Abstract
Despite aggressive management consisting of maximal safe surgical resection followed by external beam radiation therapy (60 Gy/30 fractions) with concomitant and adjuvant temozolomide, approximately 90% of WHO grade IV gliomas (glioblastomas, GBM) will recur locally within 2 years. For patients with recurrent GBM, no standard of care exists. Thanks to the continuous improvement in radiation science and technology, reirradiation has emerged as feasible approach for patients with brain tumors. Using stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT), either hypofractionated or conventionally fractionated schedules, several studies have suggested survival benefits following reirradiation of patients with recurrent GBM; however, there are still questions to be answered about the efficacy and toxicity associated with a second course of radiation. We provide a clinical overview on current status and recent advances in reirradiation of GBM, addressing relevant clinical questions such as the appropriate patient selection and radiation technique, optimal dose fractionation, reirradiation tolerance of the brain and the risk of radiation necrosis.
Similar content being viewed by others
Introduction
Glioblastoma (GBM) is the most common malignant brain tumor in adults. The standard treatment includes surgery followed by external beam radiation therapy (RT) with concomitant and maintenance temozolomide, with a reported median survival time of 14.6 months and 2-year survival rate of 26.5%, respectively [1]. However, almost all GBMs relapse within or in close proximity to the initial site of disease despite advances in surgery and chemoradiotherapy.
For patients with a recurrent GBM, no standard of care exists. Treatment options include repeated surgery, reirradiation, systemic therapy, and best supportive care [2]. A surgical approach can be utilized for locally recurrent or progressive malignant glioma with reported median survival times ranging from 6 to 17 months, with total or subtotal (extent of resection > 80%) resection being associated with longer survival [3, 4]; however, factors associated with increased postoperative morbidity, e.g. location of tumor recurrence in eloquent/critical brain regions, low performance status, and tumor volume have to be carefully evaluated. For patients who receive salvage systemic treatment at recurrence, the antiangiogenic agent bevacizumab and alkylating agents, either temozolomide or lomustine, represent common salvage therapeutic options resulting in median survival times of 6 to 12 months [5,6,7]. Bevacizumab has been the standard salvage therapeutic option for patients with recurrent GBM in the United States since its approval in 2009 by the Food and Drug Administration [5, 6]; in contrast, lomustine is the recommended second-line chemotherapy in the European Union based on a large randomized trial of 437 patients with progressive GBM that showed a similar median survival time around 9 months for those receiving lomustine plus bevacizumab versus lomustine alone [7].
Reirradiation is increasingly used in patients with recurrent GBM. Even though damage of normal brain tissue previously treated with high dose RT is of concern, technological advances in radiation techniques, including developments in treatment planning systems and dose delivery, have improved the therapeutic ratio making it possible to use reirradiation as feasible treatment option [8]. Variable median survival times of 6 to 12 months and neurological toxicity rates of 5% to 20% have been reported after stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT), using either hypofractionated or conventionally fractionated radiation schedules [9, 10]. In addition, survival benefit has been reported following reirradiation in combination with temozolomide or bevacizumab compared to reirradiation alone. We present a clinical overview on the current status and advances of reirradiation in the setting of recurrent or progressive GBM after standard treatment, with special regard to target volume delineation and impact of radiation techniques on survival and risk of radiation-induced brain necrosis.
Imaging and delineation of target volumes
A second course of radiation remains of concern for patients with a recurrent GBM because of unacceptable risk of neurological toxicity in the form of radiation necrosis. Then, the key issue for GBM reirradiation is an accurate delineation of target volumes and organs at risk (OARs) for a precise calculation of the spatial dose distribution, and for choosing the optimal radiation dose fractionation schedule. Magnetic resonance imaging (MRI), using contrast-enhanced T1-weighted and T2-weighted sequences, are routinely used because of their more accurate depiction of the extension of the tumor and brain anatomy compared to computed tomography (CT). The gross tumor volume (GTV) is generally defined as the visible lesion on MRI contrast-enhanced T1-weighted sequences. The clinical target volume (CTV) which includes areas of potential suspected microscopic tumor infiltration and potential paths of microscopic spread, is then generated by adding a variable margin of 0–5 mm to the GTV constrained at anatomical borders, e.g. tentorium, falx cerebri, and bone. In this regard, no GTV-to-CTV margins are usually utilized during SRS with the aim to limit the risk of toxicity, where margins up to 5 mm are commonly applied during hypofractionated and conventionally fractionated SRT (see below). In a few studies, CTV delineation consists of including FLAIR abnormalities around contrast-enhancing lesion in T1-weighted sequences [11, 12]. Finally, depending on radiation technique, available technology, and institutional practice, an expansion up to 3 mm is applied to generate the planning target volume (PTV) which accounts for uncertainties in treatment planning and delivery.
Positron emission tomotherapy (PET)/CT imaging with radiolabeled amino acids may help to improve target volume delineation accuracy by revealing tumor infiltration in regions with a non-specific MRI appearance [13,14,15,16,17,18]. In a trial including 44 patients with recurrent high-grade gliomas who received reirradiation with hypofractionated SRT (30 Gy/6 fractions) with or without temozolomide, Grosu et al. [13] demonstrated significantly longer median survival time using treatment planning based on (11)C-methionine (MET)-PET or (123)I-alpha-methyl-tyrosine (IMT) single-photon computed emission tomography/CT/MRI image fusion compared with treatment planning using CT/MRI alone (9 vs. 5 months; p = 0.03). Significantly improved survival using MET-PET/CT to define the GTV compared with treatment planning based on conventional MRI has been observed by others [14]. In a prospective imaging study comparing FET-PET with advanced MRI imaging in 41 patients who received SRT for recurrent GBM, Popp et al. [17] found that target volume delineation using MET-PET imaging correlated better with localization of post-reirradiation recurrences in comparison to target volume delineation based on diffusion-weighted (DWI) MRI and apparent diffusion coefficient (ADC) maps which reveal regions of high cellularity as surrogate for active tumor. Currently, a multicenter phase II trial (GLIAA, NOA-10, ARO 2013/1) is seeking to evaluate whether reirradiation planning using FET/PET improves clinical outcome in patients with recurrent GBM compared to contrast-enhanced MRI [19]. Although these studies support the use of biologic imaging as an effective strategy for target delineation of recurrent GBM, the impact of PET-based treatment planning on survival requires further investigation.
Brain tolerance to reirradiation
Normal brain tissue dose tolerance is the limiting factor when giving reirradiation. An estimated risk of symptomatic radiation necrosis has been determined following brain SRS and SRT [20, 21]. For conventional fractionation, a 5% and 10% risk of symptomatic radiation necrosis is predicted to occur at a biologically equivalent dose (BED) of 72 Gy (range, 60–84 Gy) and 90 Gy (range, 84–102 Gy) in 2-Gy fractions. For SRS, the risk of complications increases rapidly once the volume of the brain exposed to 12 Gy is more than 5–10 ml [22, 23]. Dose-volume predictors of toxicity for critical structures, e.g. optic chiasm and brainstem, have also been determined for SRS and SRT, both hypofractionation and normofractionation [24,25,26]; however, high-level evidence is lacking and current constraints should be used with caution.
