Abstract
Bovine leukemia virus (BLV) causes enzootic bovine leukosis and is closely related to the human T-lymphotropic virus. Bovine major histocompatibility complex (BoLAs) are used extensively as markers of disease and immunological traits in cattle. For BLV diagnosis, proviral load is a major diagnosis index for the determination of disease progression and transmission risk. Therefore, we investigated the frequency of BoLA-DRB3 alleles, BoLA-DQA1 alleles, and haplotypes of BoLA class II isolated from the heads of 910 BLV-infected cows out of 1290 cows assessed from BLV-positive farms, in a nationwide survey from 2011 to 2014 in Japan. Our aim was to identify BoLA class II polymorphisms associated with the BLV proviral load in the Holstein cow. The study examined 569 cows with a high proviral load and 341 cows with a low proviral load. Using the highest odds ratio (OR) as a comparison index, we confirmed that BoLA-DRB3 was the best marker for determining which cow spread the BLV (OR 13.9 for BoLA-DRB3, OR 11.5 for BoLA-DQA1, and OR 6.2 for BoLA class II haplotype). In addition, DRB3*002:01, *009:02, *012:01, *014:01, and *015:01 were determined as BLV provirus associated alleles. BoLA-DRB3*002:01, *009:02, and *014:01 were determined as resistant alleles (OR > 1), and BoLA-DRB3*012:01 and *015:01 were determined as susceptible alleles (OR < 1). In this study, we showed that BoLA-DRB3 was a good marker for determining which cow spread BLV, and we found not only one resistant allele (BoLA-DRB3*009:02), but also two other disease-resistant alleles and two disease-susceptible alleles. This designation of major alleles as markers of susceptibility or resistance can allow the determination of the susceptibility or resistance of most cows to disease. Overall, the results of this study may be useful in eliminating BLV from farms without having to separate cows into several cowsheds.
Similar content being viewed by others
Bovine leukemia virus (BLV), which is the causative agent of enzootic bovine leukosis (EBL), belongs to the family Retroviridae (genus Deltaretrovirus), together with human T-lymphotropic virus types 1 and 2 (HTLV-1 and -2) [1]. At present, BLV is widely distributed in cow populations [2,3,4,5,6,7]. The virus was identified in 1969 as an infectious retrovirus, and it induces CD5+B- cell leukemia/lymphoma in 1% to 5% of infected cows of 5 to 10 years of age [1]. Therefore, the virus was categorized as a non-severe infectious disease in several regions, including USA, South America, and some Asian countries [8]. This decision has resulted in the number of BLV-infected cows increasing in these areas; for example, in Japan, almost 40% of cows are infected [9], and in the USA, 80% of farms have become BLV-positive [8]. At present, BLV elimination is quite difficult, due to its high infection rate and the existence of heavily infected cows. Moreover, recently emerged BLV infections can cause earlier EBL onset, high accident rates, and low rates of conception and low milk production [6, 10,11,12,13,14,15]. Therefore, techniques for identifying high-risk cows are urgently needed to mitigate economic losses. It has been posited that cows classified as high-risk show a proviral load of over 14,000 copies/105 cells and 18,000 copies/105 cells in blood samples secreting BLV provirus into nasal and saliva, respectively [16]. It has been suggested that these cows may cause a high-risk for BLV transmission via coming into direct contact with healthy cows. In addition, it appears that proviral load correlates not only with BLV infection, but also with BLV disease progression [17,18,19]. Thus, BLV proviral load is an important index for estimating the stage of BLV infection.
