Abstract
Background
Information on hyperoxemia among patients with trauma has been limited, other than traumatic brain injuries. This study aimed to elucidate whether hyperoxemia during resuscitation of patients with trauma was associated with unfavorable outcomes.
Methods
A post hoc analysis of a prospective observational study was carried out at 39 tertiary hospitals in 2016–2018 in adult patients with trauma and injury severity score (ISS) of > 15. Hyperoxemia during resuscitation was defined as PaO2 of ≥ 300 mmHg on hospital arrival and/or 3 h after arrival. Intensive care unit (ICU)-free days were compared between patients with and without hyperoxemia. An inverse probability of treatment weighting (IPW) analysis was conducted to adjust patient characteristics including age, injury mechanism, comorbidities, vital signs on presentation, chest injury severity, and ISS. Analyses were stratified with intubation status at the emergency department (ED). The association between biomarkers and ICU length of stay were then analyzed with multivariate models.
Results
Among 295 severely injured trauma patients registered, 240 were eligible for analysis. Patients in the hyperoxemia group (n = 58) had shorter ICU-free days than those in the non-hyperoxemia group [17 (10–21) vs 23 (16–26), p < 0.001]. IPW analysis revealed the association between hyperoxemia and prolonged ICU stay among patients not intubated at the ED [ICU-free days = 16 (12–22) vs 23 (19–26), p = 0.004], but not among those intubated at the ED [18 (9–20) vs 15 (8–23), p = 0.777]. In the hyperoxemia group, high inflammatory markers such as soluble RAGE and HMGB-1, as well as low lung-protective proteins such as surfactant protein D and Clara cell secretory protein, were associated with prolonged ICU stay.
Conclusions
Hyperoxemia until 3 h after hospital arrival was associated with prolonged ICU stay among severely injured trauma patients not intubated at the ED.
Trial registration
UMIN-CTR, UMIN000019588. Registered on November 15, 2015.
Similar content being viewed by others
Background
Oxygen administration has a vital role in the management of critically ill patients [1, 2]. However, supraphysiological oxygen tension in the blood and/or tissue, hyperoxemia, has been reported to affect mortality and intensive care unit (ICU) length of stay in different diseases [1, 3,4,5], such as traumatic brain injury [6, 7], post-cardiac arrest syndrome [8, 9], and post-cardiac surgery [10]. Moreover, various studies revealed that unnecessarily high fraction of inspired oxygen (FiO2) was also associated with increased mortality of critically ill patients [11, 12], including sepsis [13, 14].
As several pathophysiological mechanisms behind harmful effects of hyperoxemia have been investigated, brain injury and pulmonary toxicity are emphasized as pivotal causes of unfavorable clinical outcomes in critically ill patient [15, 16]. Paradoxical reduction of oxygen delivery to the brain, due to vascular constriction and mitochondrial dysfunction, was observed in patients with traumatic/ischemic brain injury who experienced hyperoxemia [15, 17]. In addition, alveolar capillary injuries and pulmonary vasoconstriction inhibition by redundant reactive oxygen species with hyperoxemia was found in patients treated with mechanical ventilation [16, 18]. Furthermore, some basic studies suggested that hyperoxemia-induced acute lung injury (ALI) was exaggerated by inflammatory or lung-related biomarkers [19, 20].
Given that other subsets of critically ill patients, such as severely injured trauma patients, suffer from systematic inflammation, this population would be potentially affected by hyperoxemia. However, studies on clinical consequences of trauma patients who were exposed to hyperoxemia have been limited other than traumatic brain injury [21, 22]. Accordingly, this study aimed to elucidate whether hyperoxemia during resuscitation was associated with unfavorable clinical outcomes of trauma patients with severe injuries. We hypothesized that hyperoxemia exposure in the first 3 h after hospital arrival was associated with prolonged ICU stay. We also investigated the inflammatory and lung-protective biomarkers in patients with hyperoxemia to determine pathophysiological backgrounds of its potential harm.
