Abstract
Critical limb ischemia (CLI) causes severe ischemic rest pain, ulcer, and gangrene in the lower limbs. In spite of angioplasty and surgery, CLI patients without suitable artery inflow or enough vascular bed in the lesions are often forced to undergo amputation of a major limb. Cell-based therapeutic angiogenesis has the potential to treat ischemic lesions by promoting the formation of collateral vessel networks and the vascular bed. Peripheral blood mononuclear cells and bone marrow-derived mononuclear cells are the most frequently employed cell types in CLI clinical trials. However, the clinical outcomes of cell-based therapeutic angiogenesis using these cells have not provided the promised benefits for CLI patients, reinforcing the need for novel cell-based therapeutic angiogenesis strategies to cure untreatable CLI patients. Recent studies have demonstrated the possible enhancement of therapeutic efficacy in ischemic diseases by preconditioned graft cells. Moreover, judging from past clinical trials, the identification of adequate transplant timing and responders to cell-based therapy is important for improving therapeutic outcomes in CLI patients in clinical settings. Thus, to establish cell-based therapeutic angiogenesis as one of the most promising therapeutic strategies for CLI patients, its advantages and limitations should be taken into account.
Similar content being viewed by others
Background
Peripheral artery disease (PAD), also called peripheral vascular disease, is characterized by the narrowing of blood vessels, which leads to impaired blood supply to the organs. PAD is caused mostly by atherosclerosis obliterans (ASO) and thromboangiitis obliterans (TAO). Owing to changes in lifestyle, the number of TAO patients is decreasing, while that of ASO patients is increasing. Consequently, as PAD is thought to develop mostly from ASO, the worldwide prevalence of PAD is expected to increase [1].
Critical limb ischemia (CLI) is clinically defined as the chronic and severe stagnation of limb perfusion, its ultimate outcomes being tissue ulceration and gangrene. CLI is commonly caused by PAD and is the disease of arteries of all range size. It can cause diabetic microangiopathy and vasculitis, and is associated with a high risk of cerebro-cardiovascular events, including myocardial infarction and stroke. Accordingly, it presents poor prognosis and high mortality: 20% within 6 months and 50% within 5 years of the diagnosis [2–4]. Surgical bypass and angioplasty for limb revascularization are the gold standards for CLI. However, about 20–30% of patients with CLI are ineligible for these therapies because of severe calcification of the arteries, lack of suitable target arteries and vein graft, and extensive comorbidities [5, 6]. Unfortunately, major limb amputation is required within 1 year for as many as 40% of untreatable CLI patients [3, 7]. Consequently, the development of alternative therapeutic strategies for these high-risk patients is strongly desired.
Therapeutic angiogenesis, which can be induced by delivery of protein(s), gene(s), or cell(s) to ischemic tissues, offers the possibility of blood flow recovery in ischemic limbs, thus sparing CLI patients from major limb amputation [8, 9]. For example, gene delivery of vascular endothelial growth factor (VEGF) resulted in a significant improvement in hemodynamics and skin ulcer in CLI patients, even though there was no significant reduction in the amputation rate after 100 days of treatment [10]. This small randomized trial introduced the possibility of gene delivery-mediated therapeutic angiogenesis for CLI. However, virus-mediated gene therapy often supplies a transient excess of pro-angiogenic factors to ischemic and non-ischemic tissues. This increases the risk of side effects such as malignant alteration of tumors [11]. Although rapid and remarkable advances have been made in gene therapy, suitable gene delivery methods should be developed to reduce excessive pro-angiogenic factors in clinical settings. In contrast, cell delivery strategies, namely cell-based therapies, enable the stable supply of growth factors/cytokines for angiogenesis of ischemic tissues [12]. Particularly, the discovery of endothelial progenitor cells (EPCs) in bone marrow and their strong angiogenic potential encouraged many groups, including ours, to attempt cell-based therapeutic angiogenesis in CLI patients [13–19]. We performed the first human trial of transplantation of bone marrow-derived cells, also known as bone marrow mononuclear cells (BMMNCs), into CLI patients. Even though it involved only a small number of subjects, the procedure demonstrated the feasibility of cell-based therapeutic angiogenesis in CLI patients [20]. Thereafter, many institutions have performed clinical trials using bone marrow-derived cells in CLI patients. In recent years, peripheral blood mononuclear cells (PBMNCs) have also been used for cell-based therapeutic angiogenesis in CLI patients. PBMNCs can be more easily and safely isolated from patients than BMMNCs, while displaying similar therapeutic efficacy [21]. To date, BMMNCs and PBMNCs have been implemented in several trials involving CLI patients, greatly expanding the achievements and possibilities of cell-based therapeutic angiogenesis [22–27]. In particular, these trials demonstrated the safety and feasibility of cell-based therapeutic angiogenesis for CLI patients (Table 1). However, it is still unlikely that this therapeutic strategy will fulfill the promise of a general use in clinical settings because of limited therapeutic outcomes.
