Abstract
The insulin-like growth factor-I (IGF-I) signaling induces epithelial to mesenchymal transition (EMT) program and contributes to metastasis and drug resistance in several subtypes of tumors. In preclinical studies, targeting of the insulin-like growth factor-I receptor (IGF-IR) showed promising anti-tumor effects. Unfortunately, high expectations for anti-IGF-IR therapy encountered challenge and disappointment in numerous clinical trials. This review summarizes the regulation of EMT by IGF-I/IGF-IR signaling pathway and drug resistance mechanisms of targeting IGF-IR therapy. Most importantly, we address several factors in the regulation of IGF-I/IGF-IR-associated EMT progression that may be potential predictive biomarkers in targeted therapy.
Similar content being viewed by others
Background
The insulin-like growth factor-I receptor (IGF-IR) is a transmembrane tyrosine kinase receptor which regulates growth, development and metabolism by binding of the IGF-I ligands [1–3]. In recent years, mounting evidence indicates that the IGF-I/IGF-IR signaling is also involved in epithelial to mesenchymal transition (EMT)-associated tumor metastasis and drug resistance [4–9]. Overexpression of IGF-IR is associated with high risk of metastasis and poor prognosis in many cancer patients [10–14]. Therefore, IGF-IR, the key signaling component, is considered as the potential target of several investigational agents in clinical development. However, the IGF-I/IGF-IR signaling pathway seems more complex than initially thought. Failures in Phase II/III clinical trials in unselected patients prompted the scientists to pause and reevaluate the problem before conducting further trials [15–18]. In the face of these setbacks, searching for relevant biomarkers has become glaringly apparent. This review will first present EMT in tumor progression and discuss the mechanisms of IGF-I/IGF-IR signaling in regulating EMT programs in different epithelial tumor; secondly, we will consider the current strategies of anti-IGF-IR targeted therapy and analyze the reasons for treatment failure; Most importantly, we will extract candidate biomarkers and optional strategies to identify the right patients based on regulation mechanisms of IGF-I/IGF-IR-induced EMT progression.
The key role for IGF-IR signaling in IGF system
The IGF system consists of three ligands: IGF-I, IGF-II and insulin; three receptors: IGF-IR, insulin receptor (IR) and IGF-IIR; and a family of six high-affinity binding proteins IGFBPs. The IR exists in two splice variant isoforms, the IRA and IRB. Different receptors dimerize to form six receptor species that vary in their ligand affinity (Fig. 1) [19, 20]. (1) IGF-I can bind to the IGF-IR, IRA, and IGF-IR/IRA receptor hybrids [21, 22]; (2) IGF-II can bind with high affinity to the IGF-IIR /mannose-6-phosphate receptor, a non-signaling receptor, which is considered to play an important role in the clearance and degradation of IGF-II [23, 24]; (3) IGF-II binds with high affinity to the IGF-IR, IRA, hybrid IGF-IR/IR receptors but not the IRB isoform [25, 26]. (4) Insulin can bind with IGF-IR and IR [1]. IGFBPs are carrier proteins that have binding affinities for both IGF-I and IGF-II. There are, at present, six members in IGFBP superfamily (IGFBP-1 through 6). IGFBPs help lengthen the half-life of circulating IGF-I due to their higher affinity to IGF ligands than the receptors. IGFBPs are also instrumental in modulating IGF-IR biological accessibility and activity [27, 28]. In biological fluids, approximately 98% of IGF-I is normally bound to one of six binding proteins IGFBPs. However, IGFBPs have a relative lower affinity with insulin [29].
