Abstract
Background
In an enhanced recovery after surgery program, not placing a closed suction drain following routine primary total joint arthroplasty (TJA) is becoming more acceptable. However, the influence of drain use on transfusion rate and postoperative length of stay (PLOS) in TJA remains controversial. Therefore, we aimed to compare drain use with no drain in routine primary TJA to determine the differences in transfusion rate and PLOS.
Methods
We analyzed the data from 12,992 patients undergoing primary unilateral TJA: 6325 total knee arthroplasties (TKA) and 6667 total hip arthroplasties (THA). Patients were divided into two groups according to whether they received a drain postoperatively following TKA and THA. We extracted information for transfusion and PLOS from patients’ electronic health records and analyzed the data by logistic and linear regression analyses.
Results
The transfusion rate and PLOS were 15.07% and 7.75 ± 3.61 days, respectively, in the drain group and 6.72% and 6.54 ± 3.32 days, respectively, in the no-drain group following TKA. The transfusion rate and PLOS were 20.53% and 7.00 ± 3.35 days, respectively, in the drain group and 13.57% and 6.07 ± 3.06 days, respectively, in the no-drain group following THA. After adjusting for the following variables: age, gender, body mass index, orthopedic diagnoses, hypertension, type 2 diabetes, coronary heart disease, chronic obstructive pulmonary disease, preoperative hemoglobin, albumin, analgesic use, anesthesia, American Society of Anesthesiologists class, tranexamic acid use, intraoperative bleeding, operative time, and tourniquet use (for TKA), drain use correlated significantly with a higher transfusion rate (risk ratio = 2.812, 95% confidence interval (CI) 2.224–3.554, P < 0.001 for TKA and risk ratio = 1.872, 95% CI 1.588–2.207, P < 0.001 for THA) and a longer PLOS (partial regression coefficient (B) = 1.099, 95% CI 0.879–1.318, P < 0.001, standard regression coefficient (B′) = 0.139 for TKA; B = 0.973, 95% CI 0.695–1.051, P < 0.001, and B′ = 0.115 for THA). Two groups showed no significant difference in wound complications.
Conclusions
Our findings indicated that drain use was associated with a higher transfusion rate and a longer PLOS in patients undergoing routine primary TJA. The routine use of postoperative drainage is not recommended in primary unilateral TJA.
Similar content being viewed by others
Introduction
The use of closed suction drainage (CSD) in surgery has been a standard practice since the time of Hippocrates [1]. Many surgeons follow the practice based on their early training, rather than on the basis of scientific evidence [2]. Proponents believe that postoperative CSD reduces ecchymosis [3] and avoids hematoma formation, which impair wound healing by increasing wound tension and decreasing surrounding tissue blood perfusion [4]. In addition, a hematoma also may lead to superficial and deep-seated infection secondary to bacterial colonization [5]. However, the exclusion of CSD in total joint arthroplasty (TJA) is becoming increasingly accepted with enhanced recovery after surgery (ERAS) programs. Opponents of CSD propose that CSD in TJA is associated with greater blood loss because the drain prevents the tamponade effect [6] and because of the higher risk of infection secondary to retrograde bacterial migration [7]. Moreover, some studies [8, 9] also reported that the use of CSD impairs early postoperative rehabilitation and complicates postoperative nursing care.
Although some previous studies have evaluated the effect of CSD on transfusion rate and postoperative length of stay (PLOS) in total knee arthroplasty (TKA) and total hip arthroplasty (THA), CSD remains controversial because most studies had insufficient sample sizes [10]. Therefore, we based this study on a large dataset to investigate (1) whether CSD increases the postoperative transfusion rate, and (2) whether CSD is associated with a longer PLOS after routine primary TKA and THA.
Materials and methods
Data source
This study was a secondary analysis of a large database generated from a prospective multicenter study evaluating the efficacy and safety of perioperative management of TKA and THA. This study protocol was registered in the Chinese Clinical Trial Registry. The surgeons decided whether to use CSD at the end of surgery. Transfusion criteria were (1) hemoglobin level < 70 g/L and (2) hemoglobin level 70–100 g/L with symptomatic anemia appearing as lightheadedness, presyncope, fatigue, palpitation, or shortness of breath, excluding other causes. We defined PLOS data as the number of days between the date of surgery and the date of discharge. The database used in this study included patient-level hospital discharge data, which were provided by 26 university teaching hospitals in China (10 national and 16 regional hospitals) sponsored by the Chinese Health Ministry. The completeness and validity of the data were confirmed by careful comparison with data from hospital information systems. Patient consent was deemed unnecessary by each hospital’s institutional review board.