In this review, we focus mainly on the challenges and limitations of cell-based therapeutic angiogenesis raised by previous studies, and discuss potential therapeutic strategies for its clinical application in CLI.
Mechanism of cell-based therapeutic angiogenesis
In spite of yielding promising results, the mechanism of cell-based therapeutic angiogenesis remains vastly unknown. Cell-based therapeutic angiogenesis is thought to depend on a combination of secreted pro-angiogenic factors and direct differentiation of graft into vessel cells [28–30]. However, recent studies have suggested that a direct contribution of graft cells to the neovascularization of ischemic limbs is relatively rare. Instead, multiple pro-angiogenic factors secreted by graft cells are most likely responsible for the efficacy of therapeutic neovascularization [31–33].
VEGF, a dimeric glycoprotein of ~45 kDa, is an early pro-angiogenic factor in therapeutic angiogenesis [34]. VEGF binds to the FLT-1 and FLK-1 receptors on endothelial cells (ECs), activating their intracellular tyrosine kinases. This triggers phosphoinositide-3-kinase/Akt, and mitogen-activated protein kinase signaling pathways, promoting EC proliferation, migration, and survival [35, 36]. VEGF-A165, a VEGF isoform, binds also to the co-receptor neuropilin-1. In an initial clinical trial, in which the VEGF gene was delivered on a plasmid, the collateral formation of blood vessels was effectively induced in ischemic limbs [37].
Basic fibroblast growth factor (bFGF) is also a promising pro-angiogenic factor for therapeutic angiogenesis in CLI patients [9, 38]. The mechanism of action of bFGF in angiogenesis can be explained by the direct effect of FGF receptors on EC proliferation and migration [8]. Interestingly, bFGF contributes to angiogenesis in synergy with VEGF. A combination therapy with congenial pro-angiogenic factors represents a possible strategy for enhancing the effect of therapeutic angiogenesis in CLI patients [39].
Hepatocyte growth factor (HGF) also possesses angiogenic activity, which is exerted through phosphorylation of the tyrosine kinase of its specific receptor, c-Met, stimulating the motility and growth of ECs [40]. As with VEGF, direct delivery of HGF using plasmids has been tested on CLI patients in several clinical trials, demonstrating its safety and potential benefits during the early phase [41, 42].
Although the aforementioned pro-angiogenic factors act mainly on the motility of ECs to initiate vascular structures, it is thought that functional maturation of new vessels is required for the suitable recovery of blood flow in CLI patients. Platelet-derived growth factor-BB (PDGF-BB) recruits mural cells, also known as pericytes, and induces maturation of newly formed vessels [43]. Accordingly, a combination of cell-based therapeutic angiogenesis and PDGF-BB could represent an effective strategy for CLI patients.
Source of graft cells for therapeutic angiogenesis
For example, mesenchymal stem cells (MSCs) and adipose-derived stem cells (ADSCs) are potential therapeutic sources of neovascularization because of their utilities in addition to angiogenic activity. Particularly, immune-privilege of MSCs has been paid attention for autologous transplantation [44]. However, it is still controversial which cell types are best for cell-based therapeutic angiogenesis in CLI patients. After investigating the therapeutic efficacy of various cell types in animal models and patients, mononuclear cells from bone marrow and peripheral blood (e.g., BMMNCs and PBMNCs) appear to be the most realistic choice in clinical settings. Common characteristics of these cell types are the presence of EPCs and the ability to secrete various pro-angiogenic factors. Although cellular heterogeneity and differentiation capacity vary between BMMNCs and PBMNCs, their clinical outcomes are not significantly different [21, 45, 46]. In fact, the major difference between these cells is represented by their invasiveness and isolation procedure. BMMNCs are collected from the iliac bone under general anesthesia, whereas PBMNCs are obtained from peripheral blood by leukapheresis without anesthesia. Minimal invasiveness and absence of anesthesia are required for high-risk CLI patients. Therefore, PBMNCs might be more suitable than BMMNCs for cell-based therapeutic angiogenesis in CLI patients, particularly given that the therapeutic effect is similar [21].