Studies on BLV-associated host factors identified polymorphisms within the bovine major histocompatibility complex (MHC) (BoLA) [20,21,22,23,24,25,26,27,28,29]. BoLA is a highly polymorphic and tightly linked gene cluster [30]. Functionally, the BoLA class II gene is classified into two groups, DR and DQ. The DR molecule was constructed from a single DRA locus and a single DRB3 locus, and DQ molecules were constructed from at least two DQA loci and two DQB loci [31]. To date, 136 DRB3, 65 DQA, and 87 DQB alleles have been registered on the IPD-MHC database (http://www.ebi.ac.uk/ipd/mhc/bola). Recently BLV proviral load quantification methods have been developed [17, 32] and several studies have successfully identified SNPs or BoLA-DRB3 alleles that are associated with increasing or suppressing the BLV provirus load [27,28,29, 33,34,35,36]. However, the results of association studies that compared the frequencies of BoLA alleles in low proviral load cows with those in high proviral load cows were strongly affected by the allele frequencies in normal cows, and there is little information on how the allele frequencies were stable year-on-year in a countries.
For BLV diagnosis, proviral load is one of the major diagnosis indices for determining disease progression and transmission risk. Therefore, in this study, we investigated BoLA-DRB3 and BoLA-DQA1 allele frequencies in Japan over 4 years, performed an association study using the specific BoLA class II allele to determine BLV provirus load in Holstein cows, and determined proviral load-associated polymorphisms using cow which collected among 4 years.
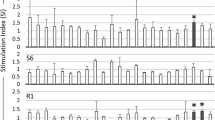
We collected blood samples from 1290 cow heads over 6 months old from BLV-positive farms in a nationwide survey in Japan from 2011 to 2014, isolated genomic DNA and sera from peripheral blood. Cows determined as BLV-positive by anti-BLV gp51 antibody ELISA kit using sera (JNC Corporation, Kanagwa Japan) (Table 1) and the BLV proviral load measured by the BLV-CoCoMo-qPCR-2 method [32] using genomic DNA. First, we confirmed the allele frequencies of BoLA-DRB3 gene in each 4 years and confirmed the allele frequency is stable in these 4 years in Holstein in Japan (Fig. 1). Next, our previous report showed that cows with a detected proviral load of over 14,000 copies/105 cells (as determined by the BLV-CoCoMo-qPCR-2 method) secreted BLV provirus into nasal secretions [16]. Thus, these cows may be high-risk transmitters. Therefore, we here categorized the 910 BLV-infected cows into two groups, as follows: (i) cows with proviral load over 10,000 copies/105 cells—high-risk BLV spreader cows, and (ii) cows with proviral load under 10,000 copies/105 cells—low-risk BLV spreader cows (Table 1). The 910 cow heads tested were separated into 341 heads of “low-risk spreaders” and 569 heads of “high-risk spreaders.”
Transition of BoLA-DRB3 allele frequencies of Holstein cow in Japan from 2011 to 2014. DNA samples were collected from the blood of 1290 Holstein cows belonging to BLV-positive commercial dairy farms located in the 23 prefectures of Japan, from 2011 to 2014 by Ohno et al. [18] and genotyped for BoLA-DRB3 alleles with PCR-sequence based typing (SBT) developed previously [37]. In total, 644 alleles were detected at 322 cows in 2011, 780 alleles at 390 cow in 2012, 580 alleles at 290 cow in 2013 and 576 alleles at 288 cow in 2014
Next, these 910 cows were subjected to BoLA-DRB3 genotyping using a PCR-sequence-based typing (PCR-SBT) method [37]. From 910 BLV-positive cows, a total of 1820 BoLA-DRB3 alleles were detected, which were classified into 23 types of known BoLA-DRB3 alleles (Fig. 2). BoLA-DRB3 allele frequencies of these two groups, i.e., 682 alleles originating from low-risk spreaders and 1138 alleles originating from high-risk spreaders, were calculated, and estimated p values and odds ratios (ORs) for each BoLA-DRB3 allele in the two spreader groups were compared (Fig. 2). If the allele which significantly low frequency in low risk spreader than high risk spreader (OR > 1), we determined that the allele was resistant allele. Moreover, in the case that the allele which significantly high frequency in high risk spreader than low risk spreader (OR > 1), the allele was determined as susceptible allele. From these 23 BoLA-DRB3 alleles, DRB3*002:01, DRB3*009:02, DRB3*012:01, DRB3*014:01:01, and DRB3*015:01 were determined as BLV provirus-associated alleles. BoLA-DRB3*002:01, DRB3*009:02, and DRB3*014:01:01 were determined to be alleles associated with BLV resistance (OR > 1), whereas BoLA-DRB3*012:01 and DRB3*015:01 were determined to be alleles associated with BLV susceptibility (OR < 1).