We recorded the following patient information: demographic characteristics, preoperative comorbidities and drug use, laboratory values, operative variables, and wound complications. Demographic characteristics comprised age, gender, body mass index (BMI), and orthopedic diagnoses. Preoperative comorbidities and drug use included hypertension, type 2 diabetes, chronic obstructive pulmonary disease (COPD), coronary heart disease, and preoperative analgesic use. Preoperative laboratory values comprised preoperative hemoglobin and albumin. We recorded the following operative variables: method of anesthesia (general or regional anesthesia), American Society of Anesthesiologists (ASA) class evaluated by an anesthetist preoperatively, the use of tranexamic acid (TXA) and drainage, operative time, intraoperative bleeding, and tourniquet use in TKA. Wound complications included non-infectious complications (fat liquefies and wound effusion, wound necrosis and hematoma) and infectious complications [superficial wound infection and periprosthetic joint infection (PJI) within a month postoperatively].
Patients’ demographics
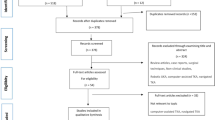
All patients, namely, 6325 patients undergoing routine primary TKA and 6667 undergoing routine primary THA, were identified by the presence of the International Classification of Diseases, Tenth Revision, Clinical Modification procedure codes. Based on discharge records, we excluded patients who had undergone previous arthroplasty or bilateral TKA or THA, patients with metastatic and/or bone cancer, and those experiencing a joint dislocation. We also excluded patients younger than age 18 years or who received an autologous blood transfusion rather than an allogeneic blood product. Patients undergoing TKA were divided into two groups based on the presence of degenerative arthritis (osteoarthritis) and inflammatory arthritis of the knee into a rheumatoid arthritis group and an ankylosing spondylitis group. We divided patients undergoing routine primary THA into three groups: a degenerative arthritis group (osteoarthritis, osteonecrosis of the femoral head, and developmental dysplasia of the hip), an inflammatory arthritis group (rheumatoid arthritis and ankylosing spondylitis of the hip joint), and a group with femoral neck fracture.
Statistical analyses
Variables were summarized as mean ± standard deviation for continuous variables and frequency (proportion) for categorical variables. We built a crude model of transfusion rate and PLOS regarding the use of CSD, using univariate analyses. We used logistic and linear regression analyses to adjust for other variables and explore the relationships between the variables. Statistical significance was set as P < 0.05. All data analyses were performed using SPSS version 21 software (IBM, Armonk, NY, USA).
Results
Patients’ demographics
The demographics for patients who underwent routine primary TKA are shown in Table 1. The TKA group included 4540 patients in the drain group and 1785 patients in the no-drain group. The total transfusion rate was 12.71%: 15.07% in the drain group and 6.72% in the no-drain group. The average PLOS was 7.41 ± 3.57 days after TKA: 7.75 ± 3.61 days in the drain group and 6.54 ± 3.32 days in the no-drain group (Table 2).
The demographics for patients who underwent routine primary THA are shown in Table 3. The THA group included 4943 patients in the drain group and 1724 patients in the no-drain group. The total transfusion rate was 18.74%: 20.53% in the drain group and 13.57% in the no-drain group. The average PLOS was 6.76 ± 3.30 days: 7.00 ± 3.35 days in the drain group and 6.07 ± 3.06 days in the no-drain group (Table 4).
The effect of drain use on transfusion rate and PLOS after TKA
As shown in Table 5, univariate analyses (crude model) showed that CSD was highly associated with the transfusion rate (relative risk (RR) = 2.461, 95% confidence interval (CI) 2.010–3.013, P < 0.001) and PLOS (partial regression coefficient (B) = 1.214, 95% CI 1.016–1.412, P < 0.001; standard regression coefficient (B′) = 0.153) after TKA. After controlling for age, gender, BMI, and orthopedic diagnoses (model 1), logistic and linear regression analyses showed that CSD remained correlated with a higher transfusion rate (RR = 2.561, 95% CI 2.090–3.139, P < 0.001) and longer PLOS (B = 1.223, 95% CI 1.025–1.422, P < 0.001; B′ = 0.154).