Methods
Study design and settings
This study was a post hoc analysis of a nationwide multicenter prospective descriptive study conducted by the Japanese Association for Acute Medicine (JAAM) Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis and Trauma (FORECAST) study group from April 1, 2016, to January 31, 2018. Patient data including blood samples were obtained from 39 emergency departments (EDs) and ICUs in tertiary hospitals [23]. The FORECAST TRAUMA study was registered at the University Hospital Medical Information Network Clinical Trial Registry on November 15, 2015 (UMIN-CTR ID, UMIN000019588). The JAAM approved this study, and all collaborating hospitals obtained approval of their individual institutional review board (IRB) for conducting research with human participants (approval number JAAM, 2014-01; approval number 014-0307 from Hokkaido University Graduate School of Medicine, Head institute of the FORECAST group; and approval number 20150056 from the Keio University School of Medicine Keio, institute of the corresponding author). This study was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained from patients or their next of kin.
Study population
The JAAM FORECAST TRAUMA study enrolled severely injured adult trauma patients (1) who were aged ≥ 16 years, (2) with injury severity score (ISS) of ≥ 16, and (3) who were directly transported from the scene. Patients without any available arterial partial pressure of oxygen (PaO2) data within 3 h after hospital arrival were excluded. The size of the study population was dependent on the study period.
Data collection and definition
Patient data were prospectively collected and entered into an online data collection portal by treating physicians or volunteer registrars designated by each hospital. Available data included patient demographics, injury mechanism, vital signs on scene and hospital arrival, abbreviated injury scale (AIS), ISS, sequential organ failure assessment score, laboratory data including arterial blood gas and inflammatory and lung-related biomarkers (soluble receptor for advanced glycation end-products [sRAGE], high mobility group box-1 (HMGB-1), surfactant protein D [SPD], Clara cell secretory protein [CCSP], and interleukin-8 [IL-8]), amount of transfusion, resuscitative procedure conducted at the ED, any surgical procedures or angiography, ICU and hospital length of stay, and survival status at discharge.
Arterial blood gas was obtained on arrival and at 3 h post-admission without any prespecified exception, and hyperoxemia was defined as PaO2 of ≥ 300 mmHg. Hyperoxemia during resuscitation was defined as hyperoxemia on hospital arrival and/or at 3 h after admission. Inflammatory and lung-related biomarkers were obtained at the ED. The Charlson index was scored to assess comorbidities [24]. Isolated brain injury was defined as a head AIS of ≥ 3 and other regions of ≤ 1.
Outcome measures
The primary outcome was ICU-free days until day 28, a composite of in-hospital mortality and ICU length of stay, defined as the number of days alive and out of the ICU between the day of hospital arrival and 28 days later. Secondary outcomes included survival to discharge and ventilator-free days until day 28.
Statistical analysis
Patients were divided into hyperoxemia and non-hyperoxemia groups. The hyperoxemia group consisted of patients who experienced hyperoxemia during resuscitation (hyperoxemia on hospital arrival and/or at 3 h after admission), whereas the non-hyperoxemia group consisted of patients in whom hyperoxemia was not observed both on hospital arrival and at 3 h after admission. Considering that oxygen exposure during resuscitation and its pathophysiological effect on the pulmonary tissue would significantly differ between patient on mechanical ventilation and those who were not, analyses were performed on the whole population and those who were divided based on the intubation status at the ED. Unadjusted analysis was performed on the ICU-free days using the Mann–Whitney U test, and between-group differences were presented using the Hodges–Lehmann estimator of the median of all paired differences with 95% confidence intervals (CIs).
To adjust patient characteristics between the two groups, inverse probability of treatment weighting (IPW) analyses with propensity scores were performed to compare primary and secondary outcomes [25]. The propensity score was developed using the logistic regression model to estimate the probability of being assigned to the hyperoxemia group compared with the non-hyperoxemia group [26]. Relevant covariates were carefully selected from known or possible unfavorable clinical outcome predictors in trauma patients based on previous studies (such as age, comorbidities, injury mechanism, ISS, degree of chest injury, and requirement of tube thoracotomy), intubation status at the ED, and vital signs on hospital arrival. All of this information was subsequently entered into the propensity model [27,28,29], in which patients with missing covariates were excluded from the propensity score calculation. The precision of propensity score discrimination was analyzed using the c-statistic [26]. IPW analyses were then performed as adjusted analyses, in which primary and secondary outcomes were compared using Mann–Whitney U tests and chi-square tests [25]. IPW was performed with restriction, in which patient data with ≤ 0.1 or ≥ 0.9 of the propensity score were not used to avoid extreme weights. Between-group differences were presented using the Hodges–Lehmann estimator with 95% CIs.