Schematic representation of the insulin and IGF receptor family. The IGF system consists of ligands (IGF-I, IGF-II and insulin), receptors (IGF-IR, IGF-II/ M6P, IR), and a family of six high-affinity IGFBPs. The IR exists in two splice variant isoforms, the IRA and IRB. Different receptors dimerize to form six receptor species those vary in their ligand affinity. Ligands binding to receptors can result in activation of different intracellular signaling cascades that regulate cell proliferation, survival, differentiation, and metabolism
The IGF-I/IGF-IR signaling is the major signal-transducing pathway in IGF family. Its activation after ligand binding mediates cell survival, proliferation, differentiation, and metabolism [30–32]. The effects of IGF-IR signaling in cancer biology are divergent. Previous studies have reported that cytoplasmic IGF-IR expression is correlated with favorable disease free survival and specific survival in estrogen receptor positive invasive ductal breast carcinoma [33]. IGF-IR expression is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib [34]. Whereas the opposite association is found in some other malignancies where IGF-1R exacerbated malignant transformation and tumor cell proliferation [14, 35]. This may be due to the complex and tightly regulated networks of IGF-I/IGF-IR signaling. As a potential drug target, the IGF-I/IGF-IR signaling has a number of appealing features. Many preclinical studies indicate that IGF-I induces EMT program and contributes to metastasis in breast, prostate, gastric and lung cancer [5, 36–39]. IGF-IR is involved in epidermal growth factor receptor (EGFR) TK inhibitor (TKI) resistance through crosstalk between IGF-IR and EMT signaling pathways in non-small cell lung cancer (NSCLC) with EGFR mutations [39, 40]. In addition, IGF-IR signaling mediates resistance to TKI drugs targeting both epidermal growth factor receptor 2 (HER-2) and EGFR in gastric cancer via EMT-like process [41]. In ovarian cell models, adaptive resistance to PI3K/mTOR inhibitors was associated with upregulation of IGF-IR and other pro-survival proteins [42]. Therefore, the close relationships between IGF-I/IGF-IR signaling and EMT progression makes it an attractive therapeutic target for cancer treatment.
EMT-an overview
EMT is a multi-step biologic process characterized by the cell-cell contacts breakdown, cell-matrix adhesion remodeling and acquisition of mesenchymal phenotype [43, 44]. EMT plays a central role in both physiological and pathological processes. It contributes to the formation of the body plan and the differentiation processes of multiple tissues and organs [43, 45]. EMT also plays as a physiological response to injury. During wound healing, keratinocytes at the border of the injury undergo EMT which maintains the loose contacts [43, 46]. As a pathological response, EMT is involved in organ degeneration, such as fibrosis [47]. Overwhelming evidence suggests that developmental of EMT program promotes the initiation of tumor metastasis and acquisition of therapeutic resistance [48, 49]. It also endows cells with stem cell properties and prevents apoptosis, which results in tumor progression [50, 51].
Initiating a transformation from an epithelial cell into a mesenchymal cell requires alterations in cell morphology, cellular architecture, adhesion and migration ability. Loss of the epithelial marker E-cadherin and gain of mesenchymal marker vimentin are considered as the fundamental event in EMT process [52]. Down-regulation of E-cadherin expression causes adherens junctions breakdown between cells, loss of cell polarity, leading to a mesenchymal phenotype with invasive abilities [53]. This dynamic process can be triggered by the complex interplay of several inducers, such as TGF-β, multiple receptor tyrosine kinases (RTKs), Wnt/β-catenin, Notch and Hedgehog signaling pathways [54–57]. Two important components of initiation of these complex signaling pathway networks are ZEB1/2 and Snail1/2. These EMT inducing transcription factors (EMT-TFs) can bind to E-boxes of E-cadherin promoter and repress its transcription [58–60]. Hence, any biological processes that will induce overexpression of ZEB or Snail are likely to down-regulate E-cadherin expression, which contributes to EMT. Also, some TFs supress E-cadherin transcription indirectly, such as Twist1/2, E2.2 and FoxC2 [61–63]. TGF-β induces EMT through the activation of Smad2 signaling or other non-canonical signaling pathways (PI3K/Akt or MAPK/ERK pathways) [64]. Activation of NF-κB signaling can induce EMT program through up-regulating Twist1/2 [65, 66]. Furthermore, activation of Notch, Wnt/β-catenin and Hedgehog signaling also contribute to the progression of EMT via regulation of Snail1/2 [67, 68]. These EMT-TFs not only repress E-cadherin, but also inhibit other tight junctional proteins transcriptionally, which facilitates EMT process. Additionally, newly published studies have highlighted the essential role of microRNA in the mediation of EMT process by regulating the inducers [69]. Commonly used EMT markers, inducers, pathways and transcription factors are summarized in Table 1 and Fig. 2. Nowadays, more and more studies are focusing on reinforcing EMT as a major driver factor on tumor progression, metastasis and drug resistance. Given that a complex network of regulators and inducers play integral roles in EMT, understanding the regulation mechanisms is helpful for designing more effective targeted therapies.