After additional adjustment for hypertension, type 2 diabetes, COPD, coronary heart disease, preoperative analgesic use, and preoperative hemoglobin and albumin (model 2), CSD remained associated with a higher transfusion rate (RR = 2.529, 95% CI 2.052–3.116, P < 0.001) and a longer PLOS (B = 1.492, 95% CI 1.290–1.694, P < 0.001; B′ = 0.188). After further controlling for all covariates (covariates in model 2 plus the method of anesthesia, ASA class, operative time, intraoperative bleeding, and TXA and tourniquet use), drain use remained correlated with a higher transfusion rate (odds ratio (OR) = 2.812, 95% CI 2.224–3.554, P < 0.001) and a longer PLOS (B = 1.099, 95% CI 0.879–1.318, P < 0.001; B′ = 0.139), as in model 3.
The effect of drain use on transfusion rate and PLOS after THA
As shown in Table 6, univariate analyses (crude model) revealed that CSD was correlated with higher transfusion rates (RR = 1.645, 95% CI 1.410–1.920, P < 0.001) and longer PLOS (B = 0.927, 95% CI 0.734–1.111, P < 0.001; B′ = 0.123) after THA. Logistic and linear regression analyses showed that CSD was correlated with a higher transfusion rate (RR = 1.657, 95% CI 1.420–1.935, P < 0.001) and a longer PLOS (B = 0.935, 95% CI 0.753–1.117, P < 0.001; B′ = 0.123) after controlling for age, gender, BMI, and orthopedic diagnoses (model 1). After further adjustment for hypertension, type 2 diabetes, COPD, coronary heart disease, preoperative analgesic use, and hemoglobin and albumin level (model 2), CSD remained associated with a higher transfusion rate (RR = 1.690, 95% CI 1.445–1.978, P < 0.001) and a longer PLOS (B = 0.931, 95% CI 0.750–1.112, P < 0.001; B′ = 0.122). After controlling for all covariates, which included the covariates in model 2 plus the method of anesthesia, ASA class, operative time, intraoperative bleeding, and TXA use, CSD remained correlated with a higher transfusion rate (OR = 1.872, 95% CI 1.588–2.207, P < 0.001) and longer PLOS (B = 0.973, 95% CI 0.695–1.051, P < 0.001; B′ = 0.115), as in model 3.
The wound complication rates between the two groups in TKA and THA
As shown in Tables 7 and 8, there are no differences in the non-infectious and infectious complication rates between the drain and no-drain group after routine primary TKA and THA (P > 0.05).
Discussion
This study evaluating data for 12,992 patients was performed to provide conclusive results regarding the effect of CSD in patients undergoing primary unilateral TJA on transfusion rate and PLOS. The most important finding was that CSD was associated with a higher transfusion rate and a longer PLOS in patients undergoing routine primary TKA and THA.
TJA is a successful and common surgical treatment for patients with end-stage knee or hip diseases because it can significantly relieve pain and improve patients’ function and quality of life [11]. Although efforts have been made to reduce perioperative blood loss, significant early postoperative total blood loss of up to 1000–1500 mL including intraoperative bleeding and hidden blood loss remains a challenge [12]. A previous study [13] based on a nationwide inpatient sample database reported that TKA and THA were among the top 10 most-frequent procedures requiring transfusion in the USA. Furthermore, blood transfusion was related to numerous serious complications such as viral infection, anaphylactic and hemolytic reactions, longer PLOS, deep vein thromboembolism, high hospitalization costs, and increased in-hospital mortality [14,15,16]. Allogeneic blood reserves are often insufficient worldwide; consequently, reducing the transfusion rate is essential for patients undergoing TKA or THA.
Previous studies [2, 9, 17, 18] have demonstrated that CSD was not associated with a higher transfusion rate in primary unilateral TJA; however, this finding may be related to the small sample sizes and insufficient data in these studies. A systematic review and meta-analysis [19] evaluating 19 randomized controlled trials reported that CSD significantly increased the risk of transfusion in primary TKA (RR = 1.38, 95% CI 1.04–1.83); we found similar results (OR = 2.812, 95% CI 2.224–3.554 in TKA; RR = 1.872, 95% CI 1.588–2.207 in THA). Furthermore, Bjerke-Kroll et al. [20] reported that CSD increased the number of allogeneic blood transfusions both in unilateral primary TKA and in THA, possibly because of the higher blood loss in the drain group. Several previous studies [2, 8, 20,21,22] reported that CSD was associated with increased total blood loss in TKA and THA. Studies [23, 24] have also revealed that hidden blood loss accounts for approximately 60% of total blood loss in TJA and the majority of the blood loss happens early postoperatively. Hidden blood loss occurs primarily because of large blood accumulation in the joint space and muscle compartments, and when a drain is placed between the joint space and muscle compartment postoperatively, bleeding continues until the drain is removed because the drain prevents the tamponade effect, which is an important step in filling the dead space in an operated wound [1, 25].
