from additional studies [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88, 90,91,92,93,94,95] . The overall results seem to differ greatly from study to study within the two different approaches, so that a greater influence on the part of the operating surgeon and the clinic can be assumed.
The mean incision length in our NMA ranged from 9.1 to 13.7 cm for DAA and from 5.8 to 10.4 cm for SuperPATH. DAA had a 4.3 cm longer incision length than SuperPATH, using a fixed and a random effect model. Since both approaches are minimally invasive, they should aim for shorter incision lengths. Nevertheless, literature is inconclusive about the importance of incision length. A 2013 meta-analysis by Xu with 14 RCTs and 1174 patients did not come to a definite overall conclusion whether mini-incision or standard incision THA is superior [96]. Another 2013 meta-analysis by Moskal with 30 studies and 3548 THAs showed that limited incision was superior to standard incision in short-term recovery after THA [97]. Incision length is also dependent on patient weight, height, and gender. Larger and more obese patients as well as women are more likely to receive longer incisions in mini-incision THA [98].
The mean intraoperative blood loss in our NMA ranged from 166 to 1344 ml for DAA and from 138 to 1108 ml for SuperPATH. There was no difference in intraoperative blood loss, using a random effect model. DAA had a 59 ml higher intraoperative blood loss than SuperPATH, using a fixed effect model. The lower blood loss is an important advantage of SuperPATH. In general, literature shows a superiority of mini-incision approaches in reducing blood loss compared to standard approaches [10, 24, 99]. A reason for the higher blood loss of DAA might be a bleeding of branches of the lateral circumflex femoral artery that cross the surgical field when operating through DAA. Sometimes, the ligation of those branches is tedious and time consuming. Other known factors besides approaches to the hip joint influencing blood loss in THA are the utilization of tranexamic acid and intraoperative active warming [100,101,102]. A 2019 meta-analysis by Qi with 10 RCTs showed that the utilization of intravenous tranexamic acid in patients with hip fracture undergoing hip surgeries reduces blood loss and allogeneic blood transfusion [100]. A 2018 NMA by Fillingham with 34 included studies came to the same conclusion in THA [101].
The mean pain VAS 1 day postoperatively in our NMA ranged from 2.6 to 4 points for DAA and from 3.1 to 8.3 points for SuperPATH. DAA had a 0.8 points higher pain VAS 1 day postoperatively than SuperPATH, using a fixed effect model. There was no difference between DAA and SuperPATH in pain VAS 1 day postoperatively, using a random effect model. Furthermore, there was no difference between DAA and SuperPATH in pain VAS 3 days postoperatively, using a fixed and a random effect model. Postoperative pain is an expected but yet undesirable side effect of all surgical interventions. It has a strong influence on the overall well-being of the patient. The lower pain VAS 1 day postoperatively is an important advantage of SuperPATH. The difference may be due to the innervation of the operation area. Branches of the femoral nerve, the obturator nerve, and cutaneal lateral femoral nerve may contribute to pain sensation, when operating through DAA. In contrast, only branches from Th12 and iliohypogastric nerves contribute to pain sensation, when operating through SuperPATH. Furthermore, the superior-lateral aspect of the capsule may play a greater role than any other region in proprioception and pain perception of the hip joint. However, greater understanding is required in regard to the distribution of capsular innervation according to its anatomical location [103]. A recent 2019 NMA by Liu found that the best way to reduce THA pain 1–2 days postoperatively are the spinal anesthesia and lumbar plexus block [104]. A 2016 NMA by Jiménez-Almonte with 35 RCTs and 2296 patients included found a slight advantage to peripheral nerve blocks compared to local infiltration analgesia and opioid consumption 24 h after THA [105].
The mean HHS 3 months postoperatively in our NMA ranged from 85.9 to 94.6 points for DAA and from 72.3 to 89.6 points for SuperPATH. There was no difference between DAA and SuperPATH in HHS 3, 6, and 12 months postoperatively, using a fixed and a random effect model. Several meta-analyses found that DAA and SuperPATH were superior to CAs in early postoperative functional outcome (HHS 3 months postoperatively) and equal to CAs in subsequent postoperative functional outcomes [23, 24, 26, 27, 29]. Functional outcome is a very important outcome parameter. HHS was developed for the assessment of the results of hip surgery, covering four relevant areas: pain, function, absence of deformity, and range of motion [34].