Basic molecular processes and signaling pathways contributing to the epithelial-mesenchymal transition (EMT). EMT is a developmental process by which epithelial cells lose their cell-cell adhesions and acquire mesenchymal cells identity. Loss of epithelial marker such as E-cadherin and the gain of mesenchymal marker such as Vimentin are considered as hallmarks in the initiation and execution of EMT. In many human tumors, the expression of multiple RTKs and their ligands induce autocrine growth factor loops. The activated RTKs induce signaling via PI3K/Akt and MAPK/ERK downstream signaling pathways, which up-regulates transcriptional factors (ZEB1/2 and Snail1/2) and causes EMT progression via binding to E-boxes of E-cadherin gene. TGF-β induces EMT through the activation of Smad2 signaling or other non-canonical signaling pathways (PI3K/Akt or MAPK/ERK pathways). Activation of NF-κB signaling can induce EMT program through up-regulating Twist1/2. In addition, activation of Notch, Wnt/β-catenin and Hedgehog signaling also contribute to the progression of EMT via regulation of Snail1/2
Molecular mechanisms of IGF-IR signaling in EMT
Recently, mounting evidence indicates that the IGF-IR signaling is also involved in EMT-mediated tumor metastasis and drug resistance. The mechanism of IGF-IR signaling in regulation of EMT is summed up in three aspects: autocrine ligand production and receptor overexpression, signal transduction by ligand binding, and cross-talk between signaling pathways.
Autocrine ligand production and receptor overexpression
IGF-I is peptide growth factor synthesized in the liver and secreted into the bloodstream under the control of growth hormone. In the circulation, the ligands of IGF-I are combined with a family of high-affinity binding proteins (six known IGFBPs), which allows growth hormone to produce more IGF-I continuously [28, 29]. Many studies have demonstrated that slight elevations in serum levels of IGF-I are correlated with an increased risk for developing prostate, breast, colon, lung, ovarian and endometrial cancer [70–77]. Interestingly, EMT process may in turn trigger autocrine IGF-I production, thus activating a positive feedback loop between IGF-IR activation and Slug expression in vitro [78]. Furthermore, IGF-IR expression is observed in up to 80% of lung cancer patients and approaching 90% of breast cancer patients [79, 80]. Overexpression of IGF-IR promotes migratory and invasive behaviors of triple negative breast cancer cell lines by activating focal adhesion kinase signaling pathway [81]. Our newly published data has implicated that elevated IGF-IR is associated with lymph node metastasis in gastric cancer patients [37]. In the light of these discoveries, strategies that are able to inhibit the functions of IGF-IR or which are able to lower plasma levels of IGF-I should be considered with the goal of inhibiting tumor development and metastasis.
Signal transduction by ligand binding
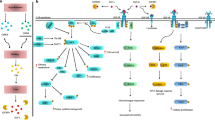
Ligand activation of IGF-IR results in intrinsic tyrosine kinase phosphorylation and activates downstream adaptor protein IRS-1 and Shc, leading to activation of two main signaling pathways, IRS-1/PI3K/Akt and Ras/Raf/ERK pathways respectively [82–84]. Activation of ERK pathway results in up-regulation of ZEB1 expression in response to IGF-I stimulation which induces EMT progression in prostate cancer [5, 85]. Our previous study demonstrated that both Akt and ERK pathways are partially involved in IGF-I-induced EMT process in gastric cancer. Inhibition of Akt/ERK pathways or knockdown of Akt/ERK gene partially reversed IGF-I-induced EMT through up-regulation of microRNA-200c which directly targets E-cadherin transcriptional repressors ZEB2 [37]. In addition to these two signaling pathways, GSK-3β is now considered as an essential EMT regulator in response to IGF-I [86]. Activation of Akt and ERK pathways result in inactivation of GSK-3β in response to paracrine/autocrine IGF-I through Ser9 phosphorylation [87, 88]. Kim et al. detected that GSK-3β was involved in direct reduction of Snail and Slug expression through proteasome-dependent degradation or NF-κB activation in response to IGF-I stimulation [89]. Zhou et al. reported that GSK-3β could bind to and phosphorylate Snail at two consensus motifs to regulate the biological functions of Snail; activation of Akt pathway led to the suppression of GSK-3β through phosphorylation of Ser9 and stabilization of Snail in response of IGF-I [90]. Our newly published data demonstrated that inhibition of Akt reversed IGF-I-induced EMT and mesenchymal phenotype in gastric cancer cells through initiating GSK-3β ability in epithelial phenotype maintenance [38]. These results indicate that the main signal transduction pathways by IGF-I ligand binding, IRS-1/Akt/GSK-3β and ERK/MAPK pathways, are potent inducers/activators in IGF-I-induced EMT process. Fig. 3 represents the relationship between the IGF-I system and the EMT process.
