Abstract
Cancers of the female reproductive system include ovarian, uterine, vaginal, cervical and vulvar cancers, which are termed gynecologic cancer. The emergence of long noncoding RNAs (lncRNAs), which are believed to play a crucial role in several different biological processes, has made the regulation of gene expression more complex. Although the function of lncRNAs is still rather elusive, their broad involvement in the initiation and progression of various cancers is clear. They are also involved in the pathogenesis of cancers of the female reproductive system. LncRNAs play a critical physiological role in apoptosis, metastasis, invasion, migration and cell proliferation in these cancers. Different expression profiles of lncRNAs have been observed in various types of tumors compared with normal tissues and between malignant and benign tumors. These differential expression patterns may lead to the promotion or suppression of cancer development and tumorigenesis. In the current review, we present the lncRNAs that show a differential expression between cancerous and normal tissues in ovarian, cervical and endometrial cancers, and highlight the associations between lncRNAs and some of the molecular pathways involved in these cancers.
Similar content being viewed by others
Background
Several organs of the female reproductive system like the uterus, cervix, ovaries, vagina and vulva are prone to be affected by cancer. The most widespread types of gynecological cancers are uterine cancer of the endometrium, followed by cervical cancer [1]. While ovarian tumors are relatively rare in comparison to other gynecologic tumors and are only the third [2] most common gynecological cancer, they represent the seventh cause of death by all cancer in women worldwide [1]. About 1–2% of all gynecologic malignancies are due to primary carcinoma of the vagina (PCV) which predominantly affects postmenopausal women [3, 4]. Vulvar cancer, which is the twentieth most common cancer in women, is very rare and is associated with HPV infection [4, 5]. Primary fallopian tube carcinoma (PFTC) causes about 0.14%–1.8% of female reproductive malignancies. However, PFTC resembles epithelial ovarian cancer (EOC) in histological and clinical terms [6] and it is thought that at least a subset of ovarian tumors could originate in the Fallopian tube. Therefore, “ovarian cancer” often refers to tumors of both ovarian and Fallopian origin [7, 8].
Endometrial carcinoma
There are two types of endometrial carcinoma (EC); type I EC and type II EC. These two types are distinguishable on the basis of their pathological and demographic properties. Type I EC, or endometrioid endometrial carcinoma (EEC), develops in hyperplastic endometrial tissue and can affect women both before and after menopause. There is a correlation between type I EC and relatively high levels of circulating estrogen [9]. On the other hand, type II EC, also known as non-Endometrioid Endometrial Carcinoma (NEEC), emerges in the post-menopausal period. Since about 50% of type II tumors recur in the 5 years following surgical resection, their prognosis is poor [10].
Cervical cancer
Cervical cancer is clinically associated with persistent infection with a ‘high-risk’ subset of human papillomaviruses (HPVs) [11, 12]. There are two subtypes of cervical cancer: squamous cell carcinoma and adenocarcinoma. The thin flat cells that cover the outer part of the cervix lead to squamous cell carcinoma. On the other hand, globular-shaped cells on the inner part of the cervix lead to the less common adenocarcinoma [11, 13]. Screening tests, including conventional cytology, are used to identify pre-cancerous lesions. This method, which is known as the Pap smear, can be utilized for their early detection, thereby helping to optimize therapy [14]. Since there are an estimated 12,990 cases of invasive cervical cancer and 4120 deaths per year worldwide, it is critical to find better biomarkers for an earlier diagnosis to improve treatment efficacy and disease outcome [15].
Ovarian cancer
The fifth leading cause of cancer death in women in developed countries is ovarian cancer. Since the introduction of platinum-based drugs in clinical practice, no significant improvements in ovarian cancer therapies has taken place over the past four decades [16, 17]. Ovarian cancer is a pathology covering a heterogeneous group of tumors originating in the epithelial cells, germ cells, mesenchyme and Fallopian tube. However, approximately 85–90% of ovarian cancers are of epithelial origin. If the tumor is diagnosed early while it is still limited to a single ovary (Stage I FIGO), five-year survival exceeds 80%. However, owing to the lack of symptoms during the early stages, diagnosis is often delayed until when the disease has already spread to the peritoneal cavity. Therefore, the average 5-year survival for ovarian cancer patients when all stages are considered does not exceed 40% [18]. The two main ovarian cancer diagnostic tests are transvaginal ultrasonography (TVUS) and the measurement of cancer antigen (CA-125) concentration in blood [19], although they lack specificity and sensitivity. The advent of accurate diagnostic tools for ovarian cancer in the early stages and the development of new therapeutic approaches are thus urgently needed to improve the outcome of this pathology.
Long non-coding RNAs (LncRNAs)
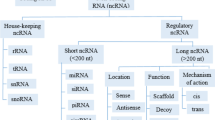
Only 2% of the human genome accounts for protein-coding regions, yet more than 70% of the human genome is transcribed in RNA that does not encode proteins [20]. Whether the entirety of the cellular transcriptional output has a functional role is still unknown, and to date only a few ncRNAs have been studied in detail. However, this non-coding part of the genome plays many key roles in the majority of the most critical biological processes such as development, differentiation and the cell cycle, as well as in cancers and other diseases [21]. NcRNAs are generally divided into two classes according to their size [22, 23]. Small ncRNAs are 20–200 nucleotides (nt) in size and long ncRNAs (lncRNAs) are longer than 200 nt, and can exceed 100,000 nt [24, 25]. These lncRNAs can be categorized as exonic, intronic, overlapping or intergenic according to their proximity to the nearest protein-coding transcripts (Fig 1).
Roles of LncRNAs in cancer
During tumorigenesis, down- or up-regulation of specific lncRNAs occurs relative to the corresponding normal tissues. These lncRNAs thus behave like tumor suppressors or oncogenes [26]. For instance, it has been found that HOTAIR lncRNA overexpression correlates with aggressive breast [27], ovarian [28], cervical [29], endometrial [30], colorectal [31], hepatocellular [32] and gastrointestinal stromal tumors [33], whereas MEG3 lncRNA may act as a tumor suppressor in a variety of human cancers such as ovarian cancer, breast cancer, hepatocarcinoma and uterine cancer [34]. The growth of several cancer cell lines (such as HeLa and MCF7) is inhibited by MEG3 overexpression [35]. Apoptosis in PLC/PRF/5, HepG2, U251 and U87 MG cells is also increased by MEG3 overexpression [34, 36, 37]. A comprehensive overview of lncRNAs in cancer in general was published recently by Evans et al. [38].