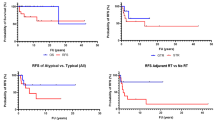
When assessing the risk of radiation necrosis following reirradiation, several factors should be taken into account, including dose and fractionation, treated volume, combined chemotherapy, and interval between radiation treatments. Previous meta-analyses of brain reirradiation found no cases of brain necrosis when the cumulative radiation dose of the two courses of radiation, calculated as biological equivalent total dose normalized to 2 Gy/fraction (EQD2) using the linear-quadratic model, was < 96 Gy [27, 28]. The median cumulative EQD2 reported in conventionally fractionated reirradiation (81.6–101.9 Gy) series was generally lower than those observed in hypofractionated SRT (90–133.9 Gy) and SRS (111.6–137.2 Gy). The estimated risk of radiation necrosis at 1 year was 2–12% for cumulative EQD2 > 96.2 Gy and up to 17% for cumulative EQD2 > 137 Gy. Our update of the literature on GBM reirradiation confirms the relationship between cumulative EQD2 and the risk of radiation necrosis, as shown in Tables 1, 2 and 3; the reported risk was about 0–3% after conventional fractionation at cumulative EQD2 < 101 Gy, 7–13% after hypofractionated SRT at cumulative EQD2 of 102–130 Gy, and up to 24.4% after SRS using a cumulative EQD2 of about 124–150 Gy.
Although the validity of the linear-quadratic model has been questioned for high radiation doses as employed during SRS and ultrahypofractionated schedules [29], current data indicates that doses exceeding 100 Gy should be used with caution, especially in case of large irradiated volumes. In contrast, other factors did not appear to be linked with an increased risk of radiation necrosis, e.g. the time interval between two radiation courses or the use of concurrent bevacizumab, the latest associated with reduced treatment toxicity [30].
Based on dose/volume data and clinical risk estimates for central nervous system (CNS), maximum doses exceeding 8–10 Gy and 12 Gy given in single fraction and 55 Gy and 54 Gy (59 Gy to < 10 cc) given in 1.8–2 Gy fractions for optic apparatus and brainstem, respectively, should be avoided in clinical practice [26]; however, limited data are available in the setting of reirradiation. Niyazi et al. [31] showed no relevant long-term toxicity in a series of 58 patients who received reirradiation for a malignant glioma using maximum cumulative EQD2 of 80.3 Gy, 79.4 Gy, and 95.2 Gy to optic chiasm, optic nerves, and brainstem, respectively. With regard to the brainstem, a few series did not observe significant neurological toxicity and/or radiological changes suggestive of brain necrosis in patients with progressive diffuse intrinsic pontine gliomas receiving a second course of SRT with doses of 20–24 Gy given in 2-Gy fractions [32]. Overall, these data suggest relatively high and fast recovery capacity of the normal human brain after a second course of RT, similarly to that seen for spine [33], and support the use of cumulative EQD2 doses around 100 Gy or even higher (up to 120 Gy), e.g. small and well defined lesions away from eloquent areas.
Few data are available on the effect of reirradiation on cognitive deterioration. Wick et al. [34] reported the results of a phase II study involving 91 patients with progressive GBM randomized to receive reirradiation (36 Gy in 2-Gy fractions) with or without the systemic agent Asunercept. Neurological status and quality of life, assessed by the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C15 PAL fatigue scale, EORTC QLQ-BN20 and Medical Research Council (MRC)-Neurological status, remained stable in both groups until progression. In another small prospective series of 15 patients who received hypofractionated SRT (35 Gy in 7-Gy fractions) for malignant gliomas, overall quality of life, physical functioning, and cognitive functioning remained stable in two thirds of the patients at a median time of 9 months. Although these results offer some reassurance about the safety of reirradiation in adult patients, caution should be applied in clinical practice when treating large volumes with large fraction size.
Survival outcomes and toxicity
SRS
For patients with recurrent GBM, SRS is usually given alone or in combination with systemic therapy. Treatment planning characteristics and clinical outcomes of sixteen selected series published from 2005 to 2020 and including 901 patients who received SRS for recurrent GBM are shown in Table 1 [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. With a median dose of 15–18 Gy for a treated volume around 4–10 ml as seen in the majority of studies, the overall survival time from the date of SRS reirradiation ranges from 7.5 to 13 months and progression-free survival time from 4.4 to 6 months. Gamma Knife remains the prevalent SRS modality, whereas hypofractionated treatments are typically delivered with Cyberknife and LINAC. Of note, a recent systematic review and meta-analysis of 50 non-comparative studies including 2095 patients treated with different SRS reirradiation modalities/technologies showed similar overall survival rates of 70% and 34% and progression-free survival rates of 40% and 16%, respectively, at 6 and 12 months [10].
With respect to the retrospective nature and small numbers of patients, available data suggest increased survival rates with SRS and systemic therapies compared to SRS alone [38, 44, 46, 48, 51]. The reported overall survival time after concurrent SRS and temozolomide is around 9–15 months; some [44, 46, 48, 51], but not all [36, 47], studies showed significant survival benefit following chemoradiation over SRS alone, especially in patients with O6-methylguanine-DNA methyltransferase (MGMT) gene promoter methylation. Since its approval by the US Food and Drug Administration for the treatment of patients with recurrent GBM in 2009, the efficacy of the anti-vascular endothelial growth factor (VEGF)-A humanized monoclonal antibody bevacizumab in combination with SRS has been evaluated in several studies [38, 50, 52]. In a series of 49 patients with recurrent GBM who received SRS with or without concurrent and adjuvant bevacizumab, Cuneo et al. [38] observed median progression-free survival and overall survival times from SRS of 6 months and 10 months, respectively. The 1-year overall survival time was 50% for patients who received SRS and bevacizumab and 22% for patients receiving SRS alone (p = 0.005), with respective median progression-free survival times of 5.2 months and 2.1 months (p = 0.014). The superiority of combined treatment versus SRS alone has been seen in other small retrospective series [50, 52]; of note, the safety of SRS plus bevacizumab has been confirmed in several studies, with patients receiving bevacizumab who were less likely to develop grade III adverse radiation effects.