Schematic representation of IGF signaling regulation in EMT. Ligand activation of IGF-IR results in intrinsic tyrosine kinase phosphorylation and activates two main signaling pathways, ①IRS-1/PI3K/Akt and ②Ras/Raf/ERK pathways. Both of these two main pathways regulate transcription factors of ZEB, Snail and Twist families those are all involved in the EMT program. In addition, Slug increases IGF-I transcription which potentiates the progression of EMT. On the other hand, crosstalk between other signaling pathways and IGF signaling are also involved in EMT program. ③IGF-I stimulates β-catenin relocation and stability through the inactivation of GSK-3β which initiates Wnt signaling. Furthermore, IGF-I cooperates with Wnt signaling pathway in the metastasis process by stimulating TCF/LEF-dependent transcription through the Akt/GSK-3β/β-catenin pathway. ④GSK-3β binds to and phosphorylates Snail at two consensus motifs to regulate the biological functions of Snail. ⑤Notch-1 directly up-regulates IGF-IR protein and mRNA expression and amplifies the mitogenic effects of IGF-IR/PI3K signaling that potentiates EMT program.⑥Shh signaling activation mediates EMT process through up-regulation of IRS-1 and Snail
Cross-talk between signaling pathways
Several lines of evidence indicate that a strict association between the canonical Wnt/β-catenin and IGF-I signaling may contribute to EMT process [91–93]. In human colon cancer cells, IGF-I stimulates β-catenin relocation and stability through the inactivation of GSK-3β, which increases cell motility and contributes to colon cancer metastasis [94, 95]. In addition, IGF-I cooperates with Wnt signaling pathway in the metastasis process by stimulating TCF/LEF-dependent transcription through the Akt/GSK-3β/β-catenin pathway [96]. Taken together, these data indicate the existence of cross-talk and positive feed-back loop between the IGF-I signaling and Wnt/β-catenin signaling, thus contributing to cell motility and EMT process. In addition to Wnt signaling pathway, activation of Notch signaling results in up-regulation of mesenchymal markers (fibronectin, α-smooth muscle actin), down-regulation of endothelial markers (vascular endothelial-cadherin, Tie1, Tie2) and increasing migration ability in endothelial cells [97–101]. The interaction between the Notch signaling and the IGF-IR pathway has been firstly demonstrated by Eliasz et al. in lung cancer cells [102]. Notch stimulates IGF-IR transcription by regulating its promoter under hypoxic conditions. Additionally, accumulating evidence demonstrates that Notch directly up-regulates IGF-IR protein and mRNA expression [103]. The evidence of cross-talk between Notch and IGF-IR signaling represents a general mechanism that contributes to tumor progression and metastasis [104]. Another signaling pathway Shh cooperates with IGF-IR has also been reported in several cancer cells. For example, Shh signaling activation induces the up-regulation of IRS-1 and phosphorylated IGF-IR, which synergizes to promote medulloblastoma formation [105]. Furthermore, Shh signaling is also demonstrated to mediate EMT process through up-regulating Snail and down-regulating E-cadherin in NSCLC cells [106]. However, the synergistic cooperation between Shh and IGF-I signaling is not exclusive and there may be multiple sites and intermediary molecules involved in this process. A scheme depicting the cross-talk between signaling pathways in IGF-IR-mediated EMT process is shown in Fig. 3. We still need other strong evidence and validation of cross-talk mechanism involved in EMT maintenance and metastasis progression.
Current treatment strategies-disappointment and challenges
Almost 30 candidate drugs have been tested in more than 70 clinical trials conducted in a wide variety of cancer patients through pharmaceutical, academia and biotechnology companies during the past 10 years. Novel anti-IGF-IR drugs include monoclonal antibodies, tyrosine kinase inhibitors, and anti-ligands antibodies [107–110]. However, initial high expectations quickly encountered challenges. Therapy with monoclonal antibodies (mAb) targeting the IGF-IR have been unsuccessful [111–113]. Recent PhaseIIand III clinical trials have reported the mAb targeting the IGF-IR even worsened overall survival in breast and pancreatic cancer patients [114, 115]. Two randomized phase III studies in advanced non-small cell lung cancer were closed ahead of time due to not meeting the primary endpoint of improving overall survival [116]. In addition, some serious adverse events such as pneumonia, hyperglycemia, asthenia, and dehydration are observed more commonly in patients receiving targeted IGF-IR therapy [117, 118]. For this reason, the treatment has not gained traction for clinical use.