Epigenetic regulation
Cancer can be considered as a disease that is caused by and/or involves aberrant expression of several sets of genes. These modifications include both mutational and epigenetic changes such as methylation, acetylation and phosphorylation of chromatin [39]. Some important cellular genes that are in charge of proliferation, apoptosis and stem cell differentiation undergo epigenetic modifications in cancer [40]. It has been demonstrated that some lncRNAs act by collaborating with Polycomb group repressive complexes (PRC1 and PRC2), which are responsible for the establishment and maintenance of transcriptionally repressive epigenetic modifications in chromatin: trimethylation on lysine 27 of histone 3 (H3K27Me3) and ubiquitinylation on lysine 119 of histone 2A (H2AK119Ub) for PRC2 and PRC1 respectively [41, 42]. Since PRC1 and 2 are believed to be oncogenic drivers in many kinds of cancer [43, 44], this collaboration is especially relevant. For example, the oncogenic lncRNA FAL1 (focally amplified lncRNA on chromosome 1) is in complex with the PRC1 complex subunit BMI1, and this interaction is necessary for FAL1 to exert its oncogenic functions [45]. Because approximately 20% of lncRNAs bind to PRC2 [45], it is thought that this mode of action if one of the main ones. However, it has been shown that PRC2 binds to lncRNAs in a promiscuous way [46]. Several recent publications have reported both specific and non-specific PRC2 binding to lncRNAs. Although lncRNA binding to PRC2 is probably not always specific, numerous cases of PRC2/lncRNA interaction have been shown to be necessary for specific functions [47, 48]. In addition, recent findings suggest that these observations are not mutually exclusive [49].
Epigenetic control of gene expression mediated by lncRNAs also involves transcriptional activation through physical interaction with the MLL complex, which is necessary for the deposition of transcriptionally activating trimethylation on lysine 4 of histone 3 (H3K4Me3) in some instances. An example of such a mode of action is the functional interaction and cooperation between the lncRNA HOTTIP and the MLL complex subunit WDR5 [50].
Interaction with miRNAs
MiRNAs are an important class of small non-coding RNAs that are involved in several diseases such as cancer [51]. Their main mode of action is through binding to target mRNAs via sequence complementarity, resulting in translation inhibition and mRNA destabilization. Several lncRNAs are capable of binding to miRNAs, thereby preventing them from acting on their target mRNAs. LncRNAs involved in such interactions are termed competing endogenous RNAs (ceRNAs), of which several examples with a role in cancer have been described. For example, MEG3 was recently reported to influence STAT3 expression by altering miR-21 expression in ovarian cancer [34]. A large number of lncRNAs/miRNAs relationships have been reported and/or suggested recently. However, to what extent these interactions are functionally relevant in cells remains a matter of debate [52].
Another relationship existing between lncRNAs and miRNAs is when a lncRNA itself is a precursor RNAs for miRNAs. For example, H19 is a precursor RNA for miR-675. Therefore, the study of the effects of such lncRNA involves taking into account the targets of its derived miRNA [53].
LncRNAs with a role in several cancers of the female reproductive system
Table 1 and Fig. 2 present the roles and functions of lncRNAs, discussed below, that have been studied in several cancers of the female reproductive system.
MALAT1
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), also known as NEAT2 (noncoding nuclear-enriched abundant transcript 2), is an 8000 nt-long lncRNA located in chr11q13.1. It is involved in several physiological processes such as epigenetic change of gene expression, nuclear organization and alternative splicing through the modulation of SR splicing factor phosphorylation. It is directly related to a variety of pathological processes ranging from diabetes to cancers [54, 55]. MALAT1 up-regulation has been observed in various types of cancer, where it is associated with tumorigenesis and a decrease in overall survival [56–58].
In endometrioid endometrial cancer, it has been shown that MALAT1 and miR-200c are reciprocally repressed and bound together. When this interaction was altered, EMT was decreased, as well as the invasive capacity of RL-952 cells, leading to a reduced in vivo endometrioid endometrial cancer cell growth in a xenograft model [59].
In addition, high levels of MALAT1 have been reported in endometrioid endometrial cancer [60], in relation with aberrant activation of the wnt/beta-catenin pathway where the wnt-effector transcription factor TCF4 interacts with the MALAT1 promoter region. This wnt/beta-catenin aberrant activation is caused by the loss of expression of the tumor suppressor PCDH10 which normally represses Wnt/beta-catenin activation. Interestingly, PCDH10 is involved in several malignancies such as hepatocellular, colorectal, bladder, nasopharyngeal and cervical cancers, [61–63], while MALAT1 is overexpressed in hepatocellular [64], colorectal [65], bladder [66] and cervical cancer [67]. Whether PDCH10 is involved in MALAT1 overexpression in all these malignancies remains to be determined.
Additionally in cervical cancer, higher levels of MALAT1 are found in cancer tissues compared to normal cervix and are associated with a poor prognosis. MALAT1 is overexpressed in the cervical cancer CaSki cell line and subsequently promotes growth and invasion as well as decreasing apoptosis [68, 69]. MALAT1 is also involved in proliferation in the same cell line, where the expression of cell cycle regulation molecules cyclin D1, cyclin E and CDK6 is decreased following MALAT1 gene knockdown. As a result, cells in the G1 phase are significantly increased [70].
MALAT1 is also overexpressed in the ovarian cancer cell line SKOV3ip, which is derived from SKOV3 with a more metastatic phenotype [71]. Furthermore, MALAT1 inhibition markedly suppresses tumorigenicity in SKOV3 ovarian cancer cells and changes the expression of several genes that are involved in cell proliferation, metastasis and apoptosis. However, the mechanisms by which MALAT1 regulates gene expression in this context is still unclear and requires more detailed evaluation [72].
HOTAIR
The HOX transcript antisense intergenic RNA (HOTAIR) lncRNA is 2158 nucleotides long and has 6 exons. It is located at the antisense strand of the HOXC gene cluster on chromosome 12q13.13, and was described by Rinn et al. for the first time for its involvement in the determination of the proximal-distal axis during development [47, 73, 74].
HOTAIR gene expression is responsive to estradiol in endometrial carcinoma [75] and when its expression is increased, it leads to increased rates of metastasis and reduced overall survival [30]. Reduction of HOTAIR expression results in a decrease in HEC-1A endometrial cancer cell tumorgenicity and decreases tumor sizes in an in vivo model of xenografted HEC-1A cells [76].