Subgroup analyses were performed to further interpretate primary results. IPW analyses on the primary outcome were repeated after excluding patients who experienced hypoxia during resuscitation, defined as PaO2 of < 60 mmHg within 3 h of hospital arrival. Another subgroup analysis was conducted after excluding patients with persistent hyperoxemia, defined as PaO2 of ≥ 300 mmHg both on hospital arrival and at 3 h after admission. Moreover, subgroup analysis was performed after excluding patients with isolated brain injury.
Furthermore, to investigate pathophysiological backgrounds of potential harm of hyperoxemia, effects of inflammatory and lung-protective biomarkers on the ICU length of stay were evaluated among patients treated with hyperoxemia. Each biomarker was analyzed along with intubation status at the ED, using ordinal logistic regression analysis after adjustment by IPW.
Descriptive statistics are presented as median (interquartile range) or number (percentage) and compared using Mann–Whitney U tests, Chi-square tests, or Fisher’s exact tests, as appropriate. Missing/ambitious values were used without manipulation. To test for all hypotheses, a two-sided α threshold of 0.05 was considered statistically significant. All statistical analyses were conducted using the SPSS, version 26.0 (IBM, Armonk, NY, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA).
Results
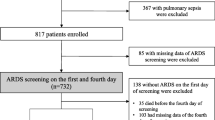
A total of 295 patients with severe injuries were registered in the JAAM FORECAST TRAUMA study. Among them, 244 with available PaO2 within 3 h of hospital arrival were eligible for this study. Figure 1 summarizes the patient flow diagram.
Patient flow diagram. A total of 295 patients with severe injuries were registered in the JAAM FORECAST TRAUMA study, which enrolled patients (1) aged ≥ 16 years, (2) with injury severity score (ISS) of ≥ 16, and (3) directly transported from the scene. Among them, 244 with available PaO2 within 3 h after hospital arrival were eligible for this study. Fifty-eight patients exposed to hyperoxemia (PaO2 ≥ 300 mmHg) within 3 h after arrival were included in the hyperoxemia group, whereas 186 not exposed to hyperoxemia were included in the non-hyperoxemia group
Fifty-eight patients were exposed to hyperoxemia (PaO2 of ≥ 300 mmHg) within 3 h of arrival and included in the hyperoxemia group, whereas 186 were not exposed to hyperoxemia and included in the non-hyperoxemia group. Table 1 summarizes patient characteristics. Compared with the non-hyperoxemia group, patients in the hyperoxemia group had lower Glasgow Coma Scale score [6 (3–13) vs 14 (11–15)] and higher ISS [29 (25–3) vs 26 (19–34)]. Furthermore, more patients in the hyperoxemia group required higher amount of blood products [red blood cell = 0 (0–4) vs 0 (0–2) and fresh frozen plasma = 0 (0–6) vs 0 (0–4)], had undergone craniotomy and angiography [19 (32.8%) vs 19 (10.5%) and 21 (36.2%) vs 37 (20.4%), respectively], and were intubated at the ED [47 (81.0%) vs 58 (31.2%)].
Arterial blood gas analyses and inflammatory biomarkers are shown in Table 2. PaO2, FiO2, and PaO2/FiO2 (P/F) ratio on hospital arrival and at 3 h after admission were higher among patients in the hyperoxemia group than those in the non-hyperoxemia group. The median PaCO2 on hospital arrival and at 3 h after admission was comparable between the two groups. Inflammatory and lung-protective biomarkers were available in 83 patients and also comparable between the two groups, except for HMGB-1 that was higher in patients with hyperoxemia than those without [20.6 (11.0–46.6) vs 13.7 (8.7–22.1)].