LOS is considered both a significant outcome measure when evaluating ERAS programs [13] and a non-substitutable marker of excellence [26]. Reducing LOS, which means improved hospital efficiency and productivity, is an important aim in many counties. Shorter LOS has obvious benefits for both hospitals and patients, namely, lower use of medical resources, significant cost savings, and excellent patient satisfaction [27, 28]. Shortening PLOS has become a vital part of shortening LOS with the development of ERAS programs. Despite some previous studies [2, 9, 17, 19] reporting that CSD was not associated with a longer PLOS, the routine use of CSD was not recommended by these studies because this empirical practice has no obvious advantages for patients undergoing primary unilateral TKA or THA. Furthermore, Sharma et al. [1] and Bjerke-Kroll et al. [20] reported that LOS was longer in their drain groups compared with the no-drain groups after TKA and THA, respectively. Our results also showed that CSD was associated with a longer PLOS after both TKA and THA. CSD interferes with early mobilization and complicates postoperative nursing care, which may contribute to the longer PLOS after TKA or THA [8, 21].
Wound complication, especially for infectious complications, would bring catastrophic consequences. Besides, non-infectious complications and superficial wound infection also may develop into PJI if they are not handled properly, which is one of the most devastating complications after TJA [29]. However, our study showed there are no differences in wound complications between the drain and no-drain groups after routine primary TKA and THA. Zhang et al. [30] conducted a systematic review and meta-analysis which enrolled 19 randomized controlled trials that showed drain use was not correlated with superficial wound infection and PJI in primary TKA, which was in accordance with our study. However, Willemen et al. [31] carried out a prospective randomized controlled trial which enrolled 41 patients who undergone TKA showing that bacteria cultures of all drain tips with cutoff at postoperative 24 h were negative, while 5 of 21 drain tips indwelling for 48 h after operation developed positive culture results (all bacteria were Staphylococci), which indicated that CSD may be a source of retrograde infection and the risk increased with the extension of indwelling time. Hence, future studies are needed to estimate the effect of CSD on wound complication, especially for infectious complications.
CSD use in TJA should balance the advantages and disadvantages, which depend mainly on the patient’s clinical condition. With the development of ERAS programs worldwide, CSD has been reconsidered by orthopedic surgeons, and excluding CSD in routine primary TKA and THA is becoming more popular [2]. Although the purchase cost of a drainage tube is relatively low, longer PLOS and higher transfusion rates caused by CSD may increase total hospitalization costs for patients undergoing TJA [20]. In addition, CSD may delay patients’ postoperative recovery because CSD interferes with early mobilization and complicates nursing care, despite the need for fewer postoperative dressing changes [9, 32]. In addition, with the associated longer incisions, longer surgery times, more bleeding, and potential for hematoma formation, revision TKA and THA are considered more complex procedures compared with routine primary TJA [33]. Abolghasemian et al. [34] reported that CSD in revision knee arthroplasty was associated with higher blood loss and a higher transfusion rate, and no differences were found regarding postoperative wound complications and knee scores compared with the no-drain group. Fichman et al. [35] also reported that CSD in revision TJA was correlated with a higher transfusion rate and a longer PLOS. Even so, future high-quality studies with longer follow-up are still needed to make a definitive conclusion regarding CSD in revision joint arthroplasty.
This study has several limitations. First, whether the drain was clamped and the clamping time were not recorded in detail in the database; thus, we were unable to determine the effect of drain clamping on transfusion rate and PLOS. Second, the drain removal criteria were not recorded, which may have affected the blood loss volume. Third, some important outcomes such as patients’ postoperative range of motion in the operated joint and the need for dressing changes were not recorded or evaluated. Fourth, the details of TXA use, other than yes or no, were not recorded in the database, and the starting time for TXA differed for each hospital, which may have affected the blood loss volume and transfusion rate. Despite these limitations, to the best of our best knowledge, our study included the largest number of patients to date and provided conclusive results regarding the use of CSD in TJA and its effect on transfusion rate and PLOS. The results of this study could become an important component of orthopedic surgeons’ decision-making regarding CSD in routine primary TKA and THA.
Conclusion
In summary, the current study found that CSD was associated with a significantly higher transfusion rate and a longer PLOS in patients undergoing primary unilateral TKA and THA. We do not recommend the routine use of CSD in primary unilateral TJA.