In epithelial ovarian carcinoma, there is a correlation between HOTAIR expression and metastatic stage. The regulation of specific matrix metalloproteinases (MMPs) and EMT-related genes is thought to be responsible for this correlation [77], suggesting that HOTAIR expression levels might constitute a prognostic factor for decreased overall survival [78]. Moreover, in several ovarian cancer cell lines, the expression of HOTAIR causes resistance to cisplatin through wnt/β-catenin pathway activation [79].
In cervical cancer, VEGF and MMP-9 expression are up-regulated by HOTAIR. These two factors play a crucial role in tumor development by increasing migration and invasion. HOTAIR is also correlated with recurrence of cervical cancer. [80, 81].
H19
H19 is a paternally imprinted gene that encodes a 2300-nt-long lncRNA located in chromosome 11p15.5. It is a bi-functional RNA, acting both as a precursor for miR-675 and a lncRNA [82, 83]. H19 is an oncofetal gene, meaning that it is expressed only in the embryo and not in adult tissue under physiological conditions. However, it is re-expressed in tumors of various origins [84]. It has a maternal expression and its neighboring gene insulin-like growth factor 2 (IGF2) has a paternal allele transcription. Together with IGF2, H19 plays a key role in the normal menstrual cycle and in early pregnancy [85]. Estradiol (E2) has a positive effect on H19 up-regulation in the endometrium. On the other hand, progesterone causes H19 to undergo a down-regulation [54, 85]. H19 induces the expression of a protein superfamily of mitogen-activated protein kinase (MAPK) that includes c-jun, JNK1/2 and the extracellular signal-regulated kinases (ERK) 1 and 2, which are involved in some aspects of the tumorigenic processes in several types of human cancers [86, 87].
Furthermore, H19 has another effect on cell-to-cell adhesion by inhibiting the expression of genes that are involved in this mechanism, including the beta-5, beta-3 and alpha-4 integrins. Thus, the increased motility and invasive potential of some tumor cells might result from this integrin down-regulation [88]. In line with these observations, H19 is overexpressed in ovarian carcinomas, which correlates with the expression of pro-metastatic genes. [89]. In ovarian cancer cells, H19 overexpression enhances migration and invasion [90]. In addition, H19 sequestering of let-7 is required for H19 to function in EMT processes such as cell invasion and migration in ovarian cancer, as well as in uterine serous carcinoma cell lines [91].
H19 expression levels increase throughout endometrial epithelium tumorigenesis. Normal endometrial epithelium has a low level of H19 expression while levels are higher in hyperplastic endometrium. The levels are very high in endometrial carcinoma and even higher during tumor tissue dedifferentiation.
Furthermore, in cervical cancer, markedly increased levels of IGF2 expression and decreased levels of H19 expression have been reported in comparison with those of normal cervical tissues. However, the mechanism promoting this dysregulation is still unclear and needs to be further investigated [92].
MEG3
Maternally expressed gene (MEG3) is a 1600-nt-long lncRNA located in the locus of DLK1-MEG3 on human chromosome 14q32.3. It was first identified as the ortholog of gene trap locus 2 (Gtl2) in mice by Schuster-Gossler [93]. Many normal human tissues express MEG3 and the loss of MEG3 expression has been reported in several types of cancer. MEG3 overexpression can lead to inhibition of proliferation and increased apoptosis in cancerous cells in several ways, either by inducing p53 or in the absence of p53 [94].
MEG3 expression is significantly reduced in ovarian cancer tissue compared to normal ovarian tissue, and its overexpression causes inhibition of growth and proliferation and induces apoptosis in the OVCAR3 ovarian cancer cell line [34, 95]. The other possible mechanism by which MEG3 suppresses tumor growth in this cancer is the RB pathway, in a way that does not depend on p53 in various other cancers such as human pituitary tumors [96].
Unlike normal adjacent tissues, cervical cancer tissues markedly express lower levels of MEG3. Furthermore, MEG3 down-regulation correlates positively with increased tumor size, advanced FIGO stage, metastasis of lymph nodes and HR-HPV positivity. In addition, growth suppression and increased apoptosis of cervical cancer cells is observed after MEG3 upregulation, which demonstrates its tumor suppressive role in this cancer [97].
CCAT2
Colon cancer-associated transcript 2 (CCAT2) is 1752 nt in size and is located at the 8q24.21 chromosomal region. This lncRNA induces tumor metastasis, progression and chromosomal instability in many different kinds of cancer including gynecologic cancers such as ovary and cervix [98].
Levels of CCAT2 gene expression are higher in tissues and cell lines of ovarian cancer than in corresponding normal tissues. Interestingly, patients who express high levels of CCAT2 have poor prognostic markers such as FIGO stage and distant metastasis. Furthermore, these patients have a much poorer prognosis than those with low CCAT2 gene expression. In addition, in the event of CCAT2 silencing in ovarian cancer cells, cell proliferation, migration and invasion are significantly suppressed [98].
When CCAT2 expression level is inhibited by transfection of siRNA in cervical cancer cells, it leads to significant suppression of their proliferation and survival. There is a correlation between CCAT2 and metastasis which reveals a poor prognosis in patients with cervical cancer [12]. However, the mechanisms mediating the mode of action of CCAT2 in cervical cancer are still unclear [99].
ANRIL
Antisense non-coding RNA in the INK4 locus (ANRIL) is a 3800-nt-long lncRNA located at the 9p21 chromosomal region. It has been shown that ANRIL regulates its neighbor tumor suppressors CDKN2A/B through epigenetic mechanisms and thereby plays a role in cell proliferation and senescence [100].
ANRIL is considered as an independent prognostic factor in ovarian cancer. By MET and MMP3 modulation, increased migration and invasion results from in vitro ANRIL overexpression in ovarian cancer cell lines. [101]. ANRIL also affects proliferation, which is correlated with the promotion of cell cycle progression and suppression of apoptosis and senescence. The reduced expression of P15INK4B and increased expression of the anti-apoptotic Bcl-2 might explain this phenotype [102, 103].
In cervical cancer cells, the lack of ANRIL expression increases p15 levels but does not influence the expression of p16 or alternative reading frame (ARF) and leads to cell-cycle arrest at the G2/M phase, resulting in suppression of proliferation [104].