Association study to determine the significance of the detection frequency of the BoLA-DRB3 allele compared with those of 682 alleles from low-risk spreaders and 1138 alleles from high-risk spreaders.. BLV-positive cows were diagnosed using an anti-BLV antibody ELISA Kit (JNC, Tokyo, Japan), which is used to detect anti-Env gp51 antibodies from serum samples, according to the manufacturer’s instructions. BLV-positive cows were subjected to BLV proviral load calculation using the BLV-CoCoMo-qPCR-2 system (RIKEN Genesis, Kanagawa, Japan) [32]. The 910 BLV-infected cows were classified into two groups: (i) cows with proviral load over 10,000 as high-risk BLV spreader cows (N = 569), and (ii) cows with proviral load under 10,000 as low-risk BLV spreader cows (N = 341). Fisher’s exact test was performed for estimating p values and odds ratios (ORs) were used for detecting the association of each BoLA-DRB3 allele and BLV proviral load, using R version 3.4.2 statistical analysis software [43]. The X-axis shows the allele number for BoLA-DRB3 and the Y-axis shows the minus multiplied common logarithm for p values. The alleles with p values < 0.00217 (0.05/23 alleles, the statistical significance threshold) were determined to be resistant when OR > 1 and susceptible when OR < 1
There are DR and DQ genes embedded in the BoLA class II region, and these genes were closely linked to each other [30]. Indeed, we previously identified 39 DRB3-DQA1 haplotypes in 507 Japanese Black cows [38]. Therefore, to determine the effect of other class II genes, we genotyped the second polymorphic class II genes, such as the DQA1 gene. The 910 Japanese Holstein cow heads were subjected to genotyping of the BoLA-DQA1 gene using a PCR-SBT method [39] and 899 cows were succeeded to genotyping for BoLA-DQA1 alleles. These 899 cows were divided into low-risk (N = 336) and high-risk spreaders (N = 563), based on whether their proviral load was under or over 10,000 copies/105 cells, respectively. In total, 1798 BoLA-DQA1 alleles were detected, and these alleles were assigned as one of 14 kinds of known BoLA-DQA1 alleles (Fig. 3). BoLA-DQA1 allele frequencies of these two groups (672 alleles originating from low-risk spreaders and 1126 alleles originating from high-risk spreaders), were analyzed using Fisher’s exact test. Three kinds of BoLA-DQA1 allele—DQA1*002:04, DQA1*012:01:01, and DQA1*014:02—were significantly associated with the high proviral load (Fig. 3). DQA1*002:04 and DQA1*014:02 showed ORs > 1 (12.8 and 2.47, respectively), as these two alleles were disease resistant. Conversely, the OR of DQA1*012:01 was 0.34 and the allele indicated disease susceptibility.