Availability of data and materials
Please contact the author for data requests.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- B :
-
Partial regression coefficient
- B′:
-
Standard regression coefficient
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CSD:
-
Closed suction drainage
- ERAS:
-
Enhanced recovery after surgery
- OR:
-
Odds ratio
- PJI:
-
Periprosthetic joint infection
- PLOS:
-
Postoperative length of stay
- RR:
-
Relative risk
- THA:
-
Total hip arthroplasty
- TJA:
-
Total joint arthroplasty
- TKA:
-
Total knee arthroplasty
References
Sharma GM, Palekar G, Tanna DD. Use of closed suction drain after primary total knee arthroplasty - an overrated practice. SICOT J. 2016;2:39.
Chen JY, Lee WC, Chan HY, Chang PC, Lo NN, Yeo SJ. Drain use in total knee arthroplasty is neither associated with a greater transfusion rate nor a longer hospital stay. Int Orthop. 2016;40(12):2505–9.
Kim YH, Cho SH, Kim RS. Drainage versus nondrainage in simultaneous bilateral total hip arthroplasties. J Arthroplasty. 1998;13(2):156–61.
Sallam HF, Shady NW. Reducing blood loss during abdominal hysterectomy with intravenous versus topical tranexamic acid: a double-blind randomized controlled trial. J Obstet Gynecol India. 2019;69(2):173–9.
Alexander JW, Korelitz J, Alexander NS. Prevention of wound infections - case for closed suction drainage to remove wound fluids deficient in opsonic proteins. Am J Surg. 1976;132(1):59–63.
Goes RFA, AFd S, Lyra FS, Loures FB, Da Palma IM, Cobra HAAB, Labronici PJ. Prospective randomized study after the use of drains in total knee arthroplasty with implant. Rev Bras Ortop (English Edition). 2013;48(3):257–62.
Minnema B, Vearncombe M, Augustin A, Gollish J, Simor AE. Risk factors for surgical-site infection following primary total knee arthroplasty. Infect Control Hosp Epidemiol. 2004;25(6):477–80.
Esler CNA, Blakeway C, Fiddian NJ. The use of a closed-suction drain in total knee arthroplasty. J Bone Joint Surg Br. 2003;85-B(2):215–7.
Keska R, Paradowski TP, Witonski D. Outcome in primary cemented total knee arthroplasty with or without drain: a prospective comparative study. Indian J Orthop. 2014;48(4):404–9.
Quinn M, Bowe A, Galvin R, Dawson P, O'Byrne J. The use of postoperative suction drainage in total knee arthroplasty: a systematic review. Int Orthop. 2015;39(4):653–8.
Kargin D, Incesoy MA, Onac O, Albayrak A, Kaygusuz MA, Bayhan IA. The effect of previous hip surgery on the outcome of hip arthroplasty in young patients. J Arthroplast. 2018;33(9):2890–2.
Ritter MA, Keating EM, Faris PM. Closed wound drainage in total hip or total knee replacement - a prospective randomized study. J Bone Joint Surg Am. 1994;76A(1):35–8.
Morton J, Anastassopoulos KP, Patel ST, Lerner JH, Ryan KJ, Goss TF, Dodd SL. Frequency and outcomes of blood products transfusion across procedures and clinical conditions warranting inpatient care: an analysis of the 2004 healthcare cost and utilization project nationwide inpatient sample database. Am J Med Qual. 2010;25(4):289–96.
Saleh A, Small T, Pillai ALPC, Schiltz NK, Klika AK, Barsoum WK. Allogenic blood transfusion following total hip arthroplasty: results from the nationwide inpatient sample, 2000 to 2009. J Bone Joint Surg Am. 2014;96A(18):e155.
Bloomfield MR, Patterson RW, Froimson MI. Complications of anticoagulation for thromboembolism in early postoperative total joint arthroplasty. Am J Orthop (Belle Mead, NJ). 2011;40(8):E148–51.
Huang Z, Huang C, Xie J, Ma J, Cao G, Huang Q, Shen B, Byers Kraus V, Pei F. Analysis of a large data set to identify predictors of blood transfusion in primary total hip and knee arthroplasty. Transfusion. 2018;58(8):1855–62.
Kumar S, Penematsa S, Parekh S. Are drains required following a routine primary total joint arthroplasty? Int Orthop. 2006;31(5):593–6.
Sundaram RO, Parkinson RW. Closed suction drains do not increase the blood transfusion rates in patients undergoing total knee arthroplasty. Int Orthop. 2007;31(5):613–6.