As shown in Table 1, the reported risk of symptomatic radiation necrosis for sixteen studies including 928 patients varies from 0 to 24.4%, being mainly related to radiation dose and treated volume. Using the cumulative EQD2 as predictor of the risk, we found that values around 120 Gy were generally associated with a risk < 10% for a median tumor volume of approximately 10 ml, whereas a higher risk up to 24% was observed for cumulative EQD2 values of > 132 Gy. Considering an EQD2 of 60 Gy for the initial standard chemoradiation, this means that SRS reirradiation doses of 15–16 Gy (EQD2 = 63.7–72 Gy) carry an acceptable risk of radiation necrosis, at least for patients with relatively small recurrent tumor volumes. In a systematic review and analysis of treatment technique of reirradiation for recurrent high-grade gliomas including results of 70 studies with 3302 patients, the median unadjusted rate of brain necrosis after SRS was 8% for patients with a median treatment volume of 10.1 ml and a median dose per fraction of 15 Gy [53]. These values are consistent with those observed in the RTOG study 90–05 that evaluated the risk of radiation necrosis following SRS reirradiation of primary brain tumors and brain metastases with doses of 15–24 Gy [54]. This means in clinical practice that small CTV/PTV margins of 0–2 mm are generally utilized during SRS reirradiation to minimize the risk of brain necrosis, especially when treating larger tumors with cumulative EQD2 exceeding 120 Gy. In contrast, the interval between radiation treatments and the use of concurrent systemic therapies did not emerge as independent factors associated with the development of radiation necrosis in several studies.
Hypofractionated SRT
Hypofractionated SRT with or without systemic therapy has been frequently used in the setting of recurrent GBM. Treatments include moderately (generally 2.5–3.5 Gy per fraction) and high-dose (5 Gy or more per fraction) hypofractionated schedules (Table 2) [12, 13, 55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Because of its higher degree of precise patient positioning and accurate dose delivery, SRT has superseded conventional RT in clinical practice in the last two decades for the treatment of patients with recurrent tumors. Results of eighteen studies including 976 patients who received stereotactic reirradiation between 2005 and 2020 are shown in Table 2. Using total doses of 30–45 Gy delivered in 2.5–4.0 Gy per fraction, ten studies including 733 patients showed a median overall survival time ranging from 7.5 to 12.5 months [12, 57,58,59, 65,66,67,68,69,70]. A similar survival time of 7.3 to 12.5 months has been observed in eight studies including 272 patients who received high-dose hypofractionated SRT at doses of 25–35 Gy in 5–7 Gy per fraction [13, 55, 56, 60,61,62,63,64]. Using a dose of 35 Gy in 10 fractions of 3.5 Gy per fraction, Fogh et al. [58] reported an overall survival time of 11 months in 105 patients with recurrent GBM. A total dose of > 35 Gy resulted in an improved overall survival with no significant differences amongst patients who had chemotherapy and those who did not. Similar survival times of 8 to11 months have been observed in large retrospective multicentric studies using either SRS or hypofractionated SRT [71, 72].
Several studies has been evaluated the use of SRT in combination with systemic therapies, as shown in Table 2. In a series of 36 patients with recurrent GBM who received moderately fractionated SRT (37.5 Gy in 15 fractions) and concurrent temozolomide at University of Rome Sapienza, Sant’Andrea Hospital, median overall survival and progression-free survival times were 9.7 months and 5 months, respectively [59]. Using hypofractionated SRT (30 Gy in six fractions) with or without concurrent temozolomide, Grosu et al. [13] observed a median survival time of 11 months after chemoradiation and 6 months after SRT alone (p = 0.04), and similar survival benefits have been reported in other few series using high-dose hypofractionated SRT (25–35 Gy in five fractions) and temozolomide [62, 63]. In all above mentioned studies, longer survival time was associated with the presence of tumors with a methylated enzyme O6-methylguanine-DNA methyltransferase (MGMT) gene promoter.
Survival benefits have been reported following fractionated SRT and concurrent bevacizumab [12, 55, 60, 64,65,66,67,68]. Gutin et al. [55] observed a median overall survival time of 12.5 months and 1-year survival rate of 54% in twenty patients who received 30 Gy in 5 fractions to the recurrent tumor with SRT; median progression-free survival time and 6-month rates were 7.4 months and 65%, respectively. In a small retrospective study comparing hypofractionated SRT (25 Gy in 5-Gy fractions) plus bevacizumab or the alkylating agent fotemustine, median survival times and 12-month survival rates were 11 months and 30% for patients treated with SRT and bevacizumab and 8.3 months and 5% for those treated with SRT and fotemustine (p = 0.01); respective median progression-free survival times were 6 and 4 months (p = 0.01). In a recent multi-institutional, prospective randomized phase II trial (NRG Oncology/Radiation Therapy Oncology Group (RTOG) trial 1205) designed to evaluate the safety and efficacy of reirradiation for recurrent GBM with modern radiation techniques, a similar survival of 10.1 months has been observed following hypofractionated SRT (35 Gy/10 fractions) and concurrent bevacizumab [68].
A risk of radiation necrosis less than 10% is generally reported after SRT for cumulative EQD2 doses less than 120 Gy following either moderately or high-dose fractionated schedules (Table 2). Of note, the risk remains low despite:—higher median treated volumes in the range of 8.5–34 ml and 33–145 ml for high-dose and moderately fractionated radiation schedules, respectively, and—the use of GTV-to-CTV margins up to 5 mm. In the respect of relatively few reported cases, radiation necrosis is usually observed in recurrent tumors that generally receive cumulative EQD2 doses > 120 Gy to volumes > 40 ml [13, 63,64,65]. Of note, the risk remains low after SRT in combination and concurrent systemic therapy, with a reported lower risk in patients receiving SRT and bevacizumab [12, 64, 68, 73]
Conventionally fractionated SRT
Several series reported on the efficacy and safety of conventionally fractionated SRT in patients with recurrent gliomas (Table 3) [30, 74,75,76,77,78,79]. Using a median dose of 36 Gy delivered in 18 fractions of 2 Gy per fraction, the reported median survival time ranges from 6.7 to 11.5 months and progression-free survival time from 2.5 to 5 months. In a large series of 172 patients with recurrent low- and high-grade gliomas treated with a second course of conventionally fractionated SRT (36 Gy in 2-Gy fractions), Combs et al. [74] reported median overall survival and progression-free survival times of 8 and 5 months, respectively, for 59 patients with GBM.
The superiority of combined chemoradiation versus radiation alone remains to be better defined [30, 76, 78, 79]. Schnell et al. [78] conducted a retrospective three-arm study of 105 patients with recurrent malignant gliomas who were treated with conventionally fractionated SRT (36 Gy in 2-Gy fractions) and concurrent bevacizumab with or without maintenance therapy, or bevacizumab/irinotecan between 2008 and 2014. The authors observed a significantly improved median post-recurrence survival time of 13.1 months for patients receiving reirradiation in combination with concurrent and maintenance bevacizumab compared to a survival time of 8 months for those receiving systemic therapy only or concurrent reirradiation and bevacizumab without maintenance therapy. A recent SRT-specific update of the same study group (n = 161) could not confirm long-term differences according to maintenance therapy; post-recurrence survival time was 9 months in both arms, but toxicity was significantly reduced among bevacizumab patients compared to those receiving reirradiation alone [30].