Association study to determine the significance of detection frequency of the BoLA-DQA1 allele compared with 672 alleles from low-risk spreaders and 1126 alleles from high-risk spreaders. The same samples as described in Fig. 1 were genotyped for BoLA-DQA1 alleles with PCR-sequence based typing (SBT) developed previously [39]. Fisher’s exact test was performed for estimating p values and odds ratios (ORs) were used to detect the association of BoLA-DQA1 alleles with BLV proviral load. The X-axis shows the allele number for BoLA-DQA and Y-axis shows the minus multiplied common logarithm for p values. The alleles with p values < 0.00357 (0.05/14 alleles) were determined as resistant when OR > 1 and susceptible when OR < 1
Notably, DRB3 and DQA1 were highly linked [38]: for example, DRB3*009:02 was linked with DQA1*002:04 and DRB3*014:01:01 was linked with DQA1*014:02 in Japanese Holstein cows [35]. Therefore, we identified that DRB3*009:02-DQA1*002:04 and DRB3*014:01:01-DQA1*014:02 haplotypes were indicated disease resistance. Table 2 shows that animals with the resistant haplotype were detected at a significantly higher level in the low proviral load group compared with the high proviral load group. However, the OR was lower when the DRB3-DQA1 haplotype was used as a marker (OR 6.16) than when the DRB3 allele alone was used (OR 13.88).
In this study, we used three markers, BoLA-DRB3, BoLA-DQA1, and BoLA class II haplotypes, to determine the risk of BLV spread through cows in the farm environment. Using the biggest OR as a comparison index, we confirmed that BoLA-DRB3 was the best marker for determining which cow spread the BLV (OR 13.9 for BoLA-DRB3, OR 11.5 for BoLA-DQA1, and OR 6.2 for BoLA class II haplotype).
The most strongly associated allele was BoLA-DRB3*009:02, which was determined to be a BLV-resistant allele in our study, and was also detected in several studies, such as those by Julliarena et al. [36], Miyasaka et al [35], Forletti et al [40], Lutzelscheab et al. [21], Carignano et al [34], and Hayashi et al [33]. Moreover, we explored the other resistant alleles, BoLA-DRB3*002:01 and DRB3*014:01:01, and susceptible alleles, DRB3*012:01 and DRB3*015:01. The effects of these alleles were weaker than that of BoLA-DRB3*009:02, but they were more frequently detected in the farm [41]. Therefore, obtaining information about these common alleles is more important than obtaining information about rare alleles. It is true that the PVL we determined is only about single time point, the PVL may be changing in future. However, in our limited data in lab, the PVL tends to be stable at least 6 months. Needs more research to confirm how long the PVL shows stable. As the BLV PVL is the most variable quantitative index for assessing the risk of BLV transmission [42], the information about disease susceptible and resistant alleles may be useful to eliminate BLV from the farm without separating cows into several sheds.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional file.
Abbreviations
- BLV:
-
bovine leukemia virus
- BoLA:
-
bovine leukocyte antigen
- EBL:
-
enzootic bovine leukosis
- HTLV:
-
human T-lymphotropic virus
- MHC:
-
major histocompatibility complex
- OR:
-
odds ratio
- PCR-SBT:
-
PCR-sequence based typing
- PVL:
-
proviral load
References
Aida Y, Murakami H, Takahashi M, Takeshima SN. Mechanisms of pathogenesis induced by bovine leukemia virus as a model for human T-cell leukemia virus. Front Microbiol. 2013;4:328.
Erskine RJ, Bartlett PC, Byrem TM, Render CL, Febvay C, Houseman JT. Using a herd profile to determine age-specific prevalence of bovine leukemia virus in michigan dairy herds. Vet Med Int. 2012;2012:350374.
Polat M, Ohno A, Takeshima SN, Kim J, Kikuya M, Matsumoto Y, Mingala CN, Onuma M, Aida Y. Detection and molecular characterization of bovine leukemia virus in Philippine cattle. Arch Virol. 2015;160:285–96.
Polat M, Takeshima SN, Hosomichi K, Kim J, Miyasaka T, Yamada K, Arainga M, Murakami T, Matsumoto Y, de la Barra Diaz V, et al. A new genotype of bovine leukemia virus in South America identified by NGS-based whole genome sequencing and molecular evolutionary genetic analysis. Retrovirology. 2016;13:4.