The mean acetabular cup anteversion angle in our NMA ranged from 17.1 to 20.1° for DAA and from 15.0 to 21.9° for SuperPATH. DAA had a 3.7° lower acetabular cup anteversion angle than SuperPATH, using a fixed and a random effect model. Both approaches stayed within the widely accepted values for acetabular cup positioning: anteversion angle from 10° to 25° [35]. The mean acetabular cup inclination angle in our NMA ranged from 37.0 to 47.1° for DAA and from 37.1 to 43.8° for SuperPATH. There was no difference between DAA and SuperPATH in acetabular cup inclination angle, using a fixed and a random effect model. Both approaches showed a slight tendency toward a flat acetabular cup inclination angle, since the widely accepted values range from 40° to 50° [35].
Intra- and postoperative fractures, especially trochanteric fractures, infections, and hip dislocations, are important complications that seem to show different patterns in certain approaches. Surgical revision rates and leg length discrepancies are also parameters often taken into consideration in comparisons of THA. Nevertheless, postoperative complications could not be compared due to lack of consistent data in the RCTs included.
Limitations
We found the following limitations to our NMA: First, the long-term outcomes in THA were not considered. Second, due to insufficient data, important outcome parameters such as hospitalization time, postoperative drainage volume, and postoperative complications could not be considered. Third, this NMA did not consider the possible influence of the surgeon operating skills, the utilization of tranexamic acid and anticoagulants, bone cement, or the types of implants for hip replacement. Fourth, part of the studies did not give any information what exact hip pathology was treated with THA. Fifth, since the SuperPATH approach is a 2-incision approach, it remains unclear whether the included RCTs reported the added incision length or the length of the larger incision, ignoring the smaller additional incision. Sixth, the direct comparison probably offers a statistically higher quality meta-analysis. Since there are no RCTs comparing DAA with SuperPATH, at this point we cannot carry out and offer anything other than an indirect comparison. Lastly, in some cases of the outcomes investigated, the heterogeneity of the included RCTs was high.
Conclusion
Our overall findings suggested that the short-term outcomes of THA through SuperPATH were superior to DAA. SuperPATH showed better results in decreasing operation time, incision length, intraoperative blood loss, and early pain intensity, using a fixed effect model. SuperPATH showed equal results to DAA in operation time, intraoperative blood loss, and early pain intensity; it showed better results than DAA in incision length, using a random effect model. DAA and SuperPATH were equal in functional outcome and acetabular cup positioning.
Availability of data and materials
The data are available from the corresponding author upon reasonable request.
Abbreviations
- CNKI:
-
China National Knowledge Infrastructure
- CI:
-
Confidence interval
- DAA:
-
Direct anterior approach
- HHS:
-
Harris hip score
- MD:
-
Mean difference
- NMA:
-
Network meta-analysis
- RCT:
-
Randomized controlled trial
- SuperPATH:
-
Supercapsular percutaneously assisted approach in total hip arthroplasty
- THA:
-
Total hip arthroplasty
- TT:
-
Traction table
- VAS:
-
Visual analog scale
References
Cardenas NC, Bellotti V, Astarita E, et al. Innovative approach in total hip arthroplasty: supercapsular percutaneously-assisted. Hip Int. 2016;26:34–7.
Sculco TP, Jordan LC, Walter WL. Minimally invasive total hip arthroplasty : the hospital for special surgery experience. Orthop Clin North Am. 2004;35:137–42.
Szendroi M, Sztrinkai G, Vass R, Kiss J. The impact of minimally invasive total hip arthroplasty on the standard procedure. Int Orthop. 2006;30:160–71.
Wall SJ, Mears SC. Analysis of published evidence on minimally invasive total hip arthroplasty. J Arthroplasty. 2008;23:55–8.
Jerosch J. Minimalinvasive Hüftendoprothetik. Deutsches Ärzteblatt. 2006;103:A3333–9.
Oinuma K, Eingartner C, Saito Y, Shiratsuchi H. Total hip arthroplasty by a minimally invasive, direct anterior approach. Oper Orthop Traumatol. 2007;19:310–26.
Rittmeister M, Peters A. Vergleich des Hüftgelenkersatzes über eine posteriore Miniinzision oder einen klassischen anterolateralen Zugang. Orthopäde. 2006;35:716–22.