Problems of cell-based therapeutic angiogenesis
Poor graft cell survival remains an unsolved problem for cell-based therapies in ischemic diseases. Reduced oxygen supply and high levels of inflammatory cytokines in ischemic tissues cause excessive production and consequent accumulation of reactive oxygen species, resulting in the death of graft cells [47, 48]. Declining cellular activities in elder patients may also contribute to reduced graft survival in ischemic tissues [49–52]. Therefore, to be effective, cell-based therapies should enhance tolerance against oxidative stress and the angiogenic potential of graft cells.
Another important problem in cell-based therapeutic angiogenesis is the maturation of newly formed vessels. These must be fully functional to supply sufficient blood flow to meet the oxygen and metabolic needs of ischemic tissues. However, newly formed vessels generated by cell transplantation are often immature, even if their number is generally sufficient [53]. Therefore, in addition to increasing the number of vessels, novel therapeutic strategies should also stimulate their maturation during neovascularization.
Hypoxic pretreatment of graft cells to augment therapeutic potential
To enhance the efficacy of cell-based therapeutic angiogenesis, several approaches have been developed and tested in pre-clinical studies [54–56]. To this end, we and others have developed a “hypoxic preconditioning” method, whereby graft cells are incubated for a short time in low oxygen prior to cell transplantation.
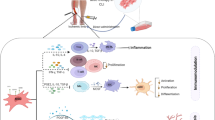
Hypoxic preconditioning enhances VEGF production of mononuclear cells (MNCs) and EPCs, resulting in successful neovascularization in a rodent hind limb ischemia model [15, 57, 58]. In addition to angiogenic activity, hypoxic preconditioning affects also resistance to oxidative stress and adhesion of graft cells to ischemic tissues [59–62]. Such increases in cellular function in preconditioned cells result from upregulation of multiple gene sets associated with cell adhesion, stress resistance, and anti-apoptosis (Fig. 1). Interestingly, hypoxic preconditioning affects neovascularization even in MNCs of aged mice [63], suggesting that this method can, at least in part, reinforce “functionally-declined” MNCs. Moreover, hypoxic preconditioning augments the cellular functions of other cell types, including mesenchymal stem cells and engineered cell sheets [64, 65]. Taken together, given its simplicity and versatility, hypoxic preconditioning is one of most feasible “boosters” of cell-based therapy.
Schematic representation of hypoxic preconditioning of peripheral blood mononuclear cells (PBMNCs). Hypoxic preconditioning of PBMNCs at 2% O2 and 33 °C for 24 h. Cell retention, cell survival, and angiogenic potency are increased by this simple method, improving efficacy of cell-based therapy in ischemic conditions
Because hypoxic preconditioning is a simple but powerful method to enhance multiple cellular functions of MNCs, it can satisfy the need for therapeutic efficacy and rapidity strongly required in clinical settings. We have recently started a clinical trial using hypoxic preconditioning whereby autologous PBMNCs were transplanted into ischemic limbs of CLI patients. A CLI patient treated with preconditioned PBMNCs was thus relieved of severe ischemic pain and showed increased blood flow in the ischemic limb (unpublished preliminary results). Briefly, the patient was categorized as Rutherford class 6 and had undergone amputation of the Lisfranc because of remaining foot gangrene following several angioplasties. In spite of the initial amputation, strong ischemic pain and progressive necrosis remained in the foot. In the present trial, we aimed to release rest pain and stop the worsening of necrosis, in addition to checking the safety of this therapeutic approach. As a result, the patient, who was injected with 5.4 × 108 preconditioned PBMNCs into the ischemic leg, was released from rest pain. Skin perfusion pressure increased (from 27 to 59 mmHg) and there were no adverse events. However, the CLI patient, who injected cells, had to be re-amputated above the ankle a month after cell transplantation because of uncontrollable necrosis and infection of the gangrenous foot (Fig. 2).