Using conventionally fractionated SRT, a low risk of radiation necrosis of 0.8% to 6.8% has been observed in six studies including 439 patients with recurrent GBM (Table 3). With a median total dose of 36 Gy delivered in 18 fractions of 2 Gy per fraction (cumulative EQD2 = 96 Gy), the risk remains low even in series with a median target volume of about 100 ml or higher and when using large safety margins up to 10 mm with the aim of including potential microscopic spread.
Prognostic factors and scores for reirradiation
Several prognostic factors have been correlated with clinical outcomes following reirradiation in patients with GBM. Age at reirradiation, Karnofsky performance status (KPS), tumor grade (grade III versus grade IV glioma) are well recognized prognostic factors associated with longer survival, whereas the role of other factors, including tumor volume, surgery before reirradiation, time interval from first course of RT, use of concurrent systemic therapy, and MGMT promoter methylation remains controversial [43, 54, 56,57,58, 64, 69, 71, 74, 75, 80,81,82,83,84,85]. In this regard, some, but not all, studies failed to demonstrate the favorable impact of surgery before reirradiation [57, 80, 82, 84, 93] and time interval between initial standard chemoradiation and reirradiation [56, 58, 64, 75, 81, 82, 85]; however, the majority of authors suggest a minimum interval of six month-interval between the two radiation courses.
Several prognostic scores have been developed to assess the clinical prognosis of patients undergoing reirradiation and to assist with patient selection [71, 75, 79, 85,86,87,88,89,90,91,92,93]. Using several prognostic factors, including WHO grade, age, gender, MGMT methylation status, time interval between first and second course of RT, and KPS, Niyazi et al. [92] developed a reirradiation risk score to independently predict survival in 565 patients from the German Cancer Consortium (DKTK)/radiation oncology group (ROG) database. Based on multivariate analysis, three prognostic groups were identified based on age, glioma grade and KPS, with longer survival in younger patients with better performance status and a grade III glioma. Another report of DKTK-ROG aimed to validate two different prognostic scores previously generated by Combs et al. [89], which included primary histology, time from primary RT to reirradiation, and age, and by Kessel et al. [90], created by adding values for KPS, irradiated volume, and performed resection. Data demonstrated a significant correlation between both scores and overall survival after reirradiation; however, salvage surgery before reirradiation and the time between radiation courses did not emerge as independent prognostic factors for survival, consistent with results of other studies [56, 57, 64, 75, 80,81,82, 84, 85, 93]. Overall, prognostic scores incorporating both tumor and patient characteristics are useful to provide recommendations to bear on clinical decisions. In this regard, the impact of other potential prognostic factors, e.g. tumor molecular markers, extent of resection, and concurrent systemic therapies, should be evaluated and validated in large series of irradiated gliomas.
Conclusions
Reirradiation is an effective and safe treatment in the management of recurrent GBM. For appropriately selected patients, both SRS and SRT, either hypofractionated or conventionally fractionated regimens, are feasible therapeutic options associated with similar median overall survival in the range of 6 to 12 months and relatively low toxicity.
Several studies have investigated the effect of reirradiation in combination with systemic therapy, although its favorable impact on survival remains controversial. Concomitant and/or adjuvant treatment with temozolomide has resulted in longer overall survival and progression-free survival times compared with radiation alone, but this is generally limited to MGMT methylated tumors [13, 48, 63, 72]; in addition, no clear survival advantages have been observed by other authors [58, 74, 85]. Some studies suggested significantly longer survival with the addition of bevacizumab to both SRS and fractionated SRT compared to reirradiation alone [38, 50, 52, 76, 78]; in contrast, other studies failed to demonstrate survival advantages [30, 68, 69, 85]. Overall, the different prognostic impact of chemoradiation over radiation alone in patients with recurrent GBM remains to determined in prospective trials.
Another important question to be solved is the potential superiority of concurrent/adjuvant systemic therapy in combination with reirradiation over systemic treatment alone. In a secondary analysis of NRG Oncology/RTOG trial 0525 evaluating dose-dense versus standard dose temozolomide in newly diagnosed GBM, Shi et al. [94] investigated the effect of reirradiation or systemic treatment after tumor progression in 637 patients who received systemic treatment (44%, bevacizumab for almost all patients), reirradiation alone (4%), combined radiation and systemic therapy (10%), or no treatment (42%). Median survival times were 4.8, 8.2, 10.6, and 12.2 months for patients who received no treatment, radiation treatment only (SRS, fractionated RT or brachytherapy), systemic therapy, or radiation and systemic therapy, respectively. Patients receiving no salvage treatment had significantly lower survival than those receiving radiation alone, systemic therapy or a combination of both; however, survival analysis showed no significant difference among patient groups who received some form of treatment. In the NRG Oncology/RTOG 1205 phase II randomized trial evaluating the efficacy and toxicity of hypofractionated SRT and concurrent bevacizumab versus bevacizumab alone in 182 patients with recurrent GBM, Tsien et al. [68] observed an equivalent median survival time of 10.1 months for patients receiving the combined treatment and 9.7 months for those receiving bevacizumab alone; however, the combined treatment was associated with better 6-month progression free survival (54% vs 29%, p < 0.001). The treatment was well tolerated with few acute (5%) and no delayed grade 3 or more toxicity, confirming the safety of reirradiation using modern RT techniques.
Appropriate patient selection is the key to improve clinical outcome. International guidelines recommend to consider reirradiation for recurrent or progressive GBM in young patients with good performance status, especially after a long period from prior radiation [95, 96]. In this regard, recent prognostic scoring models based on patient and tumor characteristics can be used as guidelines for clinical decision.
The risk of severe radiation-induced complications after reirradiation is a major concern in patients previously exposed to high doses of RT, even with the further optimization obtained with the use of stereotactic techniques. Clinical deterioration due to radiation necrosis has been reported in up to 25% of patients. Reirradiation doses and treatment volumes must both be considered when evaluating the risk of brain necrosis. This risk remains generally low (less than 10%) for cumulative EQD2 doses around 100–110 Gy, but may increase up to 25% for cumulative EQD2 > 130 Gy. Beyond the limit of linear quadratic model when comparing the relative biologic effectiveness of large fraction doses with typical conventional fractionated regimens [29], differences in cumulative EQD2 between the reirradiation techniques may, at least in part, explain the higher risk of brain necrosis after SRS and high-dose hypofractionated SRT versus conventionally fractionated or moderately hypofractionated SRT. This means in clinical practice that SRS or high-dose fractionated SRT would be used for small targets of 5–15 ml, while fractionated SRT using 1.8–2.5 Gy fractions should be preferred for large tumors, particularly if located close to eloquent structures. An advantage of fractionated schedules is the use of larger GTV-to-CTV margins to cover microscopic spread of disease compared with SRS, where the extent of tumor is usually defined by the lesion visible on postcontrast T1-weighted MR imaging; in this regard, the use of PET-CT for tumor definition may improve tumor definition and ensure better coverage of tumor [13,14,15,16,17,18,19].