OVAL
Ovarian adenocarcinoma amplified lncRNA (OVAL) is a 1489-nt-long lncRNA whose locus is on chromosome 1q25.3. Genomic evaluation of lncRNAs in high-grade serous ovarian carcinoma (HGS-OvCa) has shown an increase in DNA copy-number of OVAL in 3.9% of ovarian tumors from the TCGA dataset, which is related to an increase in expression of this lncRNA and could be associated with altered p53 activity [105].
OVAL has also been explored in endometrial cancer where type I EC overexpresses OVAL, while it is downregulated in type II EC [106]. Overall, further studies will be needed to characterize the precise roles and function of this lncRNA.
UCA1a (CUDR)
Cancer up-regulated drug resistant (CUDR) is a 2200-nt-long lncRNA located in the 19p13.1 chromosomal region. Like H19, CUDR is a feto-oncogene expressed only in fetal tissue under normal conditions, except in cardiac tissue [107]. It is re-expressed and up-regulated in cancer tissues from various malignancies including bladder [108] and breast [107]. Some reports suggests that CUDR may play an important role in drug resistance and the transformation of cells through several mechanisms, including caspase 3 down-regulation [109].
CUDR overexpression has been implicated in the resistance of ovarian cancer cells to cisplatin [107] and is associated with a poor prognosis in this malignancy [110].
UCA1 has also been implicated in cervical cancer, where its overexpression promotes resistance of cervical cancer cells to cisplatin [111]. Interestingly, since CUDR is not easily detected in normal tissues and has a low level of baseline expression in comparison with other biomarkers of cancer such as CEA, it might be an effective biomarker to identify the development of cancer and cancer therapeutic responses. However, in order to utilize CUDR expression levels as prognosis tool, further evaluations are required.
SRA
The steroid receptor RNA activator (SRA) is a 2000-nt-long LncRNA that is located in the 5q31.3 human chromosomal region. It plays a role in regulating the expression of genes induced by steroid receptors. SRA up-regulation has been observed in breast cancer and other tissues which are responsive to steroids, as well as in ovarian cancer.
Regardless of histological tumor grade, endometrial adenocarcinoma tissues consistently express SRA at higher levels than healthy reference tissue samples. This suggests an early role of SRA in the process of tumorigenesis in this tissue. Interestingly, SRA-transgenic mice have been demonstrated to have an elevated rate of apoptosis in order to counteract increased mitotic activity. This suggests that SRA upregulation in tumor tissues could be associated with a loss of its apoptosis-stimulating activities [112].
LncRNAs with a role in ovarian cancer
Table 2 combines the roles and functions of lncRNAs discussed below that have been studied specifically in ovarian cancer.
FAL1
Focally amplified lncRNA on chromosome1 (FAL1) is a 566-nt-long lncRNA and is located on chromosome 1q23.3. Cellular senescence is induced and proliferation rates are hampered in cancer cell lines from various tumoral localizations when FAL1 is knocked down [113]. FAL1 is associated with BMI1, a member of the PRC1 protein complex, and regulates its stability by inhibiting BMI1 degradation. PRC1 can thus repress target gene promoters including tumor suppressor p21. As a result, the cell cycle is dysregulated and tumorigenesis is increased. Furthermore, it has been demonstrated that FAL1 expression is correlated with reduced survival in ovarian cancer patients. Conversely, down-regulation of FAL1 by siRNA delivery in mice xenografted with ovarian carcinoma cells attenuates tumor growth, decreases cell proliferation and increases apoptosis [114].
AB073614
AB073614 is a 1900-nt-long lncRNA located on chromosome 3q24. It has recently been reported to be overexpressed in ovarian cancer tissues in comparison with adjacent normal tissue. In vitro down-regulation of AB073614 in xenografted mice results in attenuated tumor growth and causes the expression of the proliferation and invasion related proteins PCNA, MMP2 and MMP9 to be decreased. In addition, the ovarian cancer cell lines HO-8910 and OVCAR3 overexpress AB073614 at high levels in vitro, and down-regulation of this lncRNA reduces proliferation and results in cell death in these cell lines. Furthermore, in response to down-regulation of AB073614, the expression of the proliferation and invasion related proteins PCNA, MMP2 and MMP9 is reduced in these cells. Also, members of key signaling pathways such as the phosphorylated forms of AKT and ERK are decreased. AB073614 thus seems to play a key role in some critical processes of ovarian cancer, but its precise mechanisms of action have not yet been described [115].
HOST2
Human ovarian cancer-specific transcript 2 (HOST2) is 1800-nt-long and is located on chromosome 10q23.1. It is the second member of the five human ovarian cancer specific transcripts (HOSTs) that has been reported to be over-expressed in ovarian cancer, and it promotes proliferation and migration in cancer cells as well as tumor progression in xenografted mice. The tumorigenic effects of HOST2 depend on this its ability to behave as a molecular sponge for let-7b, for which it is a direct target, thereby impeding the capacity of let-7b to down-regulate target genes such as myc, hmga2, dicer and imp3 [116].
LSINCT5
Another lncRNA is human ovarian cancer-specific transcript (LSINCT5) which is 2430 nt long and is situated on chromosome 5q15.33. Many breast and ovarian cancer cell lines as well as tumor sample panels have been reported to overexpress LSINCT5. In addition, knocking down LSINCT5 has been shown to impair cellular proliferation as well as to modify the expression of a number of genes, several of which might play a crucial role in cancer progression. LSINCT5 is considered as a stress-regulated lncRNA and a novel nuclear-expressed gene that might have an important role in cellular proliferation in the development of breast and ovarian cancer [117].
PVT1
PVT1 is a 2430-nt-long lncRNA located in the 8q24 chromosomal region and neighboring myc, which is amplified in about half of ovarian carcinomas. Guan et al. reported that MYC and PVT1 play independent oncogenic roles in breast and ovarian cancer. However, this idea has recently been challenged by Tseng et al. [118]. The latter group indicated that elevated levels of MYC protein in 8q24-amplified cell lines are dependent on the expression of PVT1 RNA. Furthermore, the correlation of both pvt1 and myc duplication in almost every tumor bearing myc amplification are in favor of this co-dependence. More than 45% of 500 ovarian cancers from TCGA were shown to have amplification of both pvt1 and myc. However, fewer than 1% of cases displayed duplication of only myc or pvt1. The up-regulation of PVT1 in response to carboplatin-docetaxel treatment was recently demonstrated to be a determinant of p53 induction and TIMP1 mRNA expression as well as an associated decrease in cell proliferation. In the same study, tumor progression following down-regulation of PVT1 in tumor xenografts in mice was shown to be increased. This result is in opposition with the study by Tseng et al. who reported an oncogenic role for PVT1, thus underlining the need for further studies on the precise role of the myc/pvt1 association in ovarian cancer [119].