Polat M, Moe HH, Shimogiri T, Moe KK, Takeshima SN, Aida Y. The molecular epidemiological study of bovine leukemia virus infection in Myanmar cattle. Arch Virol. 2017;162:425–37.
Yang Y, Fan W, Mao Y, Yang Z, Lu G, Zhang R, Zhang H, Szeto C, Wang C. Bovine leukemia virus infection in cattle of China: association with reduced milk production and increased somatic cell score. J Dairy Sci. 2016;99:3688–97.
Polat M, Takeshima SN, Aida Y. Epidemiology and genetic diversity of bovine leukemia virus. Virol J. 2017;14:209.
APHIS: Bovine leukosis virus (BLV) on U.S. dairy operations, 2007. Veterinary services 2008.
Murakami K, Kobayashi S, Konishi M, Kameyama K-I, Tsutsui T. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009–2011. J Vet Med Sci. 2013;75:1123–6.
Emanuelson U, Scherling K, Pettersson H. Relationships between herd bovine leukemia-virus infection status and reproduction, disease incidence, and productivity in Swedish dairy herds. Prev Vet Med. 1992;12:121–31.
Da Y, Shanks RD, Stewart JA, Lewin HA. Milk and fat yields decline in bovine leukemia virus-infected Holstein cattle with persistent lymphocytosis. Proc Natl Acad Sci USA. 1993;90:6538–41.
Norby B, Bartlett PC, Byrem TM, Erskine RJ. Effect of infection with bovine leukemia virus on milk production in Michigan dairy cows. J Dairy Sci. 2016;99:2043–52.
Nekouei O, VanLeeuwen J, Stryhn H, Kelton D, Keefe G. Lifetime effects of infection with bovine leukemia virus on longevity and milk production of dairy cows. Prev Vet Med. 2016;133:1–9.
Sandev N, Koleva M, Binev R, Ilieva D. Influence of enzootic bovine leukosis virus upon the incidence of subclinical mastitis in cows at a different stage of infection. Vet Arh. 2004;74:411–6.
Bartlett PC, Norby B, Byrem TM, Parmelee A, Ledergerber JT, Erskine RJ. Bovine leukemia virus and cow longevity in Michigan dairy herds. J Dairy Sci. 2013;96:1591–7.
Yuan Y, Kitamura-Muramatsu Y, Saito S, Ishizaki H, Nakano M, Haga S, Matoba K, Ohno A, Murakami H, Takeshima SN, Aida Y. Detection of the BLV provirus from nasal secretion and saliva samples using BLV-CoCoMo-qPCR-2: comparison with blood samples from the same cattle. Virus Res. 2015;210:248–54.
Jimba M, Takeshima SN, Matoba K, Endoh D, Aida Y. BLV-CoCoMo-qPCR: quantitation of bovine leukemia virus proviral load using the CoCoMo algorithm. Retrovirology. 2010;7:91.
Ohno A, Takeshima SN, Matsumoto Y, Aida Y. Risk factors associated with increased bovine leukemia virus proviral load in infected cattle in Japan from 2012 to 2014. Virus Res. 2015;210:283–90.
Sato H, Watanuki S, Murakami H, Sato R, Ishizaki H, Aida Y. Development of a luminescence syncytium induction assay (LuSIA) for easily detecting and quantitatively measuring bovine leukemia virus infection. Arch Virol. 2018;163:1519–30.
Nikbakht Brujeni G, Ghorbanpour R, Esmailnejad A. Association of BoLA-DRB3.2 alleles with BLV infection profiles (Persistent Lymphocytosis/Lymphosarcoma) and Lymphocyte subsets in Iranian Holstein Cattle. Biochem Genet. 2016;54:194–207.
Lutzelschwab CM, Forletti A, Cepeda R, Esteban EN, Confalonieri O, Gutierrez SE. Co-infection with Mycobacterium bovis does not alter the response to bovine leukemia virus in BoLA DRB3*0902, genetically resistant cattle. Res Vet Sci. 2016;109:10–6.