In conclusion, reirradiation has emerged as an effective and safe treatment option for selected patients with recurrent GBM. Using similar biologically equivalent dose, different radiation techniques result in similar survival outcomes. Treatments are associated with a relatively low risk of toxicity when the appropriate radiation technique is carefully chosen on the basis of the size and location of the tumor and in the respect of recommended cumulative dose limits for the brain. Future research includes the use of advanced imaging for tumor definition and the survival impact of different dose-fractionation schedules with or without concomitant/adjuvant systemic therapy. Additionally, studies should report data on tolerance dose of normal brain tissue to reirradiation, as well neurocognitive status and quality of life of patients undergoing the treatment.
Availability of data and materials
All data supporting the results of this review are published in the cited references.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. https://doi.org/10.1056/NEJMoa043330.
Wick W, Weller M. Therapeutic options in recurrent glioblastoma-An update. Crit Rev Oncol Hematol. 2016;99:389–408. https://doi.org/10.1016/j.critrevonc.2016.01.018.
Ryken TC, Kalkanis SN, Buatti JM, Olson JJ, AANS/CNS Joint Guidelines Committee. The role of cytoreductive surgery in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118:479–88. https://doi.org/10.1007/s11060-013-1336-7.
Botros D, Dux H, Price C, Khalafallah AM, Mukherjee D. Assessing the efficacy of repeat resections in recurrent glioblastoma: a systematic review. Neurosurg Rev. 2020. https://doi.org/10.1007/s10143-020-01331-1.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. https://doi.org/10.1200/JCO.2008.19.8721.
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single- agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5. https://doi.org/10.1200/JCO.2008.16.3055.
Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, Bromberg JC, Platten M, Klein M, van den Bent MJ. Lomustine and Bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–63. https://doi.org/10.1056/NEJMoa1707358.
Scaringi C, Agolli L, Minniti G. Technical advances in radiation therapy for brain tumors. Anticancer Res. 2018;38:6041–5. https://doi.org/10.21873/anticanres.12954.
Ryu S, Buatti JM, Morris A, Kalkanis SN, Ryken TC, Olson JJ, AANS/CNS Joint Guidelines Committee. The role of radiotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118:489–99. https://doi.org/10.1007/s11060-013-1337-6.
Kazmi F, Soon YY, Leong YH, Koh WY, Vellayappan B. Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol. 2019;142:79–90. https://doi.org/10.1007/s11060-018-03064-0.
Kim EY, Yechieli R, Kim JK, Mikkelsen T, Kalkanis SN, Rock J, Rosenblum M, Ryu S. Patterns of failure after radiosurgery to two different target volumes of enhancing lesions with and without FLAIR abnormalities in recurrent glioblastoma multiforme. J Neurooncol. 2014;116:291–7. https://doi.org/10.1007/s11060-013-1290-4.
Chan J, Jayamanne D, Wheeler H, Khasraw M, Wong M, Kastelan M, Guo L, Back M. The role of large volume re-irradiation with Bevacizumab in chemorefractory high grade glioma. Clin Transl Radiat Oncol. 2020;22:33–9. https://doi.org/10.1016/j.ctro.2020.03.005.
Grosu AL, Weber WA, Franz M, Stärk S, Piert M, Thamm R, Gumprecht H, Schwaiger M, Molls M, Nieder C. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–9. https://doi.org/10.1016/j.ijrobp.2005.01.056.
Miwa K, Matsuo M, Ogawa S, Shinoda J, Yokoyama K, Yamada J, Yano H, Iwama T. Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol. 2014;9:181. https://doi.org/10.1186/1748-717X-9-181.
Moller S, Law I, Munck A, Rosenschold P, Costa J, Poulsen HS, Engelholm SA, Engelholm S. Prognostic value of 18F-FET PET imaging in re- irradiation of high-grade glioma: results of a phase I clinical trial. Radiother Oncol. 2016;121:132–7. https://doi.org/10.1016/j.radonc.2016.08.014.
Møller S, Munck A, Rosenschöld P, Costa J, Law I, Poulsen HS, Engelholm SA, Engelholm S. Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother Oncol. 2017;125:223–7. https://doi.org/10.1016/j.radonc.2017.09.039.
Popp I, Bott S, Mix M, Oehlke O, Schimek-Jasch T, Nieder C, Nestle U, Bock M, Yuh WTC, Meyer PT, Weber WA, Urbach H, Mader I, Grosu AL. Diffusion-weighted MRI and ADC versus FET-PET and GdT1w-MRI for gross tumor volume (GTV) delineation in re-irradiation of recurrent glioblastoma. Radiother Oncol. 2019;130:121–31. https://doi.org/10.1016/j.radonc.2018.08.019.
Fleischmann DF, Unterrainer M, Corradini S, Rottler M, Förster S, la Fougère C, Siepmann T, Schwaiger M, Bartenstein P, Belka C, Albert NL, Niyazi M. Report of first recurrent glioma patients examined with PET-MRI prior to re- irradiation. PLoS ONE. 2019;14:e0216111. https://doi.org/10.1371/journal.pone.0216111.
Oehlke O, Mix M, Graf E, Schimek-Jasch T, Nestle U, Götz I, Schneider-Fuchs S, Weyerbrock A, Mader I, Baumert BG, Short SC, Meyer PT, Weber WA, Grosu AL. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA)—protocol of a randomized phase II trial (NOA 10/ARO 2013–1). BMC Cancer. 2016;16:769. https://doi.org/10.1186/s12885-016-2806-z.
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–7. https://doi.org/10.1016/j.ijrobp.2009.02.091.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–9.
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. https://doi.org/10.1186/1748-717X-6-48.
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, Osti M, Enrici RM, Esposito V. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95:1142–8.
Timmerman RD. An overview of hypofractionation and i2ntroduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215–22.
Kirkpatrick JP, Marks LB, Mayo CS, Lawrence YR, Bhandare N, Ryu S. Estimating normal tissue toxicity in radiosurgery of the CNS: application and limitations of QUANTEC. J Radiosurg SBRT. 2011;1:95–107.
Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S28-35.
Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys. 2008;70:1350–60. https://doi.org/10.1016/j.ijrobp.2007.08.015.
Sminia P. Mayer R. External beam radiotherapy of recurrent glioma: radiation Cancers (Basel). 2012;4(2):379–99. https://doi.org/10.3390/cancers4020379.
Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240–3.
Fleischmann DF, Jenn J, Corradini S, Ruf V, Herms J, Forbrig R, Unterrainer M, Thon N, Kreth FW, Belka C, Niyazi M. Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiother Oncol. 2019;138:99–105. https://doi.org/10.1016/j.radonc.2019.06.009.
Niyazi M, Karin I, Söhn M, Nachbichler SB, Lang P, Belka C, Ganswindt U. Analysis of equivalent uniform dose (EUD) and conventional radiation treatment parameters after primary and re-irradiation of malignant glioma. Radiat Oncol. 2013;8:287. https://doi.org/10.1186/1748-717X-8-287.