A patient with right foot atherosclerotic gangrene after injection of preconditioned cells. a The angiography revealed a poor vascular bed in the right foot (circle). b Location of the gangrene-infected amputation site of the Lisfranc in the right foot. Skin perfusion pressure (SPP) was 27 mm Hg pre-treatment. c Hypoxic preconditioned peripheral blood mononuclear cells were transplanted into 54 points (1 × 107/0.1 mL/point) in ischemic tissue (5.4 × 108 cells). d SPP increased to 59 mmHg 7 days after treatment, however necrosis and infection gradually worsened
Consistent with this study, some trials have reported that the therapeutic effects of cell-based therapies were not as expected in CLI patients with diabetes mellitus, hemodialysis, and advanced Rutherford class 6 [66–68]. Therefore, it is important to determine the correct indication and adequate timing of cell-based therapies. In addition, we believe that a more powerful therapeutic strategy is necessary for high-risk patients.
Combination therapy to induce new vessels and their maturation
Evidence from preclinical studies using multi-growth factors supports the notion that a combination of induction and maturation of new vessels improves functional outcomes of therapeutic angiogenesis even in CLI patients [39, 69–72]. As mentioned previously, cell-based therapy is a promising strategy to induce new vessels in ischemic tissues, including CLI, and hypoxic preconditioning is a possible booster to enhance therapeutic angiogenesis. Therefore, a combination of cell transplantation that includes hypoxic preconditioning, and the use of vessel maturation-associated factors might provide a novel effective therapeutic strategy for CLI.
Angiopoietin-1 (Ang-1) and apelin are well known as vessel maturation-associated factors. Apelin, which is an endogenous ligand for the APJ receptor, regulates caliber size and stabilization of blood vessels; whereas Ang-1 contributes to EC migration during vessel maturation [73–76]. Recently, we investigated whether a combination of preconditioned cell transplantation and apelin administration could represent an effective therapeutic strategy for CLI. We found that hypoxic preconditioning enhanced the sensitivity of PBMNCs to apelin through upregulation of the APJ receptor, thereby resulting in increased PDGF-BB secretion. At the same time, apelin directly regulated proliferation and migration of vascular smooth muscle cells in ischemic blood vessels through induction of PDGF receptor-β (Fig. 3). Thus, a combination of preconditioned cell transplantation and apelin administration induced functionally matured new vessels and dramatically improved blood flow to the ischemic hind limbs in CLI animal models [53]. Our findings raise the possibility that cell-based therapeutic angiogenesis may benefit from the combined administration of vessel maturation-associated factors.
Combination therapy using hypoxic preconditioning and apelin. Hypoxic preconditioning and ischemic conditions upregulate the APJ receptor in peripheral blood mononuclear cells (PBMNCs) and vascular smooth muscle cells (VSMCs). Preconditioned PBMNCs receive exogenous apelin, leading to the secretion of platelet-derived growth factor (PDGF)-BB. Exogenous apelin induces upregulation of PDGF receptor-β in ischemic VSMCs. Subsequently, VSMCs are activated to mature newly formed vessels in ischemic tissue
Possible targets of cell-based therapeutic angiogenesis in CLI
Some clinical trials show discrepancies in the therapeutic outcomes of cell-based therapeutic angiogenesis among CLI patients. For example, the therapeutic angiogenesis using cell transplantation (TACT) trial, which was performed in patients with TAO using BMMNCs, demonstrated long-term safety and a higher therapeutic efficacy than in ASO patients [77]. Similarly, the PROVASA (intraarterial progenitor cell transplantation of bone marrow mononuclear cells for induction of neovascularization in patients with peripheral arterial occlusive disease) trial indicated greater overall therapeutic benefits in TAO compared with atherosclerotic CLI patients [78]. Moreover, other trials have also demonstrated a more efficient outcome of cell-based therapeutic angiogenesis in TAO than in ASO patients [79–82]. Such clinical evidence suggests that some targets may be more appropriate than others, although it remains to be determined why cell-based therapeutic angiogenesis is more effective in TAO than in ASO patients. Given that TAO is defined as a non-atherosclerotic and inflammatory disease, whereas ASO is associated with atherosclerosis and advanced age [49, 50, 83], these pathologies might determine the outcomes of cell-based therapeutic angiogenesis in CLI patients. Accordingly, we may be able to find targets other than TAO for effective cell-based therapeutic angiogenesis in CLI patients.