To explain clinical failures despite encouraging preliminary data, one can consider the mechanisms of drug resistance. These include abnormal autocrine or paracrine expression of ligand IGF-I, not shut down IGF-IR signaling completely or activation of alternative signaling pathway [119, 120]. IGF-IR mAbs can induce compensatory regulatory endocrine that may lead to supraphysiological levels of IGF-I and cause increased levels of insulin in blood. Moreover, insulin receptor (IR) forms heterodimers with IGF-IR. Both IGF-I and insulin may also activate insulin or hybrid receptors and transmit intracellular signaling information even in the treatment of IGF-IR mAbs [121, 122]. High IR to IGF-IR ratios are associated with higher resistance to IGF-IR blockade [120]. Besides that, receptor tyrosine kinase reciprocity and alternative signaling pathway activation may also contribute to the IGF-IR targeting resistance. An unique interaction between HER2 and IGF-IR contributes to trastuzumab resistance in breast cancer cells [123]. Increased expression and activation of various members of HER family receptors are observed after treatment with IGF-IR/InsR inhibitor in ovarian cancer cells, suggesting that up-regulation of HER pathway is sufficient to mediate resistance to IGF-IR-targeted therapy [124, 125]. Barnes et al. reported that IGF-I stimulation would heterodimerize IGF-IR and EGFR and phosphorylate EGFR signaling pathway [126]. Intracellular feedback loops may also cause to the increased of compensatory signaling through EGFR when IGF-IR signaling pathway is targeted by mAbs (Fig. 4) [127]. Above all, it appears that the IGF-IR signaling pathway is more complex than what was initially thought to be. Overoptimistic testing in unselected patients has already yielded to such failure in IGF-IR inhibitor therapy. Therefore, careful consideration and measurement on mechanisms of IGF-I-induced tumor metastasis, finding predictive biomarkers and selecting right patients are necessary to efficiently tailor anti-IGF-IR therapy.
Model of IGF system inhibition strategies and resistance mechanisms. Strategies to target the IGF-I/IGF-IR axis included increasing circulating levels of IGF-I and blocking kinase activation of the IGF-IR. The mechanisms of drug resistance are mainly in abnormal autocrine or paracrine expression of ligand IGF-I, not shut down receptor signaling completely (hybrid receptor or IR signaling) or activation of alternative signaling pathway (EGFR or HER2 signaling pathways). IR, insulin receptor; TKI, tyrosine kinase inhibitor; EGFR, epidermal growth factor receptor; HER2, epidermal growth factor 2 receptor
Potential strategies for anti-IGF-IR therapy in cancer
Select right patients with predictive markers according to EMT status
Most early clinical trials often consider serum IGF-I levels, IGF-IR or IR expression levels as the markers to predict response to IGF-IR blockade treatment [17, 114]. However, some clinical studies conclude that IGF-IR expression is necessary but not sufficient to predict the response [128–131]. In a clinical trial of IGF-IR inhibitor in osteosarcoma therapy, all of the IGF-IR mRNA expression, copy number, cell surface protein expression and gene mutation status were not associated with responsiveness to IGF-IR inhibition therapy [132]. Additionally, researchers could not find any correlations between levels of IGF-I and treatment effect to IGF-IR blockade in a negative phase 3 clinical trial for metastatic adenocarcinoma of pancreas [133]. Therefore, more effective biomarkers outside serum IGF-I level and tissue IGF-IR expression need to be utilized in fundamental research and clinical setting. Some researchers investigated whether EMT process could influence the response to IGF-IR blockade in cancers. Indeed, EMT could predict sensitivity to a dual IGF-IR/IR inhibitor OSI-906 in the hepatocellular carcinoma cell lines [8]. The combination of erlotinib (EGFR-TKI) and OSI-906 predicted synergistic inhibition of cell proliferation for hepatocellular carcinoma cells with epithelial phenotype. A subsequent molecular analysis of a negative randomized phase II/III clinical trial identified that mesenchymal phenotype was associated with dalotuzumab (a recombinant humanized mAb targeted against IGF-IR) therapy response. Hence, EMT status may be used to select those patients who are most likely to benefit from this treatment [134]. Recently, we discovered a potential biomarker for identifying lower risk of gastric cancer patients in IGF-I-induced EMT: Cbl-b [37]. Cbl-b is the second member of the E3 ubiquitin ligase Cbl family [135, 136]. Previous studies implicate that Cbl-b regulates cancer cell proliferation, drug sensitivity, and migration [137–139]. A negative correlation between Cbl-b and IGF-IR-associated tumor metastasis was recently verified [37]. Hence, patients with lower Cbl-b expression may get benefit from anti-IGF-IR mAb therapy; IGF-I/IGF-IR signaling may take advantage in tumor metastasis in these patients. In addition, Sorokin et.al reports that MEMO1 (mediator of ErbB2-driven cell motility 1) binds to insulin receptor substrate 1, activates the downstream PI3K/Akt signaling pathway, leads to up-regulation of Snail1 and thereby inducing the EMT program [140]. MEMO1 may act not only as a therapeutic target for cancer treatment but also as a potential biomarker for anti-IGF-IR therapy. Another team reports that reduction of CCN6 (WISP3) expression results in increased levels of IGF-I and activity of IGF-IR signaling pathway in mammary epithelial cells, which in turn is responsible for ZEB1-mediated EMT and invasion [141, 142]. Mutations in phosphoinositide-3-kinase, catalytic, alpha polypeptide (PIK3CA) may be associated with reduced sensitivity to IGF-IR/IR inhibitors [143]. Mucin 1 (MUC1), a transmembrane glycoprotein, as a critical downstream effector that mediates IGF-1-induced EMT in a PI3K/Akt signaling pathway-dependent manner in breast cancer [144]. Furthermore, survivin, a member of the inhibitor of apoptosis protein family, is also reported to be overexpressed in many tumor tissues. Activation of survivin by IGF-I signaling regulates IGF-I-induced EMT biomarkers and promotes migration ability in gastric cancer cells [145]. In addition, microRNAs have emerged as regulators in tumor metastasis by acting on multiple signaling pathways. Zhao et al. reported that microRNA-7 reversed EMT progression through targeting IGF-IR in gastric cancer [146]. All of these factors represent critical factors involved in IGF-IR-mediated EMT process, which may become potential biomarkers for identifying appropriate patients (Fig. 5). The potential biomarkers for anti-IGF-IR therapy that are involved in the regulation of EMT or have been indicated in clinical trials are listed in Table 2. Recently, our group has attempted to explore multiple classes of biomarkers including gene expression and mutations, which may carry greater predictive values on IGF-IR-associated tumor metastasis and survival. Future research is necessary to refine these biomarkers in preclinical studies and clinical trials on IGF-IR/IR inhibitors therapy.
The critical factors involved in IGF-IR-mediated EMT process. IGF-IR is a transmembrane tyrosine kinase receptor. Ligand binding leads to IRS-1 phosphorylation and activate downstream PI3K/Akt and ERK/MAPK signaling pathways. An Akt-GSK-3β-ZEB2 axis and an Akt/ERK-miR-200c-ZEB2 axis exist in IGF-I-induced EMT program. Ubiquitin ligase Cbl-b targets IGF-IR for degradation and further inhibits Akt/ERK-miR-200c-ZEB2 axis in IGF-I-induced EMT. CCN6 protein contributes to the maintenance of normal breast homeostasis through decreasing IGF-I levels in the extracellular medium and repression of IGF-IR signaling pathway activation. MEMO1 triggers EMT program via the activation of the IGF-IR/IRS-1 signaling pathway. Another factor MUC1 is a critical downstream effector that mediates IGF-I-induced EMT in breast cancer cells. MicroRNA-7 reversed EMT progression through targeting IGF-IR in gastric cancer. IGF-IR/FAK crosstalk increases expression of ZEB-1 and Snail with subsequent facilitation of EMT, leading to increased cell migration and invasion in TNBC. Cbl-b, casitas B cell lymphoma-b; CCN6, WNT1-inducible-signaling pathway protein 3; MEMO1, mediator of ErbB2-driven cell motility 1; MUC1, mucin-1; microRNA-7, miR-7; FAK, focal adhesion kinase; TNBC, triple negative breast cancer
Choose effective approaches to target pathway beyond the surface receptor
Since the IGF system comprises of multiple ligands and binding proteins, it has become evident that activation of other components of the IGF system may induce resistance to IGF-IR blocking therapies. The mechanism of resistance to specific IGF-IR inhibition therapy may be due to enhanced IR signaling, and co-targeting IGF-IR and IR signaling may acquire more response. Recently, the activity of an oral tyrosine kinase inhibitor (TKI) targeted IGF-IR/IR, KW-2450, was estimated in preclinical and phase I studies (NCT00921336). Four of 10 evaluable patients with advanced solid tumors showed stable disease. Single-agent was associated with modest antitumor activity and combination therapy needs further investigation in patients [147]. Huang, et al. reported that IRS-2 copy number gain, Kras and Braf mutation status were predictive biomarkers for response to the IGF-IR/IR inhibitor, BMS-754807 in colorectal cancer cell lines [148]. However, dual small-molecule TKI of the IGF-IR/IR used to exhibite undesirable outcomes in larger phase III trials [149]. Thus, more additional studies are necessary to determine whether these strategies can be translated into more clinical benefits.