The propensity model predicting allocation to the hyperoxemia group was confirmed to have appropriate discrimination (c-statistic = 0.858), in which three patients were excluded due to missing covariates for the propensity score calculation. Patient characteristics after IPW are summarized in Table 1, in which most covariates were successfully adjusted.
ICU-free days were significantly fewer among patients exposed to hyperoxemia within 3 h after admission, compared with those not exposed to hyperoxemia in the unadjusted analysis [17 (10–21) vs 23 (16–26) days; difference in median = − 4 days (95% CI = − 2 to − 7 days); p < 0.001; Table 3]. IPW analysis revealed that hyperoxemia during resuscitation was significantly associated with prolonged ICU stay among patients not intubated at the ED [ICU-free days = 16 (12–22) vs 23 (19–26); median difference = − 5 (− 3 to − 10) days; p = 0.004], but not among those intubated at the ED [ICU-free days = 18 (9–20) vs 15 (8–23); median difference = 0 (− 3 to 3) days; p = 0.777]. IPW analysis also identified that hyperoxemia exposure during resuscitation was associated with fewer ventilator-free days among patients not intubated [25 (15–26) vs 28 (23–28); p = 0.014], but not among those intubated at the ED. Survival to discharge were comparable between the two groups.
Subgroup analysis, excluding patients who experienced hypoxia (PaO2 of < 60 mmHg) within 3 h after hospital arrival, also revealed the association between hyperoxemia and prolonged ICU stay among patients not intubated at the ED [ICU-free days = 16 (12–22) vs 23 (20–26); median difference = − 5 (− 3 to − 10) days; p = 0.003; Table S1 (Additional file 1)]. Another subgroup analysis excluding patients with isolated brain injury revealed similar results [ICU-free days = 16 (12–22) vs 22 (19–26); median difference = − 5 (− 2 to − 10) days; p = 0.006; Table S1 (Additional file 1)]. Conversely, the analyses on patients without persistent hyperoxemia showed comparable ICU-free days between those with or without hyperoxemia on hospital arrival (ICU-free days = 16 (5–25) vs 22 (19–26); median difference = − 5 (− 16 to 3) days; p = 0.300).
Among patients in the hyperoxemia group, higher inflammatory biomarkers including sRAGE and HMGB-1 were associated with prolonged ICU stay [sRAGE (pg/mL), − 3.2 (− 5.1 to − 1.2) ICU-free days and HMGB-1 (ng/mL), − 1.5 (− 3.0 to − 0.1) ICU-free days; Fig. 2], whereas higher lung-protective proteins including SPD and CCSP were associated shorter ICU stay [SPD (ng/mL), 4.0 (1.7 to 6.3) ICU-free days and CCSP (ng/mL), 3.8 (1.1 to 6.4) ICU-free days; Fig. 2].
Effect of inflammatory and lung-related biomarkers on ICU-free days. Biological parameters were evaluated with multivariate regression among patients treated with hyperoxemia. CI, confidence interval; sRAGE, soluble receptor for advanced glycation end-products; HMGB-1, high mobility group box-1; SPD, surfactant protein D; CCSP, Clara cell secretory protein; IL, interleukin
Discussion
In these post hoc analyses of a nationwide multicenter prospective observational study, hyperoxemia during the initial resuscitation was found to be associated with prolonged ICU stay. This relationship was validated among patients not treated with mechanical ventilation at the ED, using IPW analyses that adjusted several prognostic factors. Notably, the observed association was consistent across several subgroup analyses.