Similar pathological characteristics, ranging from inflammation of small- and middle-size arteries to TAO, are observed also in patients with collagen vascular diseases (CVDs). It is thought that auto-immune disorders are underlying diseases commonly associated with both TAO and CVD. Patients with CVD present symptoms of vasculitis and occlusion of microvessels, resulting in rest pain, skin ulcer, and gangrene in the limbs. In spite of many attempts to find a cure, there are no effective drugs against CVD. Given the absence of a vascular bed in microcirculatory systems of the extremities, surgical treatments including bypass surgery do not provide adequate blood flow to ischemic limbs for long periods of time [84]. If cell transplantation could provide a vascular bed in ischemic limbs, then cell-based therapeutic angiogenesis would be a reasonable therapeutic strategy for untreatable CVD patients. A possible application of cell-based therapeutic angiogenesis for patients with CVD has been reported in some clinical trials [85–87]. Taken together, cell-based therapeutic angiogenesis could become a powerful tool in CLI with inflammation and a poor vascular bed. However, further investigation is required to ensure this therapeutic approach is translated into the right practical applications.
Conclusion
Efficacy and safety of cell-based therapeutic angiogenesis have been demonstrated in many clinical trials. However, therapeutic outcomes are still limited and further improvements are required for extensive clinical applications. For example, hypoxic preconditioning of graft cells and its combination with other strategies are some of the options for enhancing efficacy of cell-based therapeutic angiogenesis. Also, absence of necrosis and infection at the time of cell injection, and an appropriate selection of target diseases, such as TAO and vascular diseases caused by auto-immune disorders, should be considered when translating this approach to clinical settings.
Abbreviations
- ADSCs:
-
adipose-derived stem cells
- ASO:
-
atherosclerosis obliterans
- bFGF:
-
basic fibroblast growth factor
- BMMNCs:
-
bone marrow-derived mononuclear cells
- CLI:
-
critical limb ischemia
- CVD:
-
collagen vascular disease
- ECs:
-
endothelial cells
- EPCs:
-
endothelial progenitor cells
- HGF:
-
hepatocyte growth factor
- MNCs:
-
mononuclear cells
- MSCs:
-
mesenchymal stem cells
- PAD:
-
peripheral artery disease
- PBMNCs:
-
peripheral blood mononuclear cells
- PDGF:
-
platelet-derived growth factor
- TAO:
-
thromboangiitis obliterans
- VEGF:
-
vascular endothelial growth factor
References
Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–15.
Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Group TIW. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):5–67.
Stoyioglou A, Jaff MR. Medical treatment of peripheral arterial disease: a comprehensive review. J Vasc Interv Radiol. 2004;15:1197–207.
Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–7.
Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–53.
Powell RJ. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg. 2012;56:264–6.
Grochot-Przeczek A, Dulak J, Jozkowicz A. Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene. 2013;525:220–8.
Shimamura M, Nakagami H, Koriyama H, Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. Biomed Res Int. 2013;2013:186215.
Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006;17:683–91.
Marshall E. Clinical research. Gene therapy a suspect in leukemia-like disease. Science. 2002;298:34–5.
Raval Z, Losordo DW. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circ Res. 2013;112:1288–302.
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7.
Hamano K, Li TS, Kobayashi T, Kobayashi S, Matsuzaki M, Esato K. Angiogenesis induced by the implantation of self-bone marrow cells: a new material for therapeutic angiogenesis. Cell Transplant. 2000;9:439–43.
Hamano K, Li TS, Kobayashi T, Tanaka N, Kobayashi S, Matsuzaki M, Esato K. The induction of angiogenesis by the implantation of autologous bone marrow cells: a novel and simple therapeutic method. Surgery. 2001;130:44–54.
Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30:967–72.
Murohara T. Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors. Trends Cardiovasc Med. 2001;11:303–7.
Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–80.
Esato K, Hamano K, Li TS, Furutani A, Seyama A, Takenaka H, Zempo N. Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transpl. 2002;11:747–52.
Minamino T, Toko H, Tateno K, Nagai T, Komuro I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet. 2002;360:2083–4 (author reply 2084).
Liew A, Bhattacharya V, Shaw J, Stansby G. Cell therapy for critical limb ischemia: a meta-analysis of randomized controlled trials. Angiology. 2016;67:444–55.
Liu Y, Xu Y, Fang F, Zhang J, Guo L, Weng Z. Therapeutic efficacy of stem cell-based therapy in peripheral arterial disease: a meta-analysis. PLoS ONE. 2015;10:e0125032.
Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC, Moll FL. Bone marrow derived cell therapy in critical limb ischemia: a meta-analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg. 2015;50:775–83.
Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–35.
Teraa M, Sprengers RW, van der Graaf Y, Peters CE, Moll FL, Verhaar MC. Autologous bone marrow-derived cell therapy in patients with critical limb ischemia: a meta-analysis of randomized controlled clinical trials. Ann Surg. 2013;258:922–9.
Wang ZX, Li D, Cao JX, Liu YS, Wang M, Zhang XY, Li JL, Wang HB, Liu JL, Xu BL. Efficacy of autologous bone marrow mononuclear cell therapy in patients with peripheral arterial disease. J Atheroscler Thromb. 2014;21:1183–96.
Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–7.
Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56:593–607.
Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–36.
Cao Y, Hong A, Schulten H, Post MJ. Update on therapeutic neovascularization. Cardiovasc Res. 2005;65:639–48.
Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9.
Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8.
Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–70.
Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94.
Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–9.
Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370–4.
Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA. 1986;83:7297–301.
Lu H, Xu X, Zhang M, Cao R, Brakenhielm E, Li C, Lin H, Yao G, Sun H, Qi L, et al. Combinatorial protein therapy of angiogenic and arteriogenic factors remarkably improves collaterogenesis and cardiac function in pigs. Proc Natl Acad Sci USA. 2007;104:12140–5.
Suzuki J, Shimamura M, Suda H, Wakayama K, Kumagai H, Ikeda Y, Akazawa H, Isobe M, Komuro I, Morishita R. Current therapies and investigational drugs for peripheral arterial disease. Hypertens Res. 2016;39:183–91.
Morishita R, Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Taniyama Y, Sawa Y, Kaneda Y, Ogihara T. Phase I/IIa clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol. 2011;31:713–20.
Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, Yamaguchi T, Ogihara T, Morishita R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152–61.
Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–8.
Liew A, O’Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 2012;3:28.
Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, Sixta B, Varga M, Langkramer S, Sykova E, Jude EB. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab Res Rev. 2013;29:369–76.
Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98:1335–42.
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–22.
Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89.
Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600.
Li TS, Kubo M, Ueda K, Murakami M, Ohshima M, Kobayashi T, Tanaka T, Shirasawa B, Mikamo A, Hamano K. Identification of risk factors related to poor angiogenic potency of bone marrow cells from different patients. Circulation. 2009;120:S255–61.
Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6.
Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–51.
Samura M, Morikage N, Suehiro K, Tanaka Y, Nakamura T, Nishimoto A, Ueno K, Hosoyama T, Hamano K. Combinatorial treatment with apelin-13 enhances the therapeutic efficacy of a preconditioned cell-based therapy for peripheral ischemia. Sci Rep. 2016;6:19379.
Mima Y, Fukumoto S, Koyama H, Okada M, Tanaka S, Shoji T, Emoto M, Furuzono T, Nishizawa Y, Inaba M. Enhancement of cell-based therapeutic angiogenesis using a novel type of injectable scaffolds of hydroxyapatite-polymer nanocomposite microspheres. PLoS ONE. 2012;7:e35199.
Park A, Barrera-Ramirez J, Ranasinghe I, Pilon S, Sy R, Fergusson D, Allan DS. Use of statins to augment progenitor cell function in preclinical and clinical studies of regenerative therapy: a systematic review. Stem Cell Rev. 2016;12:327–39.