Lu VM, Welby JP, Mahajan A, Laack NN, Daniels DJ. Reirradiation for diffuse intrinsic pontine glioma: a systematic review and meta-analysis. Childs Nerv Syst. 2019;35:739–46. https://doi.org/10.1007/s00381-019-04118-y.
Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S42–9. https://doi.org/10.1016/j.ijrobp.2009.04.095.
Wick W, Krendyukov A, Junge K, Höger T, Fricke H. Longitudinal analysis of quality of life following treatment with Asunercept plus reirradiation versus reirradiation in progressive glioblastoma patients. J Neurooncol. 2019;145:531–40. https://doi.org/10.1007/s11060-019-03320-x.
Larson DA, Prados M, Lamborn KR, Smith V, Sneed PK, Chang S, Nicholas KM, Wara WM, Devriendt D, Kunwar S, Berger M, McDermott MW. Phase II study of high central dose Gamma Knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 2002;54(5):1397–404. https://doi.org/10.1016/s0360-3016(02)03743-4.
Combs SE, Widmer V, Thilmann C, Hof H, Debus J, Schulz-Ertner D. Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM). Cancer. 2005;104(10):2168–73. https://doi.org/10.1002/cncr.21429.
Kong DS, Lee JI, Park K, Kim JH, Lim DH, Nam DH. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer. 2008;112:2046–51. https://doi.org/10.1002/cncr.23402.
Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB, Friedman HS, Willett CG, Kirkpatrick JP. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(5):2018–24. https://doi.org/10.1016/j.ijrobp.2010.12.074.
Patel M, Siddiqui F, Jin JY, Mikkelsen T, Rosenblum M, Movsas B, Ryu S. Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol. 2009;92:185–91. https://doi.org/10.1007/s11060-008-9752-9.
Pouratian N, Crowley RW, Sherman JH, Jagannathan J, Sheehan JP. Gamma Knife radiosurgery after radiation therapy as an adjunctive treatment for glioblastoma. J Neurooncol. 2009;94(409):418. https://doi.org/10.1007/s11060-009-9873-9.
Skeie BS, Enger PØ, Brøgger J, Ganz JC, Thorsen F, Heggdal JI, Pedersen PH. γknife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012;78:658–69. https://doi.org/10.1016/j.wneu.2012.03.024.
Dodoo E, Huffmann B, Peredo I, Grinaker H, Sinclair G, Machinis T, Enger PO, Skeie BS, Pedersen PH, Ohlsson M, Orrego A, Kraepelien T, Barsoum P, Benmakhlouf H, Herrman L, Svensson M, Lippitz B. Increased survival using delayed gamma knife radiosurgery for recurrent high-grade glioma: a feasibility study. World Neurosurg. 2014;82:e623–32. https://doi.org/10.1016/j.wneu.2014.06.011.
Martínez-Carrillo M, Tovar-Martín I, Zurita-Herrera M, Del Moral-Ávila R, Guerrero-Tejada R, Saura-Rojas E, Osorio-Ceballos JL, Arrebola-Moreno JP, Expósito-Hernández J. Salvage radiosurgery for selected patients with recurrent malignant gliomas. Biomed Res Int. 2014;2014:657953. https://doi.org/10.1155/2014/657953.
Pinzi V, Orsi C, Marchetti M, Milanesi IM, Bianchi LC, DiMeco F, Cuccarini V, Farinotti M, Ferroli P, Finocchiaro G, Franzini A, Fumagalli M, Silvani A, Fariselli L. Radiosurgery reirradiation for high-grade glioma recurrence: a retrospective analysis. Neurol Sci. 2015;36:1431–40. https://doi.org/10.1007/s10072-015-2172-7.
Bokstein F, Blumenthal DT, Corn BW, Gez E, Matceyevsky D, Shtraus N, Ram Z, Kanner AA. Stereotactic radiosurgery (SRS) in high-grade glioma: judicious selection of small target volumes improves results. J Neurooncol. 2016;126:551–7. https://doi.org/10.1007/s11060-015-1997-5.
Frischer JM, Marosi C, Woehrer A, Hainfellner JA, Dieckmann KU, Eiter H, Wang WT, Mallouhi A, Ertl A, Knosp E, Filipits M, Kitz K, Gatterbauer B. Gamma knife radiosurgery in recurrent glioblastoma. Stereotact Funct Neurosurg. 2016;94:265–72. https://doi.org/10.1159/000448924.
Imber BS, Kanungo I, Braunstein S, Barani IJ, Fogh SE, Nakamura JL, Berger MS, Chang EF, Molinaro AM, Cabrera JR, McDermott MW, Sneed PK, Aghi MK. Indications and efficacy of gamma knife stereotactic radiosurgery for recurrent glioblastoma: 2 decades of institutional experience. Neurosurgery. 2017;80:129–39. https://doi.org/10.1227/NEU.0000000000001344.
Kim BS, Kong DS, Seol HJ, Nam DH, Lee JI. MGMT promoter methylation status as a prognostic factor or the outcome of gamma knife radiosurgery for recurrent glioblastoma. J Neurooncol. 2017;133:615–22. https://doi.org/10.1007/s11060-017-2478-9.
Sharma M, Schroeder JL, Elson P, Meola A, Barnett GH, Vogelbaum MA, Suh JH, Chao ST, Mohammadi AM, Stevens GHJ, Murphy ES, Angelov L. Outcomes and prognostic stratification of patients with recurrent glioblastoma treated with salvage stereotactic radiosurgery. J Neurosurg. 2018;131:489–99. https://doi.org/10.3171/2018.4.JNS172909.
Morris SL, Zhu P, Rao M, Martir M, Zhu JJ, Hsu S, Ballester LY, Day AL, Tandon N, Kim DH, Shepard S, Blanco A, Esquenazi Y. Gamma knife stereotactic radiosurgery in combination with bevacizumab for recurrent glioblastoma. World Neurosurg. 2019;127:e523–33. https://doi.org/10.1016/j.wneu.2019.03.193.
Conti A, Pontoriero A, Arpa D, Siragusa C, Tomasello C, Romanelli P, Cardali S, Granata F, De Renzis C, Tomasello F. Efficacy and toxicity of CyberKnife re- irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir (Wien). 2012;154:203–9. https://doi.org/10.1007/s00701-011-1184-1.
Park KJ, Kano H, Iyer A, Liu X, Niranjan A, Flickinger JC, Lieberman FS, Lunsford LD, Kondziolka D. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol. 2012;107:323–33. https://doi.org/10.1007/s11060-011-0744-9.
Shanker M, Chua B, Bettington C, Foote MC, Pinkham MB. Re-irradiation for recurrent high-grade gliomas: a systematic review and analysis of treatment technique with respect to survival and risk of radionecrosis. Neurooncol Pract. 2019;6:144–55. https://doi.org/10.1093/nop/npy019.
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys. 2000;47:291–8. https://doi.org/10.1016/s0360-3016(99)00507-6.
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–63. https://doi.org/10.1016/j.ijrobp.2008.10.043.