Wetzel R, Dorsch M. Der minimal-invasive Zugang zur Implantation der Hüftendoprothese. Der Orthopäde. 2006;35:738–43.
Migliorini F, Biagini M, Rath B, Meisen N, Tingart M, Eschweiler J. Total hip arthroplasty: minimally invasive surgery or not? Meta-analysis of clinical trials. Int Orthop. 2019;43(7):1573–82. https://doi.org/10.1007/s00264-018-4124-3 Epub 2018 Aug 31.
Cheng T, Feng JG, Liu T, Zhang XL. Minimally invasive total hip arthroplasty: a systematic review. Int Orthop. 2009;33(6):1473–81. https://doi.org/10.1007/s00264-009-0743-z.
Wang R, Li XX, Gao MX, Wang ZH, Yu LM, Li XS. Comparison of clinical efficacy between minimally invasive total hip arthroplasty and traditional total hip arthroplasty: a systematic review. Zhongguo Gu Shang. 2016;29(2):172–8 Article in Chinese.
Yang B, Li H, He X, Wang G, Xu S. Minimally invasive surgical approaches and traditional total hip arthroplasty: a meta-analysis of radiological and complications outcomes. PLoS One. 2012;7(5):e37947. https://doi.org/10.1371/journal.pone.0037947.
Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop. 2011;35(2):173–84. https://doi.org/10.1007/s00264-010-1075-8.
Wörner M, Weber M, Lechler P, Sendtner E, Grifka J, Renkawitz T. Minimally invasive surgery in total hip arthroplasty: surgical technique of the future? Orthopade. 2011;40(12):1068–74. https://doi.org/10.1007/s00132-011-1846-y Article in German.
Mahmood A, Zafar MS, Majid I, Maffulli N, Thompson J. Minimally invasive hip arthroplasty: a quantitative review of the literature. Br Med Bull. 2007;84:37–48. https://doi.org/10.1093/bmb/ldm029.
Galakatos GR. Direct anterior total hip arthroplasty. Mo Med. 2018;115(6):537–41.
Smith-Petersen MN, Larson CB, et al. Complications of old fractures of the neck of the femur; results of treatment of vitallium-mold arthroplasty. J Bone Joint Surg [Am]. 1947;29-A:41–8.
Judet J, Judet H. Anterior approach in total hip arthroplasty. Presse Med. 1985;14:1031–3 (In French).
Chow J, Penenberg B, Murphy S. Modified micro-superior percutaneously-assisted total hip: early experiences & case reports. Curr Rev Musculoskelet Med. 2011;4(3):146–50. https://doi.org/10.1007/s12178-011-9090-y.
Yue C, Kang P, Pei F. Comparison of direct anterior and lateral approaches in total hip arthroplasty: a systematic review and meta-analysis (PRISMA). Medicine (Baltimore). 2015;94(50):e2126. https://doi.org/10.1097/MD.0000000000002126.
Kyriakopoulos G, Poultsides L, Christofilopoulos P. Total hip arthroplasty through an anterior approach. EFORT Open Rev. 2018;3(11):574–83. https://doi.org/10.1302/2058-5241.3.180023.
Higgins BT, Barlow DR, Heagerty NE, Lin TJ. Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty. 2015;30(3):419–34. https://doi.org/10.1016/j.arth.2014.10.020.
Meermans G, Konan S, Das R, Volpin A, Haddad FS. The direct anterior approach in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 2017;99-B(6):732–40. https://doi.org/10.1302/0301-620X.99B6.38053.
Wang Z, Hou J, Wu C et al. A systematic review and meta-analysis of direct anterior approach versus posterior approach in total hip arthroplasty. J Orthop Surg Res 13, 229 (2018). https://doi.org/https://doi.org/10.1186/s13018-018-0929-4
Miller LE, Gondusky JS, Bhattacharyya S, Kamath AF, Boettner F, Wright J. Does surgical approach affect outcomes in total hip arthroplasty through 90 days of follow-up? A systematic review with meta-analysis. J Arthroplast. 2018;33:1296–302.
Kucukdurmaz F, Sukeik M, Parvizi J. A meta-analysis comparing the direct anterior with other approaches in primary total hip arthroplasty. Surgeon. 2019;17(5):291–9. https://doi.org/10.1016/j.surge.2018.09.001.