HOXA11-AS
HOXA11 antisense RNA (HOXA11-AS) is a 1620-nt-long lncRNA located in the 7p15.2 region. It was re-expressed in ovarian cancerous cells but not on the normal ovarian surface epithelium in a cohort study of 18 ovarian cancer patients. Furthermore, proliferation, migration and invasion decreased in the plasmid-based expression of HOXA11-AS in OVCA-433 and C19 ovarian cancer cell lines [80]. The expression of HOXA11-AS was reported to have no effect on the expression of its adjacent genes and no miRNA target site could be predicted, which suggests a possible trans-regulation of distant genes. Therefore, more studies are needed to elucidate its mechanisms [120].
Bc200
Brain cytoplasmic RNA 1 lncRNA (BCYRN1 or BC200) is 200 nt in size and its locus is on the 2p21 chromosomal region. BC200 expression decreases in ovarian cancer tissues in comparison to adjacent normal tissues, and BC200 suppression has been shown to increase the proliferation of ovarian cancer cells. Interestingly, its expression is induced by carboplatin, thus raising the sensitivity of ovarian cancer cells to this drug [121]. More studies are needed to elucidate its role in modulating sensitivity to cisplatin and its modes of regulation, which could lead to new ways of predicting and/or modulating the clinical response to this drug [122].
LncRNAs with a role in cervical cancer
Table 3 shows the roles and functions of the lncRNAs that have been studied specifically in cervical cancer.
LncRNA—EBIC
EZH2-binding lncRNA in cervical cancer (LncRNA—EBIC) is 1500 nt long and is located in the 12q22 chromosomal region. Only one study has focused on LncRNA—EBIC in cervical cancer while investigating differentially expressed lncRNAs in this pathology. LncRNA-EBIC was found to be able to increase cervical cancer cells invasion through the inhibition of E-cadherin expression. Owing to its association with EZH2, it has been suggested that it could be involved in recruiting the PRC2 complex to genes of interest, but this remains to be formally demonstrated [123].
GAS5
Growth arrest-specific transcript 5 (GAS5) lncRNA is 651 nt in size and located in the 1q25 chromosomal region [124]. GAS5 is considered to be an lncRNA with tumor-suppressor properties in cancers including breast cancer [125, 126]. Furthermore, it has been reported to be down-regulated in cervical cancer tissues and it also has a marked association with tumor development. In addition, some reports have demonstrated that it is an independent marker to predict clinical outcome in patients with cervical cancer. However, its precise mechanism of action remains to be elucidated by further studies [127].
CCHE1
Cervical carcinoma high-expressed 1 (CCHE1) lncRNA is 2500 nt in size and is located on chromosome 10. CCHE1 upregulation in cervical cancer is correlated with advanced FIGO stages, larger tumor size, invasion of the uterine corpus, and poor prognosis..CCHE1 binds to PCNA mRNA, increasing its expression and therefore cervical cancer cell proliferation, suggesting that CCHE1 could constitute a prognostic factor for cervical cancer [128].
lncRNA- LET
A recently identified lncRNA is lncRNA-Low Expression in Tumor (LET), which is 2600 nt in size and is located in the 15q24.1 chromosomal region. It is down-regulated in hepatocellular, gallbladder, esophageal and squamous cell carcinoma as well as in cervical cancer. It has been shown that there is a correlation between expression levels of LET in cervical cancer patients and their clinico-pathological parameters. LET could thus constitute an independent biomarker of prognosis, provided that these observations are confirmed by other studies [129].
Conclusion
On average, the 5-year survival of cancer patients has been drastically improved over the past decade, although several localizations still represent a major therapeutic challenge. In any case, more effective therapeutic strategies and reliable biomarkers are essential for improving the treatment of cancer.
In the case of gynecologic cancers, both endometrial and cervical cancers are more likely to be diagnosed in the early stages because of their symptoms and the availability of effective screening tools [130]. However, regarding ovarian cancer, late diagnosis owing to asymptomatic development of the disease in the early stages, as well as resistance to existing chemotherapeutic treatments, still pose major difficulties for patient care, and 5-year survival does not exceed 40% all stages considered [131, 132].
The study of lncRNAs in gynecological cancers is still in its infancy, although the last couple of years have seen an increasing number of publications on this topic. Already, the dysregulation of many lncRNAs has been reported in gynecological cancers and has been associated with clinical features, which could prove useful in the future for the design of new biomarkers for prognosis and diagnosis.
However, the exact mechanisms by which lncRNAs exert their action are only seldom studied in details, which underlines the need for more comprehensive studies on their modes of action before even considering using this knowledge to design new therapies or improve existing ones.
Whether some lncRNAs display almost ubiquitous roles in cancer, such as the protein coding p53, or if most of them will show context specific functions will be an interesting question to answer, and might influence the intensity of future efforts made to study the roles of lncRNAs in cancer.
MALAT1 for example is upregulated in many malignancies [55], and at least in ovarian cancer and cervical cancer MALAT1 promotes cell migration and invasion. In addition, MALAT1 is upregulated in EEC through PCDH10 silencing. Since PCDH10 is also silenced in cervical carcinoma, it could be of interest to investigate whether MALAT1 regulation and its effects are somewhat similar in different malignancies [60]. However, some degree of context dependency is to be expected in most cases, since the action of most of the lncRNAs is dependent on other partners, such as protein complexes like PRCs for the epigenetic regulation of gene expression and miRNAs in the case of ceRNA relationships.
The precise understanding of functions and mechanisms of action of lncRNAs may lead to the identification of new vulnerabilities in cancer cells. Direct targeting of lncRNAs through RNA interference is not possible because this technology is not yet available in routine clinical practice. However, once the pathways and proteins responsible for the actions of lncRNAs are identified, it could be possible to target them with specific drugs – possibly even already available drugs, depending on which targets are identified. Therefore, instead of targeting lncRNAs directly, it could be possible, at least in some instances, to simulate their actions in ways that are feasible in clinical practice.