Henke G, Paulsen F, Steinbach JP, Ganswindt U, Isijanov H, Kortmann RD, Bamberg M, Belka C. Hypofractionated reirradiation for recurrent malignant glioma. Strahlenther Onkol. 2009;185:113–9. https://doi.org/10.1007/s00066-009-1969-9.
Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R. Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol. 2009;185:235–40. https://doi.org/10.1007/s00066-009-1753-x.
Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, Evans JJ, Hyslop T, Pequignot E, Downes B, Comber E, Maltenfort M, Dicker AP, Werner-Wasik M. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–53. https://doi.org/10.1200/JCO.2009.25.6941.
Minniti G, Armosini V, Salvati M, Lanzetta G, Caporello P, Mei M, Osti MF, Maurizi RE. Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. J Neurooncol. 2011;103:683–91. https://doi.org/10.1007/s11060-010-0446-8.
Shapiro LQ, Beal K, Goenka A, Karimi S, Iwamoto FM, Yamada Y, Zhang Z, Lassman AB, Abrey LE, Gutin PH. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85:636–42. https://doi.org/10.1016/j.ijrobp.2012.05.031.
McKenzie JT, Guarnaschelli JN, Vagal AS, Warnick RE, Breneman JC. Hypofractionated stereotactic radiotherapy for unifocal and multifocal recurrence of malignant gliomas. J Neurooncol. 2013;113:403–9. https://doi.org/10.1007/s11060-013-1126-2.
Minniti G, Scaringi C, De Sanctis V, Lanzetta G, Falco T, Di Stefano D, Esposito V, Enrici RM. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol. 2013;111:187–94. https://doi.org/10.1007/s11060-012-0999-9.
Greenspoon JN, Sharieff W, Hirte H, Overholt A, Devillers R, Gunnarsson T, Whitton A. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. Onco Targets Ther. 2014;7:485–90. https://doi.org/10.2147/OTT.S60358.
Minniti G, Agolli L, Falco T, Scaringi C, Lanzetta G, Caporello P, Osti MF, Esposito V, Enrici RM. Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol. 2015;122:559–66. https://doi.org/10.1007/s11060-015-1745-x.
Youland RS, Lee JY, Kreofsky CR, Brown PD, Uhm JH, Laack NN. Modern reirradiation for recurrent gliomas can safely delay tumor progression. Neurooncol Pract. 2018;5:46–55. https://doi.org/10.1093/nop/npx014.
Palmer JD, Bhamidipati D, Song A, Eldredge-Hindy HB, Siglin J, Dan TD, Champ CE, Zhang I, Bar-Ad V, Kim L, Glass J, Evans JJ, Andrews DW, Werner-Wasik M, Shi W. Bevacizumab and re-irradiation for recurrent high grade gliomas: does sequence matter? J Neurooncol. 2018;140:623–8. https://doi.org/10.1007/s11060-018-2989-z.
Schernberg A, Dhermain F, Ammari S, Dumont SN, Domont J, Patrikidou A, Pallud J, Dezamis É, Deutsch É, Louvel G. Reirradiation with concurrent bevacizumab for recurrent high-grade gliomas in adult patients. Cancer Radiother. 2018;22:9–16. https://doi.org/10.1016/j.canrad.2017.06.013.
Tsien C, Pugh S, Dicker AP, Raizer JJ, Matuszak MM, Lallana E, Huang J, Algan O, Taylor N, Portelance L, Villano J, Hamm J, Oh KS, Ali AN Jr, Kim MM, Lindhorst S, Mehta MP. Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): initial outcomes and RT plan quality report. Int J Radiat Oncol Biol Phys. 2019;1(Suppl):S78.
Kaul D, Pudlitz V, Böhmer D, Wust P, Budach V, Grün A. Reirradiation of high-grade gliomas: a retrospective analysis of 198 patients based on the Charité Data Set. Adv Radiat Oncol. 2020;5:959–64. https://doi.org/10.1016/j.adro.2020.06.005.
Saeed AM, Khairnar R, Sharma AM, Larson GL, Tsai HK, Wang CJ, Halasz LM, Chinnaiyan P, Vargas CE, Mishra MV. Clinical outcomes in patients with recurrent glioblastoma treated with proton beam therapy reirradiation: analysis of the multi-institutional proton collaborative group registry. Adv Radiat Oncol. 2020;5:978–83. https://doi.org/10.1016/j.adro.2020.03.022.
Combs SE, Niyazi M, Adeberg S, Bougatf N, Kaul D, Fleischmann DF, Gruen A, Fokas E, Rödel CM, Eckert F, Paulsen F, Oehlke O, Grosu AL, Seidlitz A, Lattermann A, Krause M, Baumann M, Guberina M, Stuschke M, Budach V, Belka C, Debus J, Kessel KA. Re-irradiation of recurrent gliomas: pooled analysis and validation of an established prognostic score-report of the Radiation Oncology Group (ROG) of the German Cancer Consortium (DKTK). Cancer Med. 2018;7:1742–9. https://doi.org/10.1002/cam4.1425.
Navarria P, Minniti G, Clerici E, Tomatis S, Pinzi V, Ciammella P, Galaverni M, Amelio D, Scartoni D, Scoccianti S, Krengli M, Masini L, Draghini L, Maranzano E, Borzillo V, Muto P, Ferrarese F, Fariselli L, Livi L, Pasqualetti F, Fiorentino A, Alongi F, di Monale MB, Magrini S, Scorsetti M. Re-irradiation for recurrent glioma: outcome evaluation, toxicity and prognostic factors assessment. a multicenter study of the Radiation Oncology Italian Association (AIRO). J Neurooncol. 2019;142:59–67. https://doi.org/10.1007/s11060-018-03059-x.
Clarke J, Neil E, Terziev R, Gutin P, Barani I, Kaley T, Lassman AB, Chan TA, Yamada J, DeAngelis L, Ballangrud A, Young R, Panageas KS, Beal K, Omuro A. Multicenter, phase 1, dose escalation study of hypofractionated stereotactic radiation therapy with bevacizumab for recurrent glioblastoma and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 2017;99:797–804. https://doi.org/10.1016/j.ijrobp.2017.06.2466.
Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23(34):8863–9. https://doi.org/10.1200/JCO.2005.03.4157.
Scholtyssek F, Zwiener I, Schlamann A, Seidel C, Meixensberger J, Bauer M, Hoffmann KT, Combs SE, von Bueren AO, Kortmann RD, Müller K. Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol. 2013;8:161. https://doi.org/10.1186/1748-717X-8-161.
Flieger M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, la Fougère C, Ertl L, Linn J, Herrlinger U, Belka C, Niyazi M. Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol. 2014;117:337–45. https://doi.org/10.1007/s11060-014-1394-5.
Wick W, Fricke H, Junge K, Kobyakov G, Martens T, Heese O, Wiestler B, Schliesser MG, von Deimling A, Pichler J, Vetlova E, Harting I, Debus J, Hartmann C, Kunz C, Platten M, Bendszus M, Combs SE. A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014;20:6304–13. https://doi.org/10.1158/1078-0432.CCR-14-0951-T.