Several reasons could be considered for the relationship between hyperoxemia exposure and prolonged ICU stay among severely injured trauma patients. First, hyperoxemia might have affected study participants who had moderate-to-severe traumatic brain injury. Although results on clinical consequences have been conflicting [30, 31], previous studies on traumatic brain injury reported that improvement of mitochondrial function in the injured cerebral tissue was not obtained by increasing FiO2 to 1.0 from 0.5. In addition, supranormal oxygen levels in the cerebral blood have been reported to suppress cell metabolism, resulting in paradoxical neuronal death [30, 32]. Second, supraphysiologic FiO2, hyperoxia, could induce ALI among considerable number of patients exposed to hyperoxemia. Several studies suggested that hyperoxia-induced ALI should be considered when FiO2 exceeds 0.6–0.7 and may become problematic when FiO2 exceeds 0.8 [33, 34]. In this study, the significantly higher FiO2 on hospital arrival was observed in the hyperoxemia group [1.0 (0.8–1.0)], and the higher FiO2 remained even at 3 h after admission. It should be also emphasized that the association between higher amount of lung-protective biomarkers and shorter ICU length of stay was observed in the hyperoxemia group, including CCSP, an important protein against oxidative stress in the respiratory system [35], and SPD, a pulmonary collectin against oxidative injury [36, 37].
Furthermore, systemic and/or lung tissue inflammation following severe injuries would have affected the baseline condition before hyperoxemia exposure. Given that animal studies found pre-administration of anti-inflammatory medication attenuated hyperoxia-induced ALI [38], systemic inflammation caused by trauma and/or chest injury itself would magnify the adverse effects of hyperoxia and hyperoxemia. Indeed, this study found that higher inflammatory biomarkers such as sRAGE and HMGB-1 were associated with unfavorable outcomes among patients exposed to hyperoxemia: sRAGE is a central cell surface receptor for HMGB-1, and both sRAGE and HMGB-1 are involved in the host response to injury, infection, and inflammation [39, 40].
Although a recent retrospective study on trauma patients reported that PaO2 of ≥ 150 mmHg on hospital admission was related to decreased in-hospital mortality, several differences should be noted in this study. First, the definition of hyperoxemia is different; patients with hyperoxemia (PaO2 of ≥ 150 mmHg) in the abovementioned study included only small number of patients exposed to PaO2 of ≥ 300 mmHg [median PaO2 was 230 (186–308) mmHg], although the harmful effect of hyperoxemia has been identified at PaO2 of ≥ 300 mmHg among critically ill patients [41, 42]. Second, hyperoxemia during resuscitation (on hospital arrival and at 3 h after admission) was examined in the current study because investigating only PaO2 on arrival would reflect prehospital treatment, rather than in-hospital critical care of trauma patients. Third, FiO2- and lung-related biomarkers were not measured in the abovementioned study, although hyperoxia-induced ALI has been suggested as a potential cause of unfavorable clinical outcomes in patients who experienced hyperoxemia [16, 18].
The harm of hyperoxemia during resuscitation was not confirmed in patients intubated at the ED in this study, probably because precise control over FiO2 during the lung-protective ventilation: Minimizing the length of exposure time to hyperoxia (supraphysiologic FiO2) would have diminished the relatively small degree of deleterious effects of hyperoxemia. The comparable mortality between the hyperoxemia and non-hyperoxemia groups obtained in this study is similar to that of a retrospective study on wartime pediatric trauma patients, which revealed no survival benefits of normoxia over hyperoxemia [21]. Considering that differences in the median ICU-free days between the two groups were only a few days in this study, prospective study involving sufficient number of patients should be conducted to confirm the possible harm of hyperoxemia in trauma patients.
The results of this study must be interpreted within the context of the study design. Post hoc analyses of the FORECAST TRAUMA study were conducted, which did not record indications of oxygen administration. Thus, our results could have been different if the respiratory condition during resuscitation had contained unrecorded strong prognostic factors. Another limitation is that variables relating to neurologic and pulmonary function were not available in the database. Although hyperoxemia-induced brain injury and ALI could be considered main causes of prolonged ICU stay following hyperoxemia exposure, objective data did not directly validate such physiological mechanism. Moreover, only hyperoxemia on hospital arrival and at 3 h after admission were investigated. Previous studies on hyperoxemia in various critically ill patients reported that clinical outcomes were different depending on timing (arrival, within a few hours, or within a day), definition (PaO2 ≥ 300 mmHg, ≥ 400 mmHg, or highest quartile of observed data), and obtained data (highest, lowest, or defined time point) for hyperoxemia [1, 9, 42]. Therefore, our results would vary if PaO2 was measured at different time points or if hyperoxemia was differently defined. Furthermore, this study was not designed to examine whether hyperoxemia would be more harmful than hypoxia. Considering that various studies reported the harmfulness of hypoxia during trauma resuscitation, hypoxia should be avoided more reliably. Finally, some biases could not be adjusted with IPW: some variables for propensity score calculation such as vital signs on arrival could be intermediate variables between prehospital hyperoxemia and outcomes, and survival bias would exist because hyperoxemia was defined based on PaO2 within 3 h after admission.