In addition, the use of lncRNAs as biomarkers has triggered considerable excitement in the scientific community, and it can be reasonably expected that in the coming years some lncRNAs might become useful biomarkers for prognosis and for stratifying patients in order to tailor treatment to the needs of individual patients.
Reference
Ferlay J, et al. GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon: International Agency for Research on Cancer. 2010;2010:29.
Kalsi JK, Manchanda R, Menon U. Screening for gynecological cancers. Expert Review of Obstetrics & Gynecology. 2013;8(2):143–60.
Hildesheim A, et al. Sexually transmitted agents and risk of carcinoma of the vagina. Int J Gynecol Cancer. 1997;7(4):251–5.
Shilpa V. Molecular Profiling of Gynaecologic Malignancies: A Review. Hereditary Genetics: Current Research. 2016;2016:1–9.
Dunne EF, et al. CDC grand rounds: reducing the burden of HPV-associated cancer and disease. MMWR Morb Mortal Wkly Rep. 2014;63(4):69–72.
Heintz A, et al. Carcinoma of the fallopian tube. Int J Gynecol Obstet. 2006;95:S145–60.
Medeiros F, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30(2):230–6.
Kurman RJ, Shih I-M. The Origin and pathogenesis of epithelial ovarian cancer-a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433.
Cavanagh D, et al. Adenocarcinoma of the endometrium: an institutional review. Cancer Control. 1999;6:354–60.
Kitchener H, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet (London, England). 2009;373(9658):125–36.
Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4(1):46–55.
Kokka F, et al. Surgical treatment of stage IA2 cervical cancer. Cochrane Database Syst Rev. 2014;(5):CD010870. doi:10.1002/14651858.CD010870.pub2.
Eddy DM. Screening for cervical cancer. Ann Intern Med. 1990;113(3):214–26.
Peirson L, et al. Screening for cervical cancer: a systematic review and meta-analysis. Systematic reviews. 2013;2(1):1.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Jemal A, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
Huang L, et al. Improved survival time: What can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer. 2008;112(10):2289–300.
Kaku T, et al. Histological classification of ovarian cancer. Medical Electron Microscopy. 2003;36(1):9–17.
Force UPST. Screening for ovarian cancer: recommendation statement. The Annals of Family Medicine. 2004;2(3):260–2.
Khalil AM, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci. 2009;106(28):11667–72.
Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–504.
Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–7.
Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5(4):e1000459.
Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55.
Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13(12):1097–101.
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer discovery. 2011;1(5):391–407.
Gupta RA, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6.
Qiu J-j, et al. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol. 2014;134(1):121–8.
Huang L, et al. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch Gynecol Obstet. 2014;290(4):717–23.
He X, et al. The long non-coding RNA HOTAIR is upregulated in endometrial carcinoma and correlates with poor prognosis. Int J Mol Med. 2014;33(2):325–32.
Kogo R, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71(20):6320–6.
Yang Z, et al. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18(5):1243–50.
Niinuma T, et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72(5):1126–36.
Sheng X, et al. Promoter hypermethylation influences the suppressive role of maternally expressed 3, a long non-coding RNA, in the development of epithelial ovarian cancer. Oncol Rep. 2014;32(1):277–85.
Zhang X, et al. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. The Journal of Clinical Endocrinology & Metabolism. 2003;88(11):5119–26.
Braconi C, et al. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30(47):4750–6.
Wang P, Ren Z, Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem. 2012;113(6):1868–74.
Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Investig. 2016;126(8):2775.
Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36.
Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–59.
Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338(6113):1435–9.
Schwartz YB, Pirrotta V. A new world of Polycombs: unexpected partnerships and emerging functions. Nat Rev Genet. 2013;14(12):853–64.
Kleer CG, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci. 2003;100(20):11606–11.
Varambally S, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–9.
Hu X, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26(3):344–57.
Davidovich C, et al. Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol. 2013;20(11):1250–7.
Rinn JL, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–23.
Mondal T, et al. MEG3 long noncoding RNA regulates the TGF-[beta] pathway genes through formation of RNA-DNA triplex structures. Nat Commun. 2015;6:7743–60.
Davidovich C, et al. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol Cell. 2015;57(3):552–8.
Li Z, Rana TM. Decoding the noncoding: prospective of lncRNA-mediated innate immune regulation. RNA Biol. 2014;11(8):979–85.
Pereira DM, et al. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 2013;18(5):282–9.
McCaskill J, et al. RNA-mediated degradation of microRNAs: A widespread viral strategy? RNA Biol. 2015;12(6):579–85.
Vennin C, et al. H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-b. Oncotarget. 2015;6(30):29209–23.
Wu Y, et al. Long noncoding RNA MALAT1: insights into its biogenesis and implications in human disease. Curr Pharm Des. 2015;21(34):5017–28.
Yoshimoto R, et al. MALAT1 long non-coding RNA in cancer. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2016;1859(1):192–9.
Ji P, et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–41.
Lin R, et al. Control of RNA processing by a large non-coding RNA over-expressed in carcinomas. FEBS Lett. 2011;585(4):671–6.
Gutschner T, Hämmerle M, Diederichs S. MALAT1—a paradigm for long noncoding RNA function in cancer. J Mol Med. 2013;91(7):791–801.
Li Q, et al. Disrupting MALAT1/miR-200c sponge decreases invasion and migration in endometrioid endometrial carcinoma. Cancer Lett. 2016;383(1):28–40.
Zhao Y, et al. A novel wnt regulatory axis in endometrioid endometrial cancer. Cancer Res. 2014;74(18):5103–17.
Ying J, et al. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene. 2006;25(7):1070–80.
Zhong X, et al. Frequent epigenetic silencing of PCDH10 by methylation in human colorectal cancer. J Cancer Res Clin Oncol. 2013;139(3):485–90.
Narayan G, et al. Protocadherin PCDH10, involved in tumor progression, is a frequent and early target of promoter hypermethylation in cervical cancer. Genes Chromosom Cancer. 2009;48(11):983–92.
Lai M-c, et al. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol. 2012;29(3):1810–6.
Fűri I, et al. Cell Free DNA of Tumor Origin Induces a ‘Metastatic’Expression Profile in HT-29 Cancer Cell Line. PLoS One. 2015;10(7):e0131699.
Wu X-S, et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer biology & therapy. 2014;15(6):806–14.