Secondly, Insulin receptor substrate 1 (IRS1) is an adaptor protein that has the potential to transmit signals from IGF-IR proteins [150]. Activation of IGF-IR results in intrinsic tyrosine kinase phosphorylation and activates downstream adaptor protein IRS-1 and Shc, leading to activation of IRS-1/PI3K/Akt [84]. In addition to activation by IGF-IR, IRS1 has been reported to be stimulated by the growth hormone receptor and the ErbB family receptors independent of IGF-IR [151]. Preclinical data also shows that IRS1 promotes the induction of EMT process and cell proliferation in response to Wnt stimulation [152]. Components of IGF-IR signaling pathway such as IRS1 and IRS2 have been demonstrated to have predictive value in IGF-IR-targeting therapies in preclinical models of breast and colorectal cancer [153, 154]. Based on this data, it is reasonable to conclude that IRS1 may play a potential role in resistance to anti-IGF-IR therapy. However, more translational studies are necessary to determine whether patients with IRS1 overexpression who fail to respond to anti-IGF-IR therapy can get benefit from drugs targeting IRS1.
Thirdly, IGF-IR has extensive cross-talk with other receptor tyrosine kinases and their downstream factors, blocking of the IGF-IR signaling incompletely may be compensated by combination with other targeted therapy. Preclinical data has indicated that HER receptor signaling confers resistance to BMS-554417, an IGF-IR/IR inhibitor in both breast and ovarian cancer cells. Targeting HER-1 and HER-2 may overcome drug resistance to IGF-IR inhibitors [124]. Other researchers have investigated that treatment with combinatory IGF-IR and EGFR inhibitor therapy is synergistic in sarcoma and neuroblastoma cell lines [125]. Expression of IGF-IR predicts poor responses to EGFR TKI in NSCLC patients harboring activating EGFR mutations [14]. In addition to EGFR signaling pathway, some newly published data showed that co-targeting IGF-IR could sensitize triple-negative breast cancer to PI3K inhibition [155]. mTOR inhibitors are known to enhance IGF-IR signaling pathway leading to AKT downstream pathway activation [156]. The combination of IGF-IR inhibitor with mTOR inhibitors is currently being evaluated in clinical settings [157].
Finally yet importantly, since chemotherapy and radiation can induce IGF-IR activation and DNA repair mechanisms [158–160], combining IGF-IR targeted therapy to chemotherapy may be another potential effective strategy. It has been reported that IGF-IR TKI are capable of sensitizing wild-type and mutant BRAF melanoma cells to temozolomide [161]. Moreover, IGF-IR inhibition potentiates cytotoxic effects of chemotherapeutic agents in early stages of chemoresistant ovarian cancer cells [162]. Since these positive data are acquired from preclinical basic research, the feasibility and strategy of combining multiple targeted therapies and conventional cytotoxic medicine need to be further explored.