Schnell O, Thorsteinsdottir J, Fleischmann DF, Lenski M, Abenhardt W, Giese A, Tonn JC, Belka C, Kreth FW, Niyazi M. Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol. 2016;130:591–9. https://doi.org/10.1007/s11060-016-2267-x.
Shen CJ, Kummerlowe MN, Redmond KJ, Martinez-Gutierrez JC, Usama SM, Holdhoff M, Grossman SA, Laterra JJ, Strowd RE, Kleinberg LR. Re-irradiation for malignant glioma: toward patient selection and defining treatment parameters for salvage. Adv Radiat Oncol. 2018;3:582–90. https://doi.org/10.1016/j.adro.2018.06.005.
Arcicasa M, Roncadin M, Bidoli E, Dedkov A, Gigante M, Trovò MG. Reirradiation and lomustine in patients with relapsed high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;43:789–93. https://doi.org/10.1016/s0360-3016(98)00457-x (PMID: 10098434).
Cho KH, Hall WA, Gerbi BJ, Higgins PD, McGuire WA, Clark HB. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;45:1133–41. https://doi.org/10.1016/s0360-3016(99)00336-3.
Vordermark D, Kölbl O, Ruprecht K, Vince GH, Bratengeier K, Flentje M. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005;5:55. https://doi.org/10.1186/1471-2407-5-55.
Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–6. https://doi.org/10.1200/JCO.2006.08.1661.PMID:17577040;PMCID:PMC4118746.
Palmer JD, Siglin J, Yamoah K, Dan T, Champ CE, Bar-Ad V, Werner-Wasik M, Evans JJ, Kim L, Glass J, Farrell C, Andrews DW, Shi W. Re-resection for recurrent high-grade glioma in the setting of re-irradiation: more is not always better. J Neurooncol. 2015;124:215–21. https://doi.org/10.1007/s11060-015-1825-y.
Chapman CH, Hara JH, Molinaro AM, Clarke JL, Oberheim Bush NA, Taylor JW, Butowski NA, Chang SM, Fogh SE, Sneed PK, Nakamura JL, Raleigh DR, Braunstein SE. Reirradiation of recurrent high-grade glioma and development of prognostic scores for progression and survival. Neurooncol Pract. 2019;6:364–74. https://doi.org/10.1093/nop/npz017.
Combs SE, Edler L, Rausch R, Welzel T, Wick W, Debus J. Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol. 2013;52:147–52. https://doi.org/10.3109/0284186X.2012.692882.
Niyazi M, Flieger M, Ganswindt U, Combs SE, Belka C. Validation of the prognostic Heidelberg re-irradiation score in an independent mono-institutional patient cohort. Radiat Oncol. 2014;9:128. https://doi.org/10.1186/1748-717X-9-128.
Müller K, Henke G, Compter I, von Bueren AO, Friedrich C, Janssens G, Kramm CM, Hundsberger T, Paulsen F, Kortmann RD, Zwiener I, Baumert BG. External validation of a prognostic model estimating the survival of patients with recurrent high-grade gliomas after reirradiation. Pract Radiat Oncol. 2015;5:e143–50. https://doi.org/10.1016/j.prro.2014.10.001.
Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, Schlegel J, Meyer B, Combs SE. Modification and optimization of an established prognostic score after re-irradiation of recurrent glioma. PLoS ONE. 2017a;12:e0180457. https://doi.org/10.1371/journal.pone.0180457.
Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, Schlegel J, Meyer B, Combs SE. Validation of an established prognostic score after re-irradiation of recurrent glioma. Acta Oncol. 2017b;56:422–6. https://doi.org/10.1080/0284186X.2016.1276621.
Krauze AV, Peters C, Cheng J, Ning H, Mackey M, Rowe L, Cooley-Zgela T, Smart DD, Camphausen K. Re-irradiation for recurrent glioma- the NCI experience in tumor control, OAR toxicity and proposal of a novel prognostic scoring system. Radiat Oncol. 2017;12:191. https://doi.org/10.1186/s13014-017-0930-9.
Niyazi M, Adeberg S, Kaul D, Boulesteix AL, Bougatf N, Fleischmann DF, Grün A, Krämer A, Rödel C, Eckert F, Paulsen F, Kessel KA, Combs SE, Oehlke O, Grosu AL, Seidlitz A, Lattermann A, Krause M, Baumann M, Guberina M, Stuschke M, Budach V, Belka C, Debus J. Independent validation of a new reirradiation risk score (RRRS) for glioma patients predicting post-recurrence survival: A multicenter DKTK/ROG analysis. Radiother Oncol. 2018;127:121–7. https://doi.org/10.1016/j.radonc.2018.01.011.
Post CCB, Kramer MCA, Smid EJ, van der Weide HL, Kleynen CE, Heesters MAAM, Verhoeff JJC. Patterns of re-irradiation for recurrent gliomas and validation of a prognostic score. Radiother Oncol. 2019;130:156–63. https://doi.org/10.1016/j.radonc.2018.10.034.
Shi W, Scannell Bryan M, Gilbert MR, Mehta MP, Blumenthal DT, Brown PD, Valeinis E, Hopkins K, Souhami L, Andrews DW, Tzuk-Shina T, Howard SP, Youssef EF, Lessard N, Dignam JJ, Werner-Wasik M. Investigating the effect of reirradiation or systemic therapy in patients with glioblastoma after tumor progression: a secondary analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int J Radiat Oncol Biol Phys. 2018;100:38–44. https://doi.org/10.1016/j.ijrobp.2017.08.038.
Sulman EP, Ismaila N, Armstrong TS, Tsien C, Batchelor TT, Cloughesy T, Galanis E, Gilbert M, Gondi V, Lovely M, Mehta M, Mumber MP, Sloan A, Chang SM. Radiation therapy for glioblastoma: American Society of Clinical Oncology Clinical Practice guideline endorsement of the American Society for Radiation Oncology Guideline. J Clin Oncol. 2017;35:361–9. https://doi.org/10.1200/JCO.2016.70.7562.
Nccn guidelines version 1.2013, anaplastic gliomas/glioblastoma. 2013. http://www.Nccn.Org/professionals/physician_gls/pdf/cns.Pdf.
Acknowledgements
Not applicable.
Funding
This research received no funding.
Author information
Authors and Affiliations
Contributions
G.M. and M.N. designed and drafted the manuscript, performed literature research and data extraction. F.A., and P.N. reviewed the manuscript. C.B.: designed and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable (literature review).
Consent for publication
Not applicable.
Competing interests
CB received speaker honoraria and research grants from ELEKTA AB (Stockholm, Sweden). The other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Minniti, G., Niyazi, M., Alongi, F. et al. Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol 16, 36 (2021). https://doi.org/10.1186/s13014-021-01767-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-021-01767-9