Lewin HA, Wu MC, Stewart JA, Nolan TJ. Association between BoLA and subclinical bovine leukemia virus infection in a herd of Holstein-Friesian cows. Immunogenetics. 1988;27:338–44.
Lewin HA, Russell GC, Glass EJ. Comparative organization and function of the major histocompatibility complex of domesticated cattle. Immunol Rev. 1999;167:145–58.
Zanotti M, Poli G, Ponti W, Polli M, Rocchi M, Bolzani E, Longeri M, Russo S, Lewin HA, van Eijk MJ. Association of BoLA class II haplotypes with subclinical progression of bovine leukaemia virus infection in Holstein-Friesian cattle. Anim Genet. 1996;27:337–41.
Xu A, van Eijk MJ, Park C, Lewin HA. Polymorphism in BoLA-DRB3 exon 2 correlates with resistance to persistent lymphocytosis caused by bovine leukemia virus. J Immunol. 1993;151:6977–85.
Lewin HA, Bernoco D. Evidence for BoLA-linked resistance and susceptibility to subclinical progression of bovine leukaemia virus infection. Anim Genet. 1986;17:197–207.
Brym P, Bojarojc-Nosowicz B, Olenski K, Hering DM, Rusc A, Kaczmarczyk E, Kaminski S. Genome-wide association study for host response to bovine leukemia virus in Holstein cows. Vet Immunol Immunopathol. 2016;175:24–35.
Takeshima SN, Sasaki S, Meripet P, Sugimoto Y, Aida Y. Single nucleotide polymorphisms in the bovine MHC region of Japanese Black cattle are associated with bovine leukemia virus proviral load. Retrovirology. 2017;14:24.
Carignano HA, Roldan DL, Beribe MJ, Raschia MA, Amadio A, Nani JP, Gutierrez G, Alvarez I, Trono K, Poli MA, Miretti MM. Genome-wide scan for commons SNPs affecting bovine leukemia virus infection level in dairy cattle. BMC Genom. 2018;19:142.
Elsik CG, Unni DR, Diesh CM, Tayal A, Emery ML, Nguyen HN, Hagen DE. Bovine Genome Database: new tools for gleaning function from the Bos taurus genome. Nucleic Acids Res. 2016;44:D834–9.
Aida Y. Characterization and expression of bovine MHC class II genes. Bull Soc Fr Jpn Sci Vet. 1995;6:17–24.
Takeshima SN, Kitamura-Muramatsu Y, Yuan Y, Polat M, Saito S, Aida Y. BLV-CoCoMo-qPCR-2: improvements to the BLV-CoCoMo-qPCR assay for bovine leukemia virus by reducing primer degeneracy and constructing an optimal standard curve. Arch Virol. 2015;160:1325–32.
Hayashi T, Mekata H, Sekiguchi S, Kirino Y, Mitoma S, Honkawa K, Horii Y, Norimine J. Cattle with the BoLA class II DRB3*0902 allele have significantly lower bovine leukemia proviral loads. J Vet Med Sci. 2017;79:1552–5.
Carignano HA, Beribe MJ, Caffaro ME, Amadio A, Nani JP, Gutierrez G, Alvarez I, Trono K, Miretti MM, Poli MA. BOLA-DRB3 gene polymorphisms influence bovine leukaemia virus infection levels in Holstein and Holstein x Jersey crossbreed dairy cattle. Anim Genet. 2017;48:420–30.
Miyasaka T, Takeshima SN, Jimba M, Matsumoto Y, Kobayashi N, Matsuhashi T, Sentsui H, Aida Y. Identification of bovine leukocyte antigen class II haplotypes associated with variations in bovine leukemia virus proviral load in Japanese Black cattle. Tissue Antigens. 2013;81:72–82.