Tanaka R, Vaynrub M, Masuda H, Ito R, Kobori M, Miyasaka M, Mizuno H, Warren SM, Asahara T. Quality-control culture system restores diabetic endothelial progenitor cell vasculogenesis and accelerates wound closure. Diabetes. 2013;62:3207–17.
Akita T, Murohara T, Ikeda H, Sasaki K, Shimada T, Egami K, Imaizumi T. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Lab Invest. 2003;83:65–73.
Li TS, Hamano K, Suzuki K, Ito H, Zempo N, Matsuzaki M. Improved angiogenic potency by implantation of ex vivo hypoxia prestimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol. 2002;283:H468–73.
Kubo M, Li TS, Kamota T, Ohshima M, Qin SL, Hamano K. Increased expression of CXCR4 and integrin αM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J Cell Physiol. 2009;220:508–14.
Kubo M, Li TS, Suzuki R, Shirasawa B, Morikage N, Ohshima M, Qin SL, Hamano K. Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2008;294:H590–5.
Kudo T, Hosoyama T, Samura M, Katsura S, Nishimoto A, Kugimiya N, Fujii Y, Li TS, Hamano K. Hypoxic preconditioning reinforces cellular functions of autologous peripheral blood-derived cells in rabbit hindlimb ischemia model. Biochem Biophys Res Commun. 2014;444:370–5.
Kudo T, Kubo M, Katsura S, Nishimoto A, Ueno K, Samura M, Fujii Y, Hosoyama T, Hamano K. Hypoxically preconditioned human peripheral blood mononuclear cells improve blood flow in hindlimb ischemia xenograft model. Am J Transl Res. 2014;6:570–9.
Kubo M, Li TS, Kurazumi H, Takemoto Y, Ohshima M, Murata T, Katsura S, Morikage N, Furutani A, Hamano K. Hypoxic preconditioning enhances angiogenic potential of bone marrow cells with aging-related functional impairment. Circ J. 2012;76:986–94.
Hosoyama T, Samura M, Kudo T, Nishimoto A, Ueno K, Murata T, Ohama T, Sato K, Mikamo A, Yoshimura K, et al. Cardiosphere-derived cell sheet primed with hypoxia improves left ventricular function of chronically infarcted heart. Am J Transl Res. 2015;7:2738–51.
Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–82.
Gomez RA, Fernandez JD, Cabrera M, Marrero I, Ramirez N, Alvarez I. Possible predictors of poor angiogenesis after hematopoietic stem cell autograft for lower limb ischemia. MEDICC Rev. 2012;14:31–6.
Horie T, Onodera R, Akamastu M, Ichikawa Y, Hoshino J, Kaneko E, Iwashita C, Ishida A, Tsukamoto T, Teramukai S, et al. Long-term clinical outcomes for patients with lower limb ischemia implanted with G-CSF-mobilized autologous peripheral blood mononuclear cells. Atherosclerosis. 2010;208:461–6.
Sun L, Wu L, Qiao Z, Yu J, Li L, Li S, Liu Q, Hu Y, Xu N, Huang P. Analysis of possible factors relating to prognosis in autologous peripheral blood mononuclear cell transplantation for critical limb ischemia. Cytotherapy. 2014;16:1110–6.
Arsic N, Zentilin L, Zacchigna S, Santoro D, Stanta G, Salvi A, Sinagra G, Giacca M. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther. 2003;7:450–9.
Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13.
Kobayashi K, Kondo T, Inoue N, Aoki M, Mizuno M, Komori K, Yoshida J, Murohara T. Combination of in vivo angiopoietin-1 gene transfer and autologous bone marrow cell implantation for functional therapeutic angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1465–72.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34.
Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93.
Kidoya H, Naito H, Takakura N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood. 2010;115:3166–74.
Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T, Fujii R, Takakura N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27:522–34.
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6.
Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–8.
Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schluter M, Tonn T, Seeger F, Dimmeler S, Lindhoff-Last E, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv. 2011;4:26–37.
Durdu S, Akar AR, Arat M, Sancak T, Eren NT, Ozyurda U. Autologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II–III thromboangiitis obliterans. J Vasc Surg. 2006;44:732–9.