Liu S, et al. MiR-375 Is Epigenetically Downregulated by HPV-16 E6 Mediated DNMT1 Upregulation and Modulates EMT of Cervical Cancer Cells by Suppressing lncRNA MALAT1. PLoS One. 2016;11(9):e0163460.
Yang L, et al. High MALAT1 expression predicts a poor prognosis of cervical cancer and promotes cancer cell growth and invasion. Eur Rev Med Pharmacol Sci. 2015;19(17):3187–93.
Zhang Y, et al. Human MALAT-1 long non-coding RNA is overexpressed in cervical cancer metastasis and promotes cell proliferation, invasion and migration. Journal of BU ON: official journal of the Balkan Union of Oncology. 2015;20(6):1497.
Guo F, et al. Inhibition of metastasis-associated lung adenocarcinoma transcript 1 in CaSki human cervical cancer cells suppresses cell proliferation and invasion. Acta Biochim Biophys Sin. 2010;42(3):224–9.
Liu S-P, et al. Identification of differentially expressed long non-coding RNAs in human ovarian cancer cells with different metastatic potentials. Cancer biology & medicine. 2013;10(3):138–41.
Liu S, et al. Inhibition of the long non-coding RNA MALAT1 suppresses tumorigenicity and induces apoptosis in the human ovarian cancer SKOV3 cell line. Oncol Lett. 2016;11(6):3686–92.
Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol. 2010;7(5):582–5.
Nie Y, et al. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104(4):458–64.
Bhan A, et al. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425(19):3707–22.
Huang J, et al. Lentivirus-mediated RNA interference targeting the long noncoding RNA HOTAIR inhibits proliferation and invasion of endometrial carcinoma cells in vitro and in vivo. Int J Gynecol Cancer. 2014;24(4):635–42.
Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer biology & medicine. 2015;12(1):1–9.
Qiu J-j, et al. The long non-coding RNA HOTAIR promotes the proliferation of serous ovarian cancer cells through the regulation of cell cycle arrest and apoptosis. Exp Cell Res. 2015;333(2):238–48.
Li J, et al. Overexpression of long non-coding RNA HOTAIR leads to chemoresistance by activating the Wnt/β-catenin pathway in human ovarian cancer. Tumor Biol. 2016;37(2):2057–65.
Kim HJ, et al. Long non-coding RNA HOTAIR is associated with human cervical cancer progression. Int J Oncol. 2015;46(2):521–30.
Kim HJ, et al. Long noncoding RNA HOTAIR is associated with human cervical cancer progression. Gynecol Oncol. 2014;133:5.
Smits G, et al. Conservation of the H19 noncoding RNA and H19-IGF2 imprinting mechanism in therians. Nat Genet. 2008;40(8):971–6.
Jing W, et al. The Significance of Long Noncoding RNA H19 in Predicting Progression and Metastasis of Cancers: A Meta-Analysis. Biomed Res Int. 2016;2016
Raveh E, et al. The H19 Long non-coding RNA in cancer initiation, progression and metastasis–a proposed unifying theory. Mol Cancer. 2015;14(1):1.
Ivanga M, et al. Temporal analysis of E2 transcriptional induction of PTP and MKP and downregulation of IGF-I pathway key components in the mouse uterus. Physiol Genomics. 2007;29(1):13–23.
Ayesh S, et al. Possible physiological role of H19 RNA. Mol Carcinog. 2002;35(2):63–74.
Kroon ME, et al. Hypoxia in combination with FGF-2 induces tube formation by human microvascular endothelial cells in a fibrin matrix: involvement of at least two signal transduction pathways. J Cell Sci. 2001;114(4):825–33.
Tselepis C, et al. Desmosomal adhesion inhibits invasive behavior. Proc Natl Acad Sci. 1998;95(14):8064–9.
Tanos V, et al. Expression of the imprinted H19 oncofetal RNA in epithelial ovarian cancer. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1999;85(1):7–11.
Matouk IJ, et al. Oncofetal H19 RNA promotes tumor metastasis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2014;1843(7):1414–26.
Marsh DJ, Shah JS, Cole AJ. Histones and their modifications in ovarian cancer–drivers of disease and therapeutic targets. Front Oncol. 2014;4:144.
Lee C, et al. Alterations in Promoter Usage and Expression Levels of Insulin-like Growth Factor-II and H19 Genes in Cervical and Endometrial Cancer. Cancer Res Treat. 2003;35(4):314–22.
Schuster-Gossler K, et al. The mouseGtl 2 gene is differentially expressed during embryonic development, encodes multiple alternatively spliced transcripts, and may act as an RNA. Dev Dyn. 1998;212(2):214–28.
Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15(1):1.
Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer. 2011;129(4):773–9.
Zhang X, Zhou Y, Klibanski A. Isolation and characterization of novel pituitary tumor related genes: a cDNA representational difference approach. Mol Cell Endocrinol. 2010;326(1):40–7.
Zhang J, et al. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer biology & therapy. 2016;17(1):104–13.
Huang S, et al. The long non-coding RNA CCAT2 is up-regulated in ovarian cancer and associated with poor prognosis. Diagn Pathol. 2016;11(1):1.
Le Wu LJ, Zhang W, Zhang L. Roles of Long Non-Coding RNA CCAT2 in Cervical Cancer Cell Growth and Apoptosis. Med Sci Monit. 2016;22:875.
Congrains A, et al. ANRIL: molecular mechanisms and implications in human health. Int J Mol Sci. 2013;14(1):1278–92.
Meryet-Figuière M, et al. An overview of long non-coding RNAs in ovarian cancers. Oncotarget. 2016;7(28):44719–34.
Qiu J-j, et al. The long non-coding RNA ANRIL promotes proliferation and cell cycle progression and inhibits apoptosis and senescence in epithelial ovarian cancer. Oncotarget. 2016;7(22):32478.
Qiu J, et al. The long non-coding RNA ANRIL promotes proliferation and cell cycle progression and inhibits apoptosis and senescence in epithelial ovarian cancer. Oncotarget. 2016;7(22):32478–92.
Naemura M, et al. Long noncoding RNA ANRIL regulates proliferation of non-small cell lung cancer and cervical cancer cells. Anticancer Res. 2015;35(10):5377–82.
Akrami R, et al. Comprehensive analysis of long non-coding RNAs in ovarian cancer reveals global patterns and targeted DNA amplification. PLoS One. 2013;8(11):e80306.