Juliarena MA, Poli M, Sala L, Ceriani C, Gutierrez S, Dolcini G, Rodriguez EM, Marino B, Rodriguez-Dubra C, Esteban EN. Association of BLV infection profiles with alleles of the BoLA-DRB3.2 gene. Anim Genet. 2008;39:432–8.
Takeshima SN, Matsumoto Y, Miyasaka T, Arainga-Ramirez M, Saito H, Onuma M, Aida Y. A new method for typing bovine major histocompatibility complex class II DRB3 alleles by combining two established PCR sequence-based techniques. Tissue Antigens. 2011;78:208–13.
Miyasaka T, Takeshima SN, Sentsui H, Aida Y. Identification and diversity of bovine major histocompatibility complex class II haplotypes in Japanese Black and Holstein cattle in Japan. J Dairy Sci. 2012;95:420–31.
Takeshima S, Miki A, Kado M, Aida Y. Establishment of a sequence-based typing system for BoLA-DQA1 exon 2. Tissue Antigens. 2007;69:189–99.
Forletti A, Juliarena MA, Ceriani C, Amadio AF, Esteban E, Gutierrez SE. Identification of cattle carrying alleles associated with resistance and susceptibility to the Bovine Leukemia Virus progression by real-time PCR. Res Vet Sci. 2013;95:991–5.
Takeshima S, Saitou N, Morita M, Inoko H, Aida Y. The diversity of bovine MHC class II DRB3 genes in Japanese Black, Japanese Shorthorn, Jersey and Holstein cattle in Japan. Gene. 2003;316:111–8.
Juliarena MA, Barrios CN, Ceriani MC, Esteban EN. Hot topic: bovine leukemia virus (BLV)-infected cows with low proviral load are not a source of infection for BLV-free cattle. J Dairy Sci. 2016;99:4586–9.
Team RC: R: A Language and Environment for Statistical Computing. (Team RDC ed.: R Foundation for Statistical Computing; 2017.
Acknowledgements
The authors thank the veterinary officers of the prefectural Livestock Hygiene Service Centers for their help with blood sampling and collection of epidemiological data. We also thank Miss. Yiki Matsumoto, Mrs. Mari Kikuya, Miss. Yuka Takahashi, Mrs. Sonoko Abe, and other members of the Viral Infectious Diseases Unit, RIKEN, for technical assistance, help, and suggestions. We thank the Support Unit at the Biomaterial Analysis, RIKEN BSI Research Resource Center for help with sequence analysis.
Funding
The study was supported by Grants-in-Aid for Scientific Research (A and C) from the Japan Society for the Promotion of Science (JSPS) (Grant Nos. 16H02590 and 16K08039) and by MAFF-commissioned research “the Strategic Improvement project of the national Surveillance and Diagnosis system for Animal (SISDA)”. This research was also supported by grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution [the Special Scheme Project on Regional Developing Strategy (Grant No. 16817983) and the Special Scheme Project on Vitalizing Management Entities of Agriculture, Forestry and Fisheries (Grant No. 16930548)].
Author information
Authors and Affiliations
Contributions
Study conception and design: YA and ST. Data acquisition, analysis, and interpretation: ST and AO. Contribution of reagents/materials/analysis tools: YA. Drafting and revising the manuscript: ST and YA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animals were handled by veterinarians from the veterinary officers of the prefectural Livestock Hygiene Service Centers, and RIKEN, Japan in strict accordance with good animal practice following the guidelines of RIKEN. The study was approved by the RIKEN Animal Experiments Committee (approval number H29-2-104).
Consent for publication
Signed informed consents were obtained from the study subjects
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Takeshima, Sn., Ohno, A. & Aida, Y. Bovine leukemia virus proviral load is more strongly associated with bovine major histocompatibility complex class II DRB3 polymorphism than with DQA1 polymorphism in Holstein cow in Japan. Retrovirology 16, 14 (2019). https://doi.org/10.1186/s12977-019-0476-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12977-019-0476-z