Li J, Qiu B, Zhen D. Meta-analysis on clinical outcomes of the SuperPATH approach versus traditional approach in hip arthroplasty. J Clin Rehabil Tissue Eng Res. 22(15):2453–2460, MAY 28, 2018 DOI: https://doi.org/10.3969/j.issn.2095-4344.0194). [Article in Chinese]
Sun Zhenguo, Li Heng, Yang Honghang, Min Jikang. Systematic review on the curative effect of total hip arthroplasty through supercapsular percutaneously-assisted total hip approach versus posterolateral approach for treatment of hip diseases [J]. J Tradit Chin Orthop, 2018,30 (01): 32-37 + 40.) [Article in Chinese]
Ramadanov N, Bueschges S, Liu K, Klein R, Schultka R. Comparison of short-term outcomes between SuperPATH approach and conventional approaches in hip replacement: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2020;15(1):420. https://doi.org/10.1186/s13018-020-01884-3.
Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med. 2008;27:687–97.
EndNote [Computer program]. Version x9. Clarivate Analytics. Available from www.endnote.com (10.01.2020)
Gould D, et al. Visual Analogue Scale (VAS). J Cli Nurs. 2001;10:697–706.
Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–31.
Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–55.
Tan SC, Teeter MG, Del BC, et al. Effect of taper design on Trunnionosis in metal on polyethylene total hip arthroplasty. J Arthroplasty. 2015;30:1269–72.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898 Available from www.riskofbias.info (10.01.2020).
Centre for Evidence-Based Medicine. Levels of evidence (March 2009). Available at: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed September 24, 2020.
Seide SE, Jensen K, Kieser M. A comparison of Bayesian and frequentist methods in random-effects network meta-analysis of binary data. Res Synth Methods. 2020;11(3):363–78. https://doi.org/10.1002/jrsm.1397.
Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. Accessed 15 October 2020.
Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7(3):40–5.
Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages [published correction appears in PLoS One. 2015;10(4):e0123364]. PLoS One. 2014;9(12):e115065. https://doi.org/10.1371/journal.pone.0115065.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Barrett WP, Turner SE, Leopold JP. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J Arthroplasty. 2013;28(9):1634–8. https://doi.org/10.1016/j.arth.2013.01.034.
D'Arrigo C, Speranza A, Monaco E, Carcangiu A, Ferretti A. Learning curve in tissue sparing total hip replacement: comparison between different approaches. J Orthop Traumatol. 2009;10(1):47–54. https://doi.org/10.1007/s10195-008-0043-1.
De Anta-Díaz B, Serralta-Gomis J, Lizaur-Utrilla A, Benavidez E, López-Prats FA. No differences between direct anterior and lateral approach for primary total hip arthroplasty related to muscle damage or functional outcome. Int Orthop. 2016;40(10):2025–30. https://doi.org/10.1007/s00264-015-3108-9.
Mjaaland KE, Kivle K, Svenningsen S, Pripp AH, Nordsletten L. Comparison of markers for muscle damage, inflammation, and pain using minimally invasive direct anterior versus direct lateral approach in total hip arthroplasty: A prospective, randomized, controlled trial. J Orthop Res. 2015;33(9):1305–10. https://doi.org/10.1002/jor.22911.
Nistor DV, Caterev S, Bolboacă SD, Cosma D, Lucaciu DOG, Todor A. Transitioning to the direct anterior approach in total hip arthroplasty. Is it a true muscle sparing approach when performed by a low volume hip replacement surgeon? Int Orthop. 2017;41(11):2245–52. https://doi.org/10.1007/s00264-017-3480-8.
Reichert JC, von Rottkay E, Roth F, Renz T, Hausmann J, Kranz J, Rackwitz L, Nöth U, Rudert M. A prospective randomized comparison of the minimally invasive direct anterior and the transgluteal approach for primary total hip arthroplasty. BMC Musculoskelet Disord. 2018;19(1):241. https://doi.org/10.1186/s12891-018-2133-4.
Rykov K, Reininga IHF, Sietsma MS, Knobben BAS, Ten Have BLEF. Posterolateral vs direct anterior approach in total hip arthroplasty (POLADA Trial): a randomized controlled trial to assess differences in serum markers. J Arthroplasty. 2017;32(12):3652–3658.e1. https://doi.org/10.1016/j.arth.2017.07.008.
Zhao HY, Kang PD, Xia YY, Shi XJ, Nie Y, Pei FX. Comparison of early functional recovery after total hip arthroplasty using a direct anterior or posterolateral approach: a randomized controlled trial. J Arthroplasty. 2017;32(11):3421–8. https://doi.org/10.1016/j.arth.2017.05.056.
Hou J, Bao H, Cheng Y. Early effect observation of total hip arthroplasty by using SuperPATH technique. J Clin Orthop. 2017;20(1):50–3. https://doi.org/10.3969/j.issn.1008-0287.2017.01.023 Article in Chinese.
Meng W, Huang Z, Wang H, Wang D, Luo Z, Bai Y, et al. Supercapsular percutaneously-assisted total hip (SuperPath) versus posterolateral total hip arthroplasty in bilateral osteonecrosis of the femoral head: a pilot clinical trial. BMC Musculoskelet Disord. 2019;21(1):2. https://doi.org/10.1186/s12891-019-3023-0.
Ouyang C, Wang H, Meng W, Luo Z, Wang D, Pei F, et al. Randomized controlled trial of comparison between the SuperPATH and posterolateral approaches in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(12):1500–6. https://doi.org/10.7507/1002-1892.201807011 Article in Chinese.
Ren D, Yang G, Zhao H, et al. Effect of SuperPath minimally invasive incision total hip arthroplasty on femoral head necrosis and the quality of life. J Hebei Med Univ. 2016;37(12):1416–9. https://doi.org/10.3969/j.issn.1007-3205.2016.12.013 Article in Chinese.
Xie J, Zhang H, Wang L, Yao X, Pan Z, Jiang Q. Comparison of supercapsular percutaneously assisted approach total hip versus conventional posterior approach for total hip arthroplasty: a prospective, randomized controlled trial. J Orthop Surg Res. 2017;12(1):138. https://doi.org/10.1186/s13018-017-0636-6.
Yan T, Tian S, Wang Y, Yang X, Li T, Liu J, Pan P, Wang R, Wang D, Sun K. Comparison of early effectiveness between SuperPATH approach and Hardinge approach in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(1):17–24. https://doi.org/10.7507/1002-1892 Article in Chinese.
Yuan H, Zhu J, Sun Z, Zhang Z. Comparison of effectiveness between SuperPATH approach and posterolateral approach in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(1):14–9. https://doi.org/10.7507/1002-1892.201707121 Article in Chinese.
Zhang Z, Lin J, Xia B. Clinical research on joint function and life quality through SuperPath approach in total hip arthroplasty. China J Integrated Trad Chin Western Med. 2019;25(05):709–14 Article in Chinese.
Wills BW, Sheppard ED, Smith WR, Staggers JR, Li P, Shah A, Lee SR, Naranje SM. Impact of operative time on early joint infection and deep vein thrombosis in primary total hip arthroplasty. Orthop Traumatol Surg Res. 2018;104(4):445–8. https://doi.org/10.1016/j.otsr.2018.02.008.
Surace P, Sultan AA, George J, Samuel LT, Khlopas A, Molloy RM, Stearns KL, Mont MA. The association between operative time and short-term complications in total hip arthroplasty: an analysis of 89,802 Surgeries. J Arthroplasty. 2019;34(3):426–32. https://doi.org/10.1016/j.arth.2018.11.015.
Rasuli KJ, Gofton W. Percutaneously assisted total hip (PATH) and Supercapsular percutaneously assisted total hip (SuperPATH) arthroplasty: learning curves and early outcomes. Ann Transl Med. 2015;13:179. https://doi.org/10.3978/j.issn.2305-5839.2015.08.02.
Van Den Eeden Y, Van Den Eeden F. Learning curve of direct anterior total hip arthroplasty: a single surgeon experience. Acta Orthop Belg. 2018;84(3):321–30.
Alecci V, Valente M, Crucil M, Minerva M, Pellegrino CM, Sabbadini DD. Comparison of primary total hip replacements performed with a direct anterior approach versus the standard lateral approach: perioperative findings. J Orthop Traumatol. 2011;12(3):123–9. https://doi.org/10.1007/s10195-011-0144-0.
Berend KR, Lombardi AV Jr, Seng BE, Adams JB. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(Suppl 6):107–20. https://doi.org/10.2106/JBJS.I.00525.