Lee KB, Kang ES, Kim AK, Kim MH, Do YS, Park KB, Park HS, Um SH, Cho SW, Kim DI. Stem cell therapy in patients with thromboangiitis obliterans: assessment of the long-term clinical outcome and analysis of the prognostic factors. Int J Stem Cells. 2011;4:88–98.
Miyamoto K, Nishigami K, Nagaya N, Akutsu K, Chiku M, Kamei M, Soma T, Miyata S, Higashi M, Tanaka R, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–84.
Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam. 2013;2013:156905.
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7.
Deguchi J, Shigematsu K, Ota S, Kimura H, Fukayama M, Miyata T. Surgical result of critical limb ischemia due to tibial arterial occlusion in patients with systemic scleroderma. J Vasc Surg. 2009;49:918–23.
Kamata Y, Iwamoto M, Muroi K, Minota S. Repeated local implantation of autologous peripheral blood mononuclear cells for the treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology (Oxford). 2011;50:906–10.
Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, Muroi K, Ikeda U, Shimada K, Yoshio T, Okazaki H, Minota S. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology (Oxford). 2007;46:882–4.
Nevskaya T, Ananieva L, Bykovskaia S, Eremin I, Karandashov E, Khrennikov J, Mach E, Zaprjagaeva M, Guseva N, Nassonov E. Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology (Oxford). 2009;48:61–4.
Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–60.
Lenk K, Adams V, Lurz P, Erbs S, Linke A, Gielen S, Schmidt A, Scheinert D, Biamino G, Emmrich F, et al. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur Heart J. 2005;26:1903–9.
Arai M, Misao Y, Nagai H, Kawasaki M, Nagashima K, Suzuki K, Tsuchiya K, Otsuka S, Uno Y, Takemura G, et al. Granulocyte colony-stimulating factor: a noninvasive regeneration therapy for treating atherosclerotic peripheral artery disease. Circ J. 2006;70:1093–8.
Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, Sadamoto K, Yokoyama A, Yamanaka T, Onodera R, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857–64.
Prochazka V, Gumulec J, Jaluvka F, Salounova D, Jonszta T, Czerny D, Krajca J, Urbanec R, Klement P, Martinek J, Klement GL. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010;19:1413–24.
Murphy MP, Lawson JH, Rapp BM, Dalsing MC, Klein J, Wilson MG, Hutchins GD, March KL. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg. 2011;53(1565–1574):e1561.
Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, Teodorescu V, Wiechmann BN, Thompson C, Kraiss L, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5:821–30.
Tanaka R, Masuda H, Kato S, Imagawa K, Kanabuchi K, Nakashioya C, Yoshiba F, Fukui T, Ito R, Kobori M, et al. Autologous G-CSF-mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transpl. 2014;23:167–79.
Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I, Doevendans PA, Mali WP, Moll FL, Verhaar MC. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled rejuvenating endothelial progenitor cells via transcutaneous intra-arterial supplementation (JUVENTAS) trial. Circulation. 2015;131:851–60.
Authors’ contributions
MS, TH, and KH wrote the manuscript. NM, YT, and KU contributed to the conception of the manuscript. All authors discussed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Drs. Osamu Yamashita, Koshiro Ueda, and Yuya Tanaka for helpful discussions and comments. We also thank Yukari Hironaka for graphic design assistance.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Informed written consent for publication of data from the study was obtained from patient.
Ethics approval and consent to participate
An ethics review committee for human stem cell clinical research in ministry of health, labour and welfare approved the phase I clinical trial for the transplantation preconditioned peripheral blood mononuclear cells into patients with critical limb ischemia (UMIN000018594). Investigation was conducted in accordance with the Declaration of Helsinki. Informed written consent for participation in the study was obtained from patient.
Funding
This work was supported in part by JSPS-KAKENHI Grant-in-Aid for Scientific Research C and Grant-in-Aid for Young Scientists B (15K10244 to T.H. and 16K19967 to M.S.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Samura, M., Hosoyama, T., Takeuchi, Y. et al. Therapeutic strategies for cell-based neovascularization in critical limb ischemia. J Transl Med 15, 49 (2017). https://doi.org/10.1186/s12967-017-1153-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-017-1153-4