Zhang Q, Liu L, Sun W, Gao F, Zhang Q, Cheng L, Li Z. Are closed suction drains necessary for primary total knee arthroplasty? Medicine. 2018;97(30):e11290.
Bjerke-Kroll BT, Sculco PK, McLawhorn AS, Christ AB, Gladnick BP, Mayman DJ. The increased total cost associated with post-operative drains in total hip and knee arthroplasty. J Arthroplast. 2014;29(5):895–9.
Cheung G, Carmont MR, Bing AJF, Kuiper JH, Alcock RJ, Graham NM. No drain, autologous transfusion drain or suction drain? A randomised prospective study in total hip replacement surgery of 168 patients. Acta Orthop Belg. 2010;76(5):619–27.
Demirkale I, Tecimel O, Sesen H, Kilicarslan K, Altay M, Dogan M. Nondrainage decreases blood transfusion need and infection rate in bilateral total knee arthroplasty. J Arthroplast. 2014;29(5):993–7.
Lei Y, Huang Q, Huang Z, Xie J, Chen G, Pei F. Multiple-dose intravenous tranexamic acid further reduces hidden blood loss after total hip arthroplasty: a randomized controlled trial. J Arthroplast. 2018;33(9):2940–5.
Liu X, Zhang X, Chen Y, Wang Q, Jiang Y, Zeng B. Hidden blood loss after total hip arthroplasty. J Arthroplast. 2011;26(7):1100–1105 e1101.
Li C, Nijat A, Askar M. No clear advantage to use of wound drains after unilateral total knee arthroplasty: a prospective randomized, controlled trial. J Arthroplasty. 2011;26(4):519–22.
Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–41.
Brock TM, Baker PN, Rushton S, Bardgett M, Deehan D. Length of stay and its impact upon functional outcomes following lower limb arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2676–81.
van den Belt L, van Essen P, Heesterbeek PJ, Defoort KC. Predictive factors of length of hospital stay after primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(6):1856–62.
Wyss T, Schuster AJ, Christen B, Wehrli U. Tension controlled ligament balanced total knee arthroplasty: 5-year results of a soft tissue orientated surgical technique. Arch Orthop Trauma Surg. 2008;128(2):129–35.
Zhang Q, Liu L, Sun W, Gao F, Zhang Q, Cheng L, Li Z. Are closed suction drains necessary for primary total knee arthroplasty?: a systematic review and meta-analysis. Medicine (Baltimore) Jul. 2018;97(30):e11290.
Willemen D, Paul J, White SH, Crook DWM. Closed suction drainage following knee arthroplasty - effectiveness and risks. Clin Orthop Relat Res. 1991;(264):232–4.
임수재 신, 김민영 차, 제한웅 김, 서유성 이. The efficacy of suction drains after total hip arthroplasty. Hip and Pelvis. 2006;18(3):110–5.
Sharma S, Cooper H, Ivory JP. An audit on the blood transfusion requirements for revision hip arthroplasty. Ann R Coll Surg Engl. 2002;84(4):269–72.
Abolghasemian M, Huether TW, Soever LJ, Drexler M, MacDonald MP, Backstein DJ. The use of a closed-suction drain in revision knee arthroplasty may not be necessary: a prospective randomized study. J Arthroplasty. 2016;31(7):1544–8.
Fichman SG, Makinen TJ, Lozano B, Rahman WA, Safir O, Gross AE, Backstein D, Kuzyk PR. Closed suction drainage has no benefits in revision total hip arthroplasty: a randomized controlled trial. Int Orthop. 2016;40(3):453–7.
Acknowledgements
We acknowledge the data entry clerk from 26 hospitals in China for their support during the study period. We thank Jane Charbonneau, DVM, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This study was funded by the Ministry of Health of the People’s Republic of China (no. 201302007). And the funding body was not involved in the study, including the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
HX and YTL carried out the study design and writing of the manuscript. QH confirmed the completeness and validity of the data. ZYH and JWX participated in the data collection and analysis. FXP conceived of the study, participated in its study design, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. This study was a secondary analysis of a large database and deemed exempt by the hospital’s institutional review board (2012-268).
Consent for publication
Not applicable. No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, H., Xie, J., Lei, Y. et al. Closed suction drainage following routine primary total joint arthroplasty is associated with a higher transfusion rate and longer postoperative length of stay: a retrospective cohort study. J Orthop Surg Res 14, 163 (2019). https://doi.org/10.1186/s13018-019-1211-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-019-1211-0