Suppressing cancer stem cell-like cells with over-activation of IGF-IR signaling
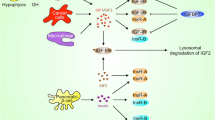
Cancer stem cells (CSCs) are the other major contributor to tumor metastasis and drug resistance [49]. Recently, it has been observed that CSCs manifest EMT phenotype [163]; some of the EMT cells can acquire CSC-like properties which contributes to the metastasis and drug resistance [164]. For instance, overexpressing transcription factors of EMT, Snail and Twist, or under TGF-β exposure will induce stem cell features in non-tumorigenic human mammary embryonic cell [163, 165]. Disseminated breast cancer cells from pleural effusions are enriched with CSC-like population [166]. On the other hand, high expression of EMT markers are positively correlated with stem cell properties in colorectal and ovarian cancers [167, 168]. Therefore, suppressing CSC-like cells may be useful for inhibiting tumor metastasis and reversing multidrug resistance. Of note, IGF system has been demonstrated to play an important role in cancer progenitor/stem cells. Knockdown of IGF-IR or inhibition of its downstream pathway, PI3K/Akt/mTOR, can reduce the breast cancer stem cells populations and suppress EMT process in breast cancer cells [169]. Similarly, chemoresistant colon cancer cells exhibit CSC phenotype and hyperactive IGF-IR signaling. Treating this subtype of CSCs may enhance sensitivity to IGF-IR-targeted therapy [170]. Nanog is considered as a stemness maintainer and EMT facilitator. Yao. et al has reported that IGF/STAT3/Nanog/Slug axis induces the progression of EMT and self-renewal of CSCs, and may serve as potential therapeutic targets for colon cancer therapy [171]. Moreover, NANOG-positive CSCs isolated from hepatocellular carcinoma cells display higher levels of IGF-IR expression and exhibit resistance to therapeutic agents and high capacity for metastasis (Fig. 6) [172]. In summary, mounting evidence highlights the emerging role of IGF-IR signaling in cancer stem cell biology; IGF-IR can be considered as a marker of stemness. For the future development of anti-IGF-IR targeted therapy, it may be possible to produce specific inhibition agents targeted to CSC-like cells with over-activation of IGF-IR signaling.
IGF-IR signaling in CSCs-like cells biology. Schematic summary of the IGF-IR signaling in the regulation of CSCs-like cells biology. After long term of EMT-associated factors effects, some of the EMT cells acquire CSCs-like properties with over-activation of IGF-IR signaling. IGF-IR/PI3K/Akt/mTOR signaling pathway activation increases the CSCs population, which promotes EMT process. The activation of IGF/STAT3/Nanog/Slug axis induces the progression of EMT and self-renewal of CSCs. CSCs, cancer stem cells; STAT3, signal transducer and activator of transcription 3; mTOR, mammalian target of rapamycin
Conclusions
A growing body of evidence shows that the role of IGF-I/IGF-IR signaling is complex and multifactorial in the development and progression of tumor metastasis. Although data based on cellular and animal models have explored some mechanisms on IGF-I-induced EMT and tumor metastasis, complexity of cancer biology and heterogeneous of tumor bring a slew of setbacks for the IGF-IR-targeted therapies. The approach of treatment with the same drug to all patients and hoping for the best response seems unrealistic. In order to choose the optimal regime for each patient, we require a better understanding of which tumor is actually driven by IGF-I/IGF-IR signaling. This is equivalent to select advantage patients who can get benefit from anti-IGF-IR therapy according to predictive biomarkers. Therefore, it is necessary to explore more potential biomarkers via research on the mechanisms of IGF-I/IGF-IR regulating tumor metastasis and drug resistance. Hopefully, clinical trials involving anti-IGF-IR strategies will be designed with this principle in mind and more selected patients will get benefit from it.
Abbreviations
- Cbl-b:
-
E3 ubiquitin ligase Casitas B cell lymphoma-b
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-mesenchymal transition
- GSK-3β:
-
Glycogen synthase kinase‐3 β
- HER-2:
-
Epidermal growth factor receptor 2
- IGFBPs:
-
Insulin-like growth factor binding proteins
- IGF-IR:
-
Insulin-like growth factor-I receptor
- IR:
-
Insulin receptor
- MEMO1:
-
Mediator of ErbB2-driven cell motility 1
- MUC1:
-
Mucin 1
- NSCLC:
-
Non-small cell lung cancer
- PDGF:
-
Platelet-derived growth factor
- PIK3CA:
-
Phosphoinositide-3-kinase catalytic, alpha polypeptide
- RTKs:
-
Receptor tyrosine kinases
- TKI:
-
Tyrosine kinase inhibitor
References
Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623.
Le Roith D. The insulin-like growth factor system. Exp Diabesity Res. 2003;4(4):205–12.
Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21(3):215–44.
Chiu L, Hsin I, Yang T, Sung W, Chi J, Chang J, Ko J, Sheu G. The ERK–ZEB1 pathway mediates epithelial–mesenchymal transition in pemetrexed resistant lung cancer cells with suppression by vinca alkaloids. Oncogene. 2016. [Epub ahead of print].
Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O’Regan RM. Insulin-like growth factor-I–dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68(7):2479–88.
Motallebnezhad M, Aghebati-Maleki L, Jadidi-Niaragh F, Nickho H, Samadi-Kafil H, Shamsasenjan K, