Abstract
Background
This aimed to evaluate the effects of self-monitoring of daily steps with or without counselling support on HbA1c, other cardiometabolic risk factors and objectively measured physical activity (PA) during a 2-year intervention in a population with prediabetes or type 2 diabetes.
Methods
The Sophia Step Study was a three-armed parallel randomised controlled trial. Participants with prediabetes or type 2 diabetes were recruited in a primary care setting. Allocation (1:1:1) was made to a multi-component intervention (self-monitoring of steps with counselling support), a single-component intervention (self-monitoring of steps without counselling support) or standard care. Data were collected for primary outcome HbA1c at baseline and month 6, 12, 18 and 24. Physical activity was assessed as an intermediate outcome by accelerometer (ActiGraph GT1M) for 1 week at baseline and the 6-, 12-, 18- and 24-month follow-up visits. The intervention effects were evaluated by a robust linear mixed model.
Results
In total, 188 subjects (64, 59, 65 in each group) were included. The mean (SD) age was 64 (7.7) years, BMI was 30.0 (4.4) kg/m2 and HbA1c was 50 (11) mmol/mol, 21% had prediabetes and 40% were female. The dropout rate was 11% at 24 months. Effect size (CI) for the primary outcome (HbA1c) ranged from -1.3 (-4.8 to 2.2) to 1.1 (-2.4 to 4.6) mmol/mol for the multi-component vs control group and from 0.3 (-3.3 to 3.9) to 3.1 (-0.5 to 6.7) mmol/mol for the single-component vs control group. Effect size (CI) for moderate-to-vigorous physical activity ranged from 8.0 (0.4 to 15.7) to 11.1 (3.3 to 19.0) min/day for the multi-component vs control group and from 7.6 (-0.4 to 15.6) to 9.4 (1.4 to 17.4) min/day for the single-component group vs control group.
Conclusion
This 2-year intervention, including self-monitoring of steps with or without counselling, prevented a decrease in PA but did not provide evidence for improved metabolic control and cardiometabolic risk factors in a population with prediabetes or type 2 diabetes.
Trial registration
ClinicalTrials.gov, NCT02374788. Registered 2 March 2015—Retrospectively registered.
Similar content being viewed by others
Background
For individuals with or at risk of type 2 diabetes, metabolic control is of utmost importance to reduce the risk of cardiovascular disease and other complications [1]. Aerobic exercise improves metabolic function and reduces cardiovascular risk factors in individuals with prediabetes or type 2 diabetes [2]. Regular physical activity (PA) is therefore central in diabetes self-management and prevention, and health care providers are encouraged to give support for PA in individuals with prediabetes or type 2 diabetes [1]. Yet, most people with prediabetes or type 2 diabetes do not reach the recommended levels of PA [3, 4].
Walking is an activity that most people can perform and is a useful therapeutic tool for individuals with type 2 diabetes [5]. Step counting is a measure of locomotor movement (e.g., walking) and daily steps correlate well with both total PA and moderate-to-vigorous PA (MVPA) [6]. Several systematic reviews and meta-analyses show that self-monitoring of PA (e.g., step counting) as a motivational tool has positive short-term effects on PA levels [7,8,9,10,11,12,13] and weight [8, 14] in people with type 2 diabetes. The use of step counters is recommended to encourage and maintain PA [15, 16]. However, evidence for the effect of self-monitoring of steps on metabolic control and cardiovascular risk factors is inconsistent [7, 9,10,11, 14].
There is evidence for stronger PA effects with resource-demanding counselling support than minimal interventions, including using step counters only [10]. However, the evidence is conflicting [9, 11, 16]. Moreover, the effectiveness of long-term (> 12 months) interventions implemented in a primary care setting remains unclear [10, 12, 17].
The Sophia Step Study was undertaken as a three-armed randomised controlled trial (RCT) to evaluate self-monitoring of daily steps with and without counselling in individuals with prediabetes or type 2 diabetes in a primary care context [18]. The study hypothesised that both intervention groups would increase PA levels and subsequently improve metabolic control and reduce cardiovascular risk factors. We also hypothesied that the multi-component group (including counselling) would maintain the effects at a higher level over time than the single-component intervention group (without counselling). The primary outcome was HbA1c as a measure of metabolic control. Secondary outcomes included several clinical and anthropometric measures of cardiovascular risk. Objectively measured PA (MVPA, light-intensity PA (LPA), time spent in sedentary behaviours (SB) and daily steps) were applied as intermediate outcomes. The intervention duration was 2 years.
Methods
Aim
This study aimed to evaluate the effects of self-monitoring of steps with or without counselling support on HbA1c, other cardiometabolic risk factors and PA during a 24-month intervention in individuals with prediabetes or type 2 diabetes.
Study design, setting and materials
The study was a three-armed parallel randomised RCT. It was performed according to the CONSORT guidelines for reporting non-pharmacological treatment interventions and multi-arm parallel group randomised trials. Patients at two urban and one rural primary care centre were recruited by their diabetes specialist nurse. Eight rounds of recruitments were made, varying across seasons between April 2013 and January 2018. Some 385 persons were invited to enrol in the study during regular visits to the primary care centre or by mailed invitation. Before baseline measurements, patients were examined by a general practitioner to ensure study eligibility. Inclusion and exclusion criteria are listed in Table 1. Participants were randomly assigned to a multi-component intervention group (self-monitoring of steps with counselling support), a single-component intervention group (self-monitoring of steps without counselling support) or a control group (standard care). Randomization was conducted using sealed envelopes prepared by project staff and distributed by the diabetes specialist nurses, stratified by gender, at an allocation ratio of 1:1:1. Demographics were collected by a questionnaire at baseline, web- or paper-based as the participant preferred. Data on health conditions and medications were obtained from medical records and asked for at the baseline assessment. Specific details on methods are published in a study protocol [18].
Interventions
Details and theoretical framework of the 2-year intervention are described in the study protocol [18]. The fidelity of the intervention is outlined in a process evaluation [20]. Participants in the two intervention groups were offered step counters (Yamax Digiwalker SW 200: Yamax Corporation, Tokyo, Japan) and directed to a website for self-monitoring of steps [21]. The participants of the multi-component group were, in addition to pedometers and the website, offered 12 group consultations (10 during the first year) and nine individual face-to-face consultations (seven during the first year) by their diabetes specialist nurse. The group sessions were led by project staff (the urban centres) and a diabetes specialist nurse (rural centre). The programme for the group sessions was guided by the health belief model [22], social cognitive theory [23] and the transtheoretical model of change [24]. The programme considered several techniques for behaviour change [18]. Individual consultations were based on a motivational interviewing technique [25]. Standard care included meeting a diabetes specialist nurse and a physician once a year, or more often if needed. All participants were offered study assessments at baseline and at follow-up (2, 3, 4, 6, 9, 12, 18 and 24 months), including feedback on health outcomes. The multi-component group had 45 min with their diabetes nurse consisting of counselling and study assessments. The single-component and control group had 15 min with their diabetes nurse for study assessment.
Outcome measures
Cardiometabolic risk factors
Data on biomarkers collected at baseline and at the 6-, 12-, 18- and 24-month follow-up were used for study analysis. Biomarkers included HbA1c (mmol/mol), fasting blood glucose (mmol/l), triglycerides (mmol/l), high-density lipoprotein cholesterol (HDL) (mmol/l), low-density lipoprotein cholesterol (LDL) (mmol/l), ApoB/ApoA1 (g/l) and C-peptide (nmol/l). Data collected at baseline and follow-up (6, 12, 18 and 24 months) were used for resting systolic blood pressure (mmHg), resting diastolic blood pressure (mmHg) and anthropometric variables: weight (kg), body fat (%), waist circumference (cm) and sagittal abdominal diameter (cm). Blood samples were analysed by standardised methods at the Research laboratory, Karolinska Hospital according to the manufacturer’s kit test instructions. HbA1c was determined using a Variant II Turbo HbA1c analyser (Bio-Rad Laboratories, USA). Plasma glucose, triglycerides, total cholesterol, HDL, LDL, Apolipoprotein-A1 and Apolipoprotein B and C-peptide were determined using analysers from Beckman Coulter, Inc. USA and Roche Diagnostics, Switzerland. Detailed descriptions of measuring and analysis methods are provided elsewhere [18].
Physical activity and sedentary behaviours
PA spent in MVPA and LPA, SB and daily steps were measured for 1 week at baseline and the 6-, 12-, 18- and 24-month follow-up with a hip-worn ActiGraph GT1M accelerometer (ActiGraph, Pensacola, FL). MVPA was chosen as the main physical activity outcome [2]. Data collection and data processing procedures have been published previously [26]. Non-wear time was determined at 90 min with consecutive zero counts, allowing for 2 min interval of nonzero counts [27]. Participants providing data of ≥ 10 h per day for at least 3 days were included in the analyses [28]. Commonly applied count-based thresholds of SB < 100 counts per minute (cpm) [29], LPA (100–1951 cpm) and MVPA (≥ 1952 cpm) [30] were applied.
Statistical analyses
Statistical analyses were performed using the R statistical software (version 4.0.3) by an external statistician blinded to group allocation. The data were examined for normality, outliers and missing values. Change from baseline to the follow-up visits was examined for each randomisation group by computing the mean and 95% confidence interval for the participants’ difference in follow-up value and baseline values.
The between group comparisons were performed with an intent-to-treat approach using linear mixed model including subject as a random effect (random intercept) and age, randomization group, time as well as a randomization group and time interaction as fixed factors [31]. Due to deviations from the normality assumption for the residuals, a robust variant of the linear mixed model was fitted. The robust linear mixed model applies weighting to the observations by giving less weight to individuals with large deviations from normality [32]. Analyses of SB were adjusted for wear time. Sensitivity analysis were conducted for participants with type 2 diabetes (excluding prediabetes participants). The study was designed to have 80% power (alpha = 0.05) to detect a difference of 4 mmol/mol (0.6%) in HbA1c between the groups at 12 months, revealing that we required a sample size of at least 56 participants in each group.
Results
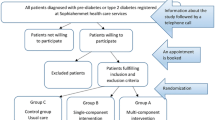
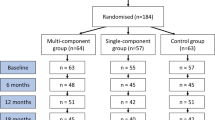
Of the 385 invited patients, 203 (53%) agreed to participate. By November 2018, 188 patients from three primary care centres fulfilled the inclusion criteria and were randomised into one of the intervention groups or the control group. The dropout rate was 9% at 12 months and 11% at 24 months. The response rate of invited and eligible individuals was 49%. A process evaluation with details on the context, reasons for declining and adherence to the intervention has been published elsewhere [20]. Figure 1 depicts the number of participants recruited, excluded, declined, consented and dropouts per intervention group, as well as reasons for exclusion and termination.
Flowchart of enrollment and participation in the Sophia Step Study. Clinical presents number participants with valid data for HbA1c, Anthr = Anthropometrics presents number participants with valid data for weight, PA = Physical activity presents number participants with valid data for moderate-to-vigorous physical activity
Table 2 describes participant baseline characteristics by allocated group. The mean age in all groups was 64 ± 7.7 years, 21% had prediabetes, 40% were female, 72% lived with a partner, 51% had a university education, mean BMI was 30.0 ± 4.4 and mean HbA1c was 50 ± 11.4 mmol/mol. Additional file 1 provides characteristics by the completers and dropouts and Additional file 2 provides baseline characteristics by diagnosis (prediabetes or type 2 diabetes). Medications and onset of diseases during the interventions in all three groups are described in Additional file 3. No adverse health events due to participation were reported.
Mean change in HbA1c, MVPA and daily steps for each group over 24 months are illustrated in Figs. 2 and 3. Additional files 4 and 5 show the mean change in HbA1c, MVPA and daily steps for each group over 24 months with confidence intervals (CI). Additional file 6 reports mean (CI) within-group differences between baseline and each follow-up. There is a trend towards improved HbA1c values in both intervention groups at 6 months, which is not seen in the control group. After 12 months, the improvement in HbA1c had reversed and at 24 months exceeded baseline levels. Mean MVPA and daily steps increased during the first 6 months for both intervention groups and showed a mixed pattern of improvement-maintenance during the intervention period. The control group decreased mean levels of MVPA and daily steps during the intervention period.
The intervention effects (between-group differences) for HbA1c, MVPA, LPA, SB and daily steps are displayed in Tables 3 and 4. There was no intervention effect on any of the cardiometabolic variables (Additional file 7).
For the PA variables, the two intervention groups showed a comparable pattern over time, with improved levels at a similar range compared to the control group. Some variations occurred in the increase in PA, reaching statistically significant levels only for MVPA in the multi-component intervention group versus the control group. The result from the sensitivity analyses, including only participants with type 2 diabetes, did not differ from the main result, except for a more pronounced intervention effect on daily steps at 6 months for both intervention groups. The intervention was delivered as intended, and no unintended effects were reported.
Discussion
This paper describes the results of a 2-year, three-armed RCT conducted in a primary health care context. The study sought to evaluate self-monitoring of daily steps with (multi-component) and without (single-component) counselling versus a control group in individuals with prediabetes or type 2 diabetes on clinical, anthropometric and behaviour outcomes. The hypothesis tested was that both intervention groups would increase PA levels and subsequently improve metabolic control and reduce cardiovascular risk factors compared to the control group, and the multi-component group would maintain the effects ata higher level over time than the single-component group.
The main finding is that this study does not provide evidence of an intervention effect in either intervention group on the primary outcome (HbA1c) or the other measured cardiometabolic risk factors. Both intervention groups showed tendencies for beneficial changes in HbA1c at 6 months, but the distribution of improvement was large, and the findings did not reach clinically relevant levels for most individuals. The favourable changes in HbA1c returned to baseline levels by month 12 and exceeded baseline levels at 24 months. The initial beneficial change in HbA1c can be explained by the increase in PA shown at 6 months when the intervention was most intense for the intervention components. However, it is unknown whether the changes in PA and HbA1c were causally linked to the intervention. To establish such a relationship subgroup analyses are needed. Yet, another explanation for the initial beneficial change is that the participants were highly motivated for behaviour change at the start of the intervention but failed to maintain the motivation for long-term behaviour change. To uphold metabolic control is known to be challenging due to the progressive nature of the disease, even with improvement in the quality of diabetes care [33].
The lack of evidence for an effect on metabolic control and cardiometabolic risk factors in this study adds to the body of conflicting evidence documenting the effect of step counters on metabolic control and cardiovascular risk factors [7, 9,10,11, 14]. The study indicates that step counters may be ineffective in improving metabolic control. However, with regard to the levels of PA and HbA1c, some clarifications can be made. The high level of baseline PA in this sample may explain the rather beneficial baseline clinical values, reaching a ceiling effect for many individuals. A recent meta-analysis suggests that every 1000 steps/day that individuals take compared to reference individuals reduces the risk of cardiovascular morbidity or mortality by 5–21% [34]. An intervention of long duration should consider trends and natural changes made in society. A comparison can be made to trends in metabolic control occurring during the intervention period that reflect improvements in diabetes care. The National Diabetes Registry data show that mean HbA1c in the general Swedish type 2 diabetes population decreased from 54.1 mmol/mol in 2015 to 52.8 mmol/mol in 2020 [35].
Due to multiple testing, interpretations of the results should be made with care. Still, there is a tendency towards an intervention effect on the maintenance of PA in both intervention groups, slightly favouring the multi-component intervention. The statistically significant effect on PA was robust over time for MVPA. The effect size (mean difference of MVPA in the multi-component intervention group compared to the control group) was 8–11 min across the 2 years follow-up. Daily steps show the same pattern as MVPA, with a variation of 754–943 steps/day between the multi-component intervention group and the control group over the 2 years but did not reach statistically significant levels. The effect of the single-component intervention did not reach statistically significant levels but followed the same pattern as the multi-component intervention (8–9 min/day for MVPA and 567–1074 for steps/day). The between-group differences are mainly explained by the intervention groups maintaining mean PA levels over the 2 years, while the control group decreased the mean PA levels and increased mean sedentary time.
While not part of the hypotheses tested, the mean level of increase in PA by the intervention groups varied over the 2 years. The increase after 6 months (876 steps/day in the multi-component intervention group and 1009 steps/day in the single-component intervention group) was somewhat lower than that found in previous similar studies using step counters that range from 1281 to 2744 steps/day [36,37,38]. The increase after 12 months (89 steps/day in the multi-component and 272 steps/day in the single-component intervention group) corresponds to an increase of 91 to 1220 steps/day [38,39,40,41,42] in previous 12-month interventions. An increase of 500 steps/day or a 5–6-min walk/day is a clinically meaningful increase that can reduce cardiovascular morbidity and mortality in inactive individuals [43]. Note that the control group reduced the mean level of PA by nearly 1000 steps/day and nearly 10 min/day of MVPA from the baseline period to month 24. While the intervention groups had a small decrease in the number of daily steps taken from baseline to 24 months, both groups maintained their minutes of MVPA at month 24 compared to baseline values. These differences between interventions and control group underline the importance of providing support for PA in this adult population with prediabetes or type 2 diabetes. While this study does not provide sufficient evidence for intervention effects on metabolic control, self-monitoring of steps may still be an acceptable, low-cost, low-risk approach to improving PA.
There was no benefit from the multi-component intervention in this study compared to the single-component intervention. Studies with comparable interventions and populations show somewhat conflicting findings, as do two recent meta-analyses with broader populations [10, 16]. In the PACE-UP trial the group receiving pedometers only increased daily steps to the same level as the group receiving pedometers and three counselling sessions [41]. Although of varying lengths and support intensity, other similar trials reported superior effects in intervention groups receiving step counting and counselling versus step counting alone [44,45,46].
A process evaluation of the Sophia Step Study has been undertaken, providing details of the intervention delivery and context, as well as evidence for feasibility and a high degree of reach of the interventions [20]. Thus, the lack of intervention effect in this study cannot be explained by unsuccessful intervention delivery. However, the study sample was small (from 59–64 per group) and both baseline PA levels and PA changes varied appreciably in the study participants. Secondary analyses are planned to analyse PA patterns over time and responsiveness in metabolic control and cardiovascular risk of subgroups based on initial PA levels and changes in PA. Further analyses are also planned to assess the effect of the intervention on health-related quality of life and aspects of mental health.
The main strength of this study is the long intervention duration and repeated measures of both objectively measured PA and variation of cardiometabolic risk factor. The randomisation was successful with a between-group variation at baseline for waist circumference among women and systolic blood pressure only. Linear mixed model analyses included baseline values and between-group differences were thus accounted for. Both genders were included, representing the Swedish diabetes population with a majority of men. Recruitment was conducted during autumn, winter and spring to reduce seasonal variations in physical activity.
The study has some limitations. We aimed to target people with low levels of PA but apparently also reached individuals that were already sufficiently active in accordance with the recommended level of a minimum of 150 min of MVPA per week. The accelerometer used has limitations as it is not sensitive enough to detect certain activities (e.g., swimming and bicycling). However, the likelihood is low that the detection limit of the accelerometer may have affected the effect on PA between the groups. Although the Sophia Step Study was an RCT, internal validation could have been violated. The repeated study assessments for all three groups may have influenced motivation to comply with a healthy lifestyle and medication regimen in the intervention groups and among the control participants. The same diabetes specialist nurse met all participants, regardless of intervention allocation. The nurses were trained in motivational interviewing and we cannot rule out that there was a diffusion effect among the intervention groups. The dropouts had a slightly detrimental health status and less time in MVPA at baseline and might have benefited the most from increasing PA. Power was calculated based on the primary outcome, HbA1c, and we did not fully reach the intended sample size. There is thus a risk of a type 2 error when interpreting the findings. The study includes individuals with prediabetes and type 2 diabetes and was underpowered to detect an effect on HbA1c on the diagnostic groups separately. Finally, the study covered three primary care centres with slightly different catchment areas. However, the educational level of the sample is quite high and therefore the results may not generalise to all populations and settings.
Conclusion
This 2-year intervention, including self-monitoring of steps with or without counselling, prevented a decrease in PA but did not provide evidence for improved metabolic control and cardiometabolic risk factors in a population with prediabetes or type 2 diabetes.
Availability of data and materials
The datasets generated or analysed during the current study are not publicly available because data can be traced back to the study participants. According to Swedish and EU data legislation, access can only be granted upon a reasonable request. The request should be addressed to the PI and will be handled on a case-by-case basis. Any sharing of data will be regulated via a data transfer and use agreement with the recipient.
References
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2019;41(2):255–323.
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–79.
Swindell N, Mackintosh K, McNarry M, Stephens JW, Sluik D, Fogelholm M, et al. Objectively measured physical activity and sedentary time are associated with cardiometabolic risk factors in adults with prediabetes: the PREVIEW study. Diabetes Care. 2018;41(3):562–9.
Kennerly AM, Kirk A. Physical activity and sedentary behaviour of adults with type 2 diabetes: a systematic review. Pract Diabetes. 2018;35(3):86-+.
Moghetti P, Balducci S, Guidetti L, Mazzuca P, Rossi E, Schena F. Walking for subjects with type 2 diabetes: a systematic review and joint AMD/SID/SISMES evidence-based practical guideline. Nutr Metab Cardiovasc Dis. 2020;30(11):1882–98.
Bassett DR Jr, Toth LP, LaMunion SR, Crouter SE. Step counting: a review of measurement considerations and health-related applications. Sports Med. 2017;47(7):1303–15.
Qiu S, Cai X, Chen X, Yang B, Sun Z. Step counter use in type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Med. 2014;12:36.
Vaes AW, Cheung A, Atakhorrami M, Groenen MT, Amft O, Franssen FM, et al. Effect of ‘activity monitor-based’ counseling on physical activity and health-related outcomes in patients with chronic diseases: a systematic review and meta-analysis. Ann Med. 2013;45(5–6):397–412.
Baskerville R, Ricci-Cabello I, Roberts N, Farmer A. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34(5):612–20.
Hodkinson A, Kontopantelis E, Adeniji C, van Marwijk H, McMillan B, Bower P, et al. Accelerometer- and pedometer-based physical activity interventions among adults with cardiometabolic conditions: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(10):e1912895.
Franssen WMA, Franssen GHLM, Spaas J, Solmi F, Eijnde BO. Can consumer wearable activity tracker-based interventions improve physical activity and cardiometabolic health in patients with chronic diseases? A systematic review and meta-analysis of randomised controlled trials. Int J Behav Nutr Phys Act. 2020;17(1):57.
Alothman S, Yahya A, Rucker J, Kluding PM. Effectiveness of interventions for promoting objectively measured physical activity of adults with Type 2 diabetes: a systematic review. J Phys Act Health. 2017;14(5):408–15.
Hodkinson A, Kontopantelis E, Adeniji C, van Marwijk H, McMillian B, Bower P, et al. Interventions using wearable physical activity trackers among adults with cardiometabolic conditions: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(7):e2116382.
Cai X, Qiu SH, Yin H, Sun ZL, Ju CP, Zugel M, et al. Pedometer intervention and weight loss in overweight and obese adults with Type 2 diabetes: a meta-analysis. Diabet Med. 2016;33(8):1035–44.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–8.
Chaudhry UAR, Wahlich C, Fortescue R, Cook DG, Knightly R, Harris T. The effects of step-count monitoring interventions on physical activity: systematic review and meta-analysis of community-based randomised controlled trials in adults. Int J Behav Nutr Phys Act. 2020;17(1):129.
Wahlich C, Chaudhry UAR, Fortescue R, Cook DG, Hirani S, Knightly R, et al. Effectiveness of adult community-based physical activity interventions with objective physical activity measurements and long-term follow-up: a systematic review and meta-analysis. BMJ Open. 2020;10(5):e034541.
Rossen J, Yngve A, Hagstromer M, Brismar K, Ainsworth BE, Iskull C, et al. Physical activity promotion in the primary care setting in pre- and type 2 diabetes - the Sophia step study, an RCT. BMC Public Health. 2015;15:647.
Taylor-Piliae RE, Norton LC, Haskell WL, Mahbouda MH, Fair JM, Iribarren C, et al. Validation of a new brief physical activity survey among men and women aged 60–69 years. Am J Epidemiol. 2006;164(6):598–606.
Rossen J, Hagströmer M, Yngve A, Brismar K, Ainsworth B, Johansson UB. Process evaluation of the Sophia Step Study- a primary care based three-armed randomized controlled trial using self-monitoring of steps with and without counseling in prediabetes and type 2 diabetes. 2021.
Select Wellness. http://steg.se/en/start.aspx. Accessed 17 June 2021.
Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15(2):175–83.
Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice-Hall; 1986.
Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–14.
Miller WR, Rollnick S. Motivational interviewing: helping people change. New York: Guilford Press; 2013.
Rossen J, Buman MP, Johansson U-B, Yngve A, Ainsworth B, Brismar K, et al. Reallocating bouted sedentary time to non-bouted sedentary time, light activity and moderate-vigorous physical activity in adults with prediabetes and type 2 diabetes. PLoS One. 2017;12(7):e0181053.
Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64.
Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–81.
Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81.
Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61(3):310–7.
Koller M. robustlmm: an R package for robust estimation of linear mixed-effects models. J Stat Softw. 2016;75(6):24.
Nazu N, Lindström J, Rautiainen P, Tirkkonen H, Wikström K, Repo T, et al. Maintenance of good glycaemic control is challenging - a cohort study of type 2 diabetes patient in North Karelia Finland. Int J Clin Pract. 2019;73:e13313.
Hall KS, Hyde ET, Bassett DR, Carlson SA, Carnethon MR, Ekelund U, et al. Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. Int J Behav Nutr Phys Act. 2020;17(1):78.
Swedish National Diabetes Registry. https://www.ndr.nu/#/knappen. Accessed 17 June 2021.
Johnson ST, Mundt C, Qiu W, Soprovich A, Wozniak L, Plotnikoff RC, et al. Increase in daily steps after an exercise specialist led lifestyle intervention for adults with type 2 diabetes in primary care: a controlled implementation trial. J Phys Act Health. 2015;12(11):1492–9.
De Greef KP, Deforche BI, Ruige JB, Bouckaert JJ, Tudor-Locke CE, Kaufman JM, et al. The effects of a pedometer-based behavioral modification program with telephone support on physical activity and sedentary behavior in type 2 diabetes patients. Patient Educ Couns. 2011;84(2):275–9.
Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the early ACTID randomised controlled trial. Lancet. 2011;378(9786):129–39.
Dasgupta K, Rosenberg E, Joseph L, Cooke AB, Trudeau L, Bacon SL, et al. Physician step prescription and monitoring to improve ARTERial health (SMARTER): a randomized controlled trial in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2017;19(5):695–704.
Yates T, Edwardson CL, Henson J, Gray LJ, Ashra NB, Troughton J, et al. Walking away from type 2 diabetes: a cluster randomized controlled trial. Diabet Med. 2017;34(5):698–707.
Harris T, Kerry SM, Limb ES, Victor CR, Iliffe S, Ussher M, et al. Effect of a primary care walking intervention with and without nurse support on physical activity levels in 45- to 75-year-olds: the Pedometer And Consultation Evaluation (PACE-UP) cluster randomised clinical trial. PLoS Med. 2017;14(1):e1002210.
Plotnikoff RC, Karunamuni N, Courneya KS, Sigal RJ, Johnson JA, Johnson ST. The Alberta Diabetes and Physical Activity Trial (ADAPT): a randomized trial evaluating theory-based interventions to increase physical activity in adults with type 2 diabetes. Ann Behav Med. 2013;45(1):45–56.
Rowlands A, Davies M, Dempsey P, Edwardson C, Razieh C, Yates T. Wrist-worn accelerometers: recommending ~1.0 mg as the minimum clinically important difference (MCID) in daily average acceleration for inactive adults. Br J Sports Med. 2020. https://doi.org/10.1136/bjsports-2020-102293.
Chudowolska-Kiełkowska M, Małek ŁA. A nurse-led intervention to promote physical activity in sedentary older adults with cardiovascular risk factors: a randomized clinical trial (STEP-IT-UP study). Eur J Cardiovasc Nurs. 2020;19(7):638–45.
Guglani R, Shenoy S, Sandhu JS. Effect of progressive pedometer based walking intervention on quality of life and general well being among patients with type 2 diabetes. J Diabetes Metab Disord. 2014;13(1):110.
Balducci S, D’Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, et al. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: the IDES_2 randomised clinical trial. JAMA. 2019;321(9):880–90.
Acknowledgements
Thanks to:
The diabetes specialist nurses and study participants engaged in the Sophia Step Study.
Funding
This work was supported by the Sophiahemmet Foundation, The Swedish Diabetes Foundation, the King Gustav V and Queen Victoria’s Order of Freemason’s research foundation, Forte (Vårdalsstiftelsen) and the Skandia and Sibling Svensson’s Foundation for Medical Research. The funders had no role in the study design or conduct, data collection, management, analysis or interpretation, manuscript preparation, review or approval. Open access funding provided by Sophiahemmet University College.
Author information
Authors and Affiliations
Contributions
AY, BA, JR, KB, MH and U-B J contributed to the design and evaluation plan of the Sophia Step Study. JR and LÅ collected the data with help from the diabetes specialist nurses. KL analysed the physical activity data from the accelerometer, double-checked and cleaned the data files. An external statistician analysed the data and JR, KL, MH and U-B J assisted in the validation and interpretation of the analyses. JR developed the manuscript in close collaboration with KL. AY, BA, KB, MH, LÅ and U-B J reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Regional Ethical Review Board (Dnr.2012/1570–31/3) and registered at ClinicalTrials.gov (NCT02374788). All participants signed informed consent before study participation. The research was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table.
Description of dropouts.
Additional file 2: Table.
Description per diagnose (prediabetes and type 2 diabetes).
Additional file 3: Table.
Description of changes in medications.
Additional file 4: Figure.
Mean change in HbA1c for each group over 24 months with confidence intervals included.
Additional file 5: Figure.
Mean change in MVPA and daily steps for each group over 24 months with confidence intervals included.
Additional file 6: Table.
Description of changes in each group.
Additional file 7: Table.
Effects on secondary outcomes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rossen, J., Larsson, K., Hagströmer, M. et al. Effects of a three-armed randomised controlled trial using self-monitoring of daily steps with and without counselling in prediabetes and type 2 diabetes—the Sophia Step Study. Int J Behav Nutr Phys Act 18, 121 (2021). https://doi.org/10.1186/s12966-021-01193-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-021-01193-w