Smolle MA, et al. Long Non-Coding RNAs in Endometrial Carcinoma. Int J Mol Sci. 2015;16(11):26463–72.
Xue M, Chen W, Li X. Urothelial cancer associated 1: a long noncoding RNA with a crucial role in cancer. J Cancer Res Clin Oncol. 2016;142(7):1407–19.
Wang X-S, et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12(16):4851–8.
Tsang WP, et al. Induction of drug resistance and transformation in human cancer cells by the noncoding RNA CUDR. RNA. 2007;13(6):890–8.
Zhang L, et al. UCA1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother Pharmacol. 2016;77(3):629–34.
Wang B, et al. Expression of Long Noncoding RNA Urothelial Cancer Associated 1 Promotes Cisplatin Resistance in Cervical Cancer. Cancer Biother Radiopharm. 2017;32(3):101–10.
Lanz RB, et al. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Mol Cell Biol. 2003;23(20):7163–76.
Zhong X, Hu X, Zhang L. Oncogenic long noncoding RNA FAL1 in human cancer. Molecular & Cellular Oncology. 2015;2(2):e977154.
Sahu A, Singhal U, Chinnaiyan AM. Long noncoding RNAs in cancer: from function to translation. Trends in cancer. 2015;1(2):93–109.
Cheng Z, et al. A long noncoding RNA AB073614 promotes tumorigenesis and predicts poor prognosis in ovarian cancer. Oncotarget. 2015;6(28):25381.
Gao Y, et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum Mol Genet. 2015;24(3):841–52.
Silva JM, et al. LSINCT5 is over expressed in breast and ovarian cancer and affects cellular proliferation. RNA Biol. 2011;8(3):496–505.
Guan Y, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13(19):5745–55.
Tseng Y-Y, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512(7512):82–6.
Richards EJ, et al. A functional variant in HOXA11-AS, a novel long non-coding RNA, inhibits the oncogenic phenotype of epithelial ovarian cancer. Oncotarget. 2015;6(33):34745.
Wu D, et al. Downregulation of BC200 in ovarian cancer contributes to cancer cell proliferation and chemoresistance to carboplatin. Oncol Lett. 2016;11(2):1189–94.
Zhao M, et al. long non-coding RNAs involved in gynecological cancer. Int J Gynecol Cancer. 2014;24(7):1140–5.
Sun N-x, et al. Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS One. 2014;9(7):e100340.
Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54(6):787–93.
Pickard MR, Williams GT. Molecular and cellular mechanisms of action of tumour suppressor GAS5 LncRNA. Genes. 2015;6(3):484–99.
Pickard MR, Williams GT. The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget. 2016;7(9):10104.
Cao S, et al. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014;7(10):6776–83.
Yang M, et al. Long noncoding RNA CCHE1 promotes cervical cancer cell proliferation via upregulating PCNA. Tumor Biol. 2015;36(10):7615–22.
Jiang S, Wang H-L, Yang J. Low expression of long non-coding RNA LET inhibits carcinogenesis of cervical cancer. Int J Clin Exp Pathol. 2015;8(1):806–11.
Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007;25(20):2873–83.
Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Coleman RL, et al. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10(4):211–24.
Liu S, et al. MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells. Tumor Biol. 2016;37(1):633–40.
Lu H, et al. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumor Biol. 2016;37(2):1683–91.
Liao L-M, et al. Low expression of long noncoding XLOC_010588 indicates a poor prognosis and promotes proliferation through upregulation of c-Myc in cervical cancer. Gynecol Oncol. 2014;133(3):616–23.
Wu Y, et al. Long noncoding RNA HOTAIR involvement in cancer. Tumor Biol. 2014;35(10):9531–8.
Ariel I, et al. Genomic Imprinting and the Endometrial Cycle: The Expression of the Imprinted Gene H19 in the Human Female Reproductive Organs. Diagn Mol Pathol. 1997;6(1):17–25.
Tanos V, et al. H19 and IGF2 gene expression in human normal, hyperplastic, and malignant endometrium. Int J Gynecol Cancer. 2004;14(3):521–5.
Ghazal S, et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO molecular medicine. 2015;7(8):996–1003.
Douc-Rasy S, et al. High incidence of loss of heterozygosity and abnormal imprinting of H19 and IGF2 genes in invasive cervical carcinomas. Uncoupling of H19 and IGF2 expression and biallelic hypomethylation of H19. Oncogene. 1996;12(2):423–30.
Yan L, et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene. 2015;34(23):3076–84.
Qin R, et al. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma. 2012;60(5):486–92.
Chen X, Liu L, Zhu W. Up-regulation of long non-coding RNA CCAT2 correlates with tumor metastasis and poor prognosis in cervical squamous cell cancer patients. Int J Clin Exp Pathol. 2015;8(10):13261.
Qiu J-J, et al. Long non-coding RNA ANRIL predicts poor prognosis and promotes invasion/metastasis in serous ovarian cancer. Int J Oncol. 2015;46(6):2497–505.
Teschendorff AE, et al. HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome medicine. 2015;7(1):1.
Zhou M, et al. Prioritizing candidate disease-related long non-coding RNAs by walking on the heterogeneous lncRNA and disease network. Mol BioSyst. 2015;11(3):760–9.
Rodriguez A, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14(10a):1902–10.
Cao R, Zhang Y. The functions of E (Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14(2):155–64.
Acknowledgments
The research review described in this paper is part of the Ph.D. thesis of ESH. The present work was supported financially by grant No. 94123 from Kashan University of Medical Sciences, Kashan, Iran, and grant No. 95819873 from Iran National Science Foundation. We also thank the Deputy of Research and Technology, Ministry of Health and Medical Education of Iran for research grant support. MMF is a recipient of a post-doctoral fellowship from the “Région Normandie”, France.
Availability of data and materials
All the authors confirm the availability of data and materials.
Authors’ contributions
ESH and HN provided direction and guidance throughout the preparation of this manuscript. ESH and MMF conducted the literature review and drafted the manuscript. HS, ESH, MMF, HHK reviewed the manuscript and made significant revisions on the drafts. All authors read and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All the authors confirm that the manuscript represents their honest work and agree to consent to its publication in Molecular Cancer.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hosseini, E.S., Meryet-Figuiere, M., Sabzalipoor, H. et al. Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol Cancer 16, 107 (2017). https://doi.org/10.1186/s12943-017-0671-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-017-0671-2