Bergin PF, Doppelt JD, Kephart CJ, Benke MT, Graeter JH, Holmes AS, Haleem-Smith H, Tuan RS, Unger AS. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392–8. https://doi.org/10.2106/JBJS.J.00557.
Brismar BH, Hallert O, Tedhamre A, Lindgren JU. Early gain in pain reduction and hip function, but more complications following the direct anterior minimally invasive approach for total hip arthroplasty: a randomized trial of 100 patients with 5 years of follow up. Acta Orthop. 2018;89(5):484-9. https://doi.org/10.1080/17453674.2018.1504505.
Cheng TE, Wallis JA, Taylor NF, Holden CT, Marks P, Smith CL, Armstrong MS, Singh PJ. A Prospective Randomized Clinical Trial in Total Hip Arthroplasty-Comparing Early Results Between the Direct Anterior Approach and the Posterior Approach. J Arthroplasty. 2017;32(3):883–90. https://doi.org/10.1016/j.arth.2016.08.027
Hananouchi T, Takao M, Nishii T, Miki H, Iwana D, Yoshikawa H, Sugano N. Comparison of navigation accuracy in THA between the mini-anterior and -posterior approaches. Int J Med Robot. 2009;5(1):20–5. https://doi.org/10.1002/rcs.226.
Hozack W, Klatt BA. Minimally invasive two-incision total hip arthroplasty: is the second incision necessary? Semin Arthrop. 2008;19:205–8. https://doi.org/10.1053/j.sart.2008.02.009.
Ilchmann T, Gersbach S, Zwicky L, Clauss M. Standard transgluteal versus minimal invasive anterior approach in hip arthroplasty: a prospective, consecutive cohort study. Orthop Rev (Pavia). 2013;5(4):e31. https://doi.org/10.4081/or.2013.e31.
Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849–54. https://doi.org/10.1016/j.arth.2012.10.029.
Mayr E, Nogler M, Benedetti MG, Kessler O, Reinthaler A, Krismer M, Leardini A. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech (Bristol, Avon). 2009;24(10):812–8. https://doi.org/10.1016/j.clinbiomech.2009.07.010.
Nakata K, Nishikawa M, Yamamoto K, Hirota S, Yoshikawa H. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24(5):698–704. https://doi.org/10.1016/j.arth.2008.04.012.
Parvizi J, Rasouli MR, Jaberi M, Chevrollier G, Vizzi S, Sharkey PF, Hozack WJ. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357–62. https://doi.org/10.1007/s00264-013-2093-0.
Pogliacomi F, De Filippo M, Paraskevopoulos A, Alesci M, Marenghi P, Ceccarelli F. Mini-incision direct lateral approach versus anterior mini-invasive approach in total hip replacement: results 1 year after surgery. Acta Biomed. 2012;83(2):114–21.
Pogliacomi F, Paraskevopoulos A, Costantino C, Marenghi P, Ceccarelli F. Influence of surgical experience in the learning curve of a new approach in hip replacement: anterior mini-invasive vs. standard lateral. Hip Int. 2012;22(5):555–61. https://doi.org/10.5301/HIP.2012.9710.
Rathod PA, Bhalla S, Deshmukh AJ, Rodriguez JA. Does fluoroscopy with anterior hip arthroplasty decrease acetabular cup variability compared with a nonguided posterior approach? Clin Orthop Relat Res. 2014;472(6):1877–85. https://doi.org/10.1007/s11999-014-3512-2.
Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25(5):671–9.e1. https://doi.org/10.1016/j.arth.2010.02.002.
Rodriguez JA, Deshmukh AJ, Rathod PA, Greiz ML, Deshmane PP, Hepinstall MS, Ranawat AS. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res. 2014;472(2):455–63. https://doi.org/10.1007/s11999-013-3231-0.
Schweppe ML, Seyler TM, Plate JF, Swenson RD, Lang JE. Does surgical approach in total hip arthroplasty affect rehabilitation, discharge disposition, and readmission rate? Surg Technol Int. 2013;23:219–27.
Sebečić B, Starešinić M, Culjak V, Japjec M. Minimally invasive hip arthroplasty: advantages and disadvantages. Med Glas (Zenica). 2012;9:160–5.
Sendtner E, Borowiak K, Schuster T, Woerner M, Grifka J, Renkawitz T. Tackling the learning curve: comparison between the anterior, minimally invasive (Micro-hip®) and the lateral, transgluteal (Bauer) approach for primary total hip replacement. Arch Orthop Trauma Surg. 2011;131(5):597–602. https://doi.org/10.1007/s00402-010-1174-4.
Seng BE, Berend KR, Ajluni AF, Lombardi AV Jr. Anterior-supine minimally invasive total hip arthroplasty: defining the learning curve. Orthop Clin North Am. 2009;40(3):343–50. https://doi.org/10.1016/j.ocl.2009.01.002.
Spaans AJ, van den Hout JA, Bolder SB. High complication rate in the early experience of minimally invasive total hip arthroplasty by the direct anterior approach. Acta Orthop. 2012;83(4):342–6. https://doi.org/10.3109/17453674.2012.711701.
Wayne N, Stoewe R. Primary total hip arthroplasty: a comparison of the lateral Hardinge approach to an anterior mini-invasive approach. Orthop Rev (Pavia). 2009;1(2):e27. https://doi.org/10.4081/or.2009.e27.
Zawadsky MW, Paulus MC, Murray PJ, Johansen MA. Early outcome comparison between the direct anterior approach and the mini-incision posterior approach for primary total hip arthroplasty: 150 consecutive cases. J Arthroplasty. 2014;29(6):1256–60. https://doi.org/10.1016/j.arth.2013.11.013.
Cai Z, Pan J, Huang C, Chen G. SuperPath minimally invasive and conventional total hip replacement surgery: comparative study on clinical efficacy of femoral neck fracture. Zhejiang Traum Surg. 2017;22(2). https://doi.org/10.3969/j.issn.1009-7147.2017.02.065.
He Q, Qiao J, Liu Y. Comparison of early curative effect between SuperPath minimally invasive total hip arthroplasty and conventional total hip replacement. 2016;34(3). https://doi.org/10.3969/j.issn.1005-4057.2016.03.018
Huang G, Xia J, Wei Y, Wang S, Wu J, Chen F, Chen J, Shi J. Short-term efficacy of hip arthroplasty through the SUPERPATH approach for femoral neck fractures in very elderly patients. Int J Orthop. 2016;37(5). https://doi.org/10.3969/j.issn.1673-7083.2016.05.014
Más Martínez J, Sanz-Reig J, Morales-Santías M, Bustamante Suarez de Puga D, Verdu Roman C, Martinez Gimenez E. Estudio de cohortes comparativo del abordaje Superpath con abordaje convencional posterior en cirugía protésica primaria de cadera no cementada: curva de aprendizaje y resultados a corto plazo. Rev Esp Cir Ortop Traumatol. 2019. https://doi.org/10.1016/j.recote.2019.07.002
Li J, Huang Q, Xu H, Yao X, Sun Z, Lin X, Zheng Z. Comparison of clinical efficacy of SuperPATH and posterolateral small incision approach in primary THA for treatment of Ischemic necrosis of femoral head. Chin J Bone Joint Injury. 2017;32(3). https://doi.org/10.7531/j.issn.1672-9935.2017.03.006
Wang X, Lan H, Hu Z, Li K, Wang Z, Luo J, Long X. SuperPATH minimally invasive approach to total hip arthroplasty of femoral neck fractures in the elderly: preliminary clinical results. Orthopaedic Surgery. 2020;12:74–85. https://doi.org/10.1111/os.12584.
Yun D, He Q, Liu K, Ding C, Ding W, Zhang H, Tang H, Qin S, Wu X, Wang G, Ji F. SuperPATH approach hip replacement surgery: evaluation of short-term curative effect of femoral neck fracture. Chin J Bone Joint Inj. 2017;32(3). https://doi.org/10.7531/j.issn.1672-9935.2017.03.032.
Xu CP, Li X, Song JQ, Cui Z, Yu B. Mini-incision versus standard incision total hip arthroplasty regarding surgical outcomes: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8(11):e80021. https://doi.org/10.1371/journal.pone.0080021.
Moskal JT, Capps SG. Is limited incision better than standard total hip arthroplasty? A meta-analysis. Clin Orthop Relat Res. 2013;471(4):1283–94. https://doi.org/10.1007/s11999-012-2717-5.