Conclusions
This study identified that hyperoxemia within 3 h after hospital arrival was associated with prolonged ICU stay among severely injured trauma patients not intubated at the ED. Further research is necessary to elucidate the harmful effect of different degrees and durations of hyperoxemia exposure.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICU:
-
Intensive care unit
- FiO2 :
-
Fraction of inspired oxygen
- ALI:
-
Acute lung injury
- JAAM:
-
Japanese Association for Acute Medicine
- FORECAST:
-
Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis, and Trauma
- ED:
-
Emergency department
- UMIN-CTR:
-
University Hospital Medical Information Network Clinical Trial Registry
- IRB:
-
Institutional review board
- ISS:
-
Injury severity score
- PaO2 :
-
Partial pressure of oxygen
- AIS:
-
Abbreviated injury scale
- sRAGE:
-
Soluble receptor for advanced glycation end-products
- HMGB-1:
-
High mobility group box-1
- SPD:
-
Surfactant protein D
- CCSP:
-
Clara cell secretory protein
- IL-8:
-
Interleukin-8
- IPW:
-
Inverse probability of treatment weighting
References
Helmerhorst HJF, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43(7):1508–19. https://doi.org/10.1097/CCM.0000000000000998.
Angus DC. Oxygen therapy for the critically ill. N Engl J Med. 2020;382(11):1054–6. https://doi.org/10.1056/NEJMe2000800.
Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18(6):711. https://doi.org/10.1186/s13054-014-0711-x.
Barbateskovic M, Schjørring OL, Russo Krauss S, Jakobsen JC, Meyhoff CS, Dahl RM, et al. Higher versus lower fraction of inspired oxygen or targets of arterial oxygenation for adults admitted to the intensive care unit. Cochrane Database Syst Rev. 2019;2019(11):CD012631. https://doi.org/10.1002/14651858.CD012631.pub2.
Palmer E, Post B, Klapaukh R, Marra G, MacCallum NS, Brealey D, et al. The association between supraphysiologic arterial oxygen levels and mortality in critically ill patients a multicenter observational cohort study. Am J Respir Crit Care Med. 2019;200(11):1373–80. https://doi.org/10.1164/rccm.201904-0849OC.
Diringer MN. Hyperoxia: good or bad for the injured brain? Curr Opin Crit Care. 2008;14(2):167–71. https://doi.org/10.1097/MCC.0b013e3282f57552.
Tolias CM, Reinert M, Seiler R, Gilman C, Scharf A, Bullock MR. Normobaric hyperoxia-induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J Neurol Surg. 2004;101:435–44.
Roberts BW, Kilgannon JH, Hunter BR, Puskarich MA, Pierce L, Donnino M, et al. Association between early hyperoxia exposure after resuscitation from cardiac arrest and neurological disability: prospective multicenter protocol-directed cohort study. Circulation. 2018;137(20):2114–24. https://doi.org/10.1161/CIRCULATIONAHA.117.032054.
Patel JK, Kataya A, Parikh PB. Association between intra- and post-arrest hyperoxia on mortality in adults with cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2018;127:83–8. https://doi.org/10.1016/j.resuscitation.2018.04.008.
Sutton ADJ, Bailey M, Bellomo R, Eastwood GM, Pilcher DV. The association between early arterial oxygenation in the ICU and mortality following cardiac surgery. Anaesthesiol Intensive Care. 2014;42(6):730–5. https://doi.org/10.1177/0310057X1404200608.
Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit the oxygen-icu randomized clinical trial. JAMA. 2016;316(15):1583–9. https://doi.org/10.1001/jama.2016.11993.
Chu DK, Kim LHY, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–705. https://doi.org/10.1016/S0140-6736(18)30479-3.
Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX). Intensive Care Med. 2020;46(1):17–26. https://doi.org/10.1007/s00134-019-05857-x.
Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5(3):180–90. https://doi.org/10.1016/S2213-2600(17)30046-2.
Watson NA, Beards SC, Altaf N, Kassner A, Jackson A. The effect of hyperoxia on cerebral blood flow: a study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur J Anaesthesiol. 2000;17(3):152–9. https://doi.org/10.1097/00003643-200003000-00004.
Reinhart K, Bloos F, König F, Bredle D, Hannemann L. Reversible decrease of oxygen consumption by hyperoxia. Chest. 1991;99(3):690–4. https://doi.org/10.1378/chest.99.3.690.
Angelos MG, Yeh ST, Aune SE. Post-cardiac arrest hyperoxia and mitochondrial function. Resuscitation. 2011;82:S48–51. https://doi.org/10.1016/S0300-9572(11)70151-4.
Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32(12):2496–501. https://doi.org/10.1097/01.CCM.0000148231.04642.8D.
Dias-Freitas F, Metelo-Coimbra C, Roncon-Albuquerque R. Molecular mechanisms underlying hyperoxia acute lung injury. Respir Med. 2016;119:23–8. https://doi.org/10.1016/j.rmed.2016.08.010.
Locy ML, Rogers LK, Prigge JR, Schmidt EE, Arnér ES, Tipple TE. Thioredoxin reductase inhibition elicits Nrf2-mediated responses in clara cells: implications for oxidant-induced lung injury. Antioxid Redox Signal. 2012;17(10):1407–16. https://doi.org/10.1089/ars.2011.4377.
Naylor JF, Borgman MA, April MD, Hill GJ, Schauer SG. Normobaric hyperoxia in wartime pediatric trauma casualties. Am J Emerg Med. 2020;38(4):709–14. https://doi.org/10.1016/j.ajem.2019.06.005.
Baekgaard JS, Abback PS, Boubaya M, Moyer JD, Garrigue D, Raux M, et al. Early hyperoxemia is associated with lower adjusted mortality after severe trauma: results from a French registry. Crit Care. 2020;24(1):604. https://doi.org/10.1186/s13054-020-03274-x.
Gando S, Shiraishi A, Wada T, Yamakawa K, Fujishima S, Saitoh D, et al. A multicenter prospective validation study on disseminated intravascular coagulation in trauma-induced coagulopathy. J Thromb Haemost. 2020;18(9):2232–44. https://doi.org/10.1111/jth.14931.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res. 2017;26(4):1654–70. https://doi.org/10.1177/0962280215584401.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786.
Yamamoto R, Cestero RF, Muir MT, Jenkins DH, Eastridge BJ, Funabiki T, et al. Delays in surgical intervention and temporary hemostasis using resuscitative endovascular balloon occlusion of the aorta (REBOA): influence of time to operating room on mortality. Am J Surg. 2020;220(6):1485–91. https://doi.org/10.1016/j.amjsurg.2020.07.017.
Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma Inj Infect Crit Care. 2006;60:S3–S11.
Moore L, Lavoie A, Abdous B, Le Sage N, Liberman M, Bergeron E, et al. Unification of the revised trauma score. J Trauma Inj Infect Crit Care. 2006;61(3):718–22; discussion 722. https://doi.org/10.1097/01.ta.0000197906.28846.87.
Davis DP, Meade W, Sise MJ, Kennedy F, Simon F, Tominaga G, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26(12):2217–23. https://doi.org/10.1089/neu.2009.0940.
Raj R, Bendel S, Reinikainen M, Kivisaari R, Siironen J, Lång M, et al. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013;17(4):R177. https://doi.org/10.1186/cc12856.
Bissonnette B, Bickler PE, Gregory GA, Severinghaus JW. Intracranial pressure and brain redox balance in rabbits. Can J Anesth. 1991;38(5):654–9. https://doi.org/10.1007/BF03008204.
Fisher AB, Beers MF. Hyperoxia and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(6):L1066. https://doi.org/10.1152/ajplung.90486.2008.
Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008. https://doi.org/10.1056/NEJMoa1916431.
Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann NY Acad Sci. 2000;923:68–77. https://doi.org/10.1111/j.1749-6632.2000.tb05520.x.
Du J, Abdel-Razek O, Shi Q, Hu F, Ding G, Cooney RN, et al. Surfactant protein D attenuates acute lung and kidney injuries in pneumonia-induced sepsis through modulating apoptosis, inflammation and NF-κB signaling. Sci Rep. 2018;8(1):15393. https://doi.org/10.1038/s41598-018-33828-7.
Bezerra FS, Ramos CO, Castro TF, Araújo NPDS, de Souza ABF, Bandeira ACB, et al. Exogenous surfactant prevents hyperoxia-induced lung injury in adult mice. Intensive Care Med Exp. 2019;7(1):19. https://doi.org/10.1186/s40635-019-0233-6.
Kawaguchi T, Yanagihara T, Yokoyama T, Suetsugu-Ogata S, Hamada N, Harada-Ikeda C, et al. Probucol attenuates hyperoxia-induced lung injury in mice. PLOS ONE. 2017;12(4):e0175129. https://doi.org/10.1371/journal.pone.0175129.
Zhang F, Su X, Huang G, Xin XF, Cao EH, Shi Y, et al. sRAGE alleviates neutrophilic asthma by blocking HMGB1/RAGE signalling in airway dendritic cells. Sci Rep. 2017;7(1):14268. https://doi.org/10.1038/s41598-017-14667-4.
Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–4. https://doi.org/10.4049/jimmunol.165.6.2950.
Yamamoto R, Yoshizawa J. Oxygen administration in patients recovering from cardiac arrest: a narrative review. J Intensive Care. 2020;8(1):60. https://doi.org/10.1186/s40560-020-00477-w.
Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, et al. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation. 2014;85(9):1142–8. https://doi.org/10.1016/j.resuscitation.2014.05.021.
Acknowledgements
This study was supported by the Japanese Association for Acute Medicine (JAAM, 2014-01). The JAAM FORECST Study Group thanks Shuta Fukuda for his special assistance in completing the study.
Funding
This study was supported by the Japanese Association for Acute Medicine (grant number 2014-01).
Author information
Authors and Affiliations
Consortia
Contributions
RY, SF, and JS contributed to the acquisition of data, conceived and designed the study, interpreted the data, drafted the manuscript, and revised the manuscript for important intellectual content. SG, DS, AS, SK, and TA contributed to the acquisition of data, interpreted the data, and revised the manuscript for important intellectual content. All authors contributed to the acquisition of the data and reviewed and discussed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All collaborating hospitals obtained approval of their individual Institutional Review Board (IRB) for conducting research with human participants (approval number JAAM, 2014-01 from Japanese Association for Acute Medicine; approval number 014-0307 from Hokkaido University Graduate School of Medicine, head institute of the FORECAST group; and approval number 20150056 from the Keio University School of Medicine Keio, institute of the corresponding author).
Consent for publication
Not applicable.
Competing interests
Dr. Fujishima reports grants and personal fees from Asahi Kasei Japan Co.; grants from Shionogi Co, Ltd.; grants from Chugai Pharmaceuticals Co., Ltd.; grants from Otsuka Pharmaceutical Co., Ltd.; grants from Teijin Pharma, Ltd.; grants from Pfizer Inc.; grants from Tsumura & Co.; grants from Astellas Pharma Inc.; and personal fees from Takeda Pharmaceutical Co., Ltd., outside the submitted work. Dr. Gando reports personal fees from ASAHIKASEI PHARMA AMERICA, personal fees from ASAHIKASEI PHARMA JAPAN, and personal fees from GRIFOLS, outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Hyperoxemia and ICU-free days in subgroups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yamamoto, R., Fujishima, S., Sasaki, J. et al. Hyperoxemia during resuscitation of trauma patients and increased intensive care unit length of stay: inverse probability of treatment weighting analysis. World J Emerg Surg 16, 19 (2021). https://doi.org/10.1186/s13017-021-00363-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13017-021-00363-2