McGrory BJ, Finch ME, Furlong PJ, Ruterbories J. Incision length correlates with patient weight, height, and gender when using a minimal-incision technique in total hip arthroplasty. J Surg Orthop Adv. 2008;17(2):77–81.
Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision posterior approach to total hip arthroplasty. J Arthroplasty. 2014;29(10):1970–82. https://doi.org/10.1016/j.arth.2014.05.021.
Qi YM, Wang HP, Li YJ, Ma BB, Xie T, Wang C, Chen H, Rui YF. The efficacy and safety of intravenous tranexamic acid in hip fracture surgery: a systematic review and meta-analysis. J Orthop Translat. 2019;19:1–11. https://doi.org/10.1016/j.jot.2019.03.007.
Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. The efficacy of tranexamic acid in total hip arthroplasty: a network meta-analysis. J Arthroplasty. 2018;33(10):3083–3089.e4. https://doi.org/10.1016/j.arth.2018.06.023.
Yi J, Liang H, Song R, Xia H, Huang Y. Maintaining intraoperative normothermia reduces blood loss in patients undergoing major operations: a pilot randomized controlled clinical trial. BMC Anesthesiol. 2018;18(1):126. https://doi.org/10.1186/s12871-018-0582-9.
Tomlinson J, Zwirner J, Ondruschka B, Prietzel T, Hammer N. Innervation of the hip joint capsular complex: a systematic review of histological and immunohistochemical studies and their clinical implications for contemporary treatment strategies in total hip arthroplasty. PLoS One. 2020;15(2):e0229128. https://doi.org/10.1371/journal.pone.0229128.
Liu P, Wu Y, Liang Z, Deng Y, Meng Q. Comparing the efficacy of pain managements after total hip arthroplasty: a network meta-analysis. J Cell Biochem. 2019;120(3):4342–54. https://doi.org/10.1002/jcb.27720.
Jiménez-Almonte JH, Wyles CC, Wyles SP, Norambuena-Morales GA, Báez PJ, Murad MH, Sierra RJ. Is Local Infiltration Analgesia Superior to Peripheral Nerve Blockade for Pain Management After THA: A Network Meta-analysis. Clin Orthop Relat Res. 2016;474(2):495-516. https://doi.org/10.1007/s11999-015-4619-9.
Acknowledgements
My acknowledgements go to Prof. Wilhelm Behringer and Prof. Gunther Hofmann.
Funding
No fundings. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
NR and PL wrote the manuscript. SB and NR did the statistics. KL helped with Chinese translation. IM, NR, and PL checked the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Appendix
Appendix
SuperPATH vs. CAs
I. Search strategy PubMed:
((SuperPATH) OR (Supercapsular Percutaneously-Assisted Total Hip)) ti,ab.
II. Search strategy CNKI:
(SuperPATH) OR (Supercapsular Percutaneously-Assisted Total Hip) in Title
III. Search strategy Cochrane Library:
((SuperPATH) OR (Supercapsular Percutaneously-Assisted Total Hip)) in Title Abstract Keyword
IV. Search Strategy Google Scholar:
(SuperPATH) OR (Supercapsular Percutaneously-Assisted Total Hip)
V. Search strategy Clinical Trials:
(SuperPATH) OR (Supercapsular Percutaneously-Assisted Total Hip)
DAA vs. CAs
I. Search strategy PubMed:
(((AMIS) OR anterior minimally invasive surgery) OR direct anterior Approach) AND ((total hip replacement) OR total hip arthroplasty)
II. Search strategy CNKI:
(直接前路入路)
III. Search strategy Cochrane Library:
(((AMIS) OR anterior minimally invasive surgery) OR direct anterior Approach) AND ((total hip replacement) OR total hip arthroplasty)
IV. Search strategy Google Scholar:
AMIS direct anterior approach versus conventional approach total hip replacement randomized controlled trial
V. Search strategy Clinical Trials:
(((AMIS) OR anterior minimally invasive surgery) OR direct anterior Approach) AND ((total hip replacement) OR total hip arthroplasty)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ramadanov, N., Bueschges, S., Liu, K. et al. Comparison of short-term outcomes between direct anterior approach (DAA) and SuperPATH in total hip replacement: a systematic review and network meta-analysis of randomized controlled trials. J Orthop Surg Res 16, 324 (2021). https://doi.org/10.1186/s13018-021-02315-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-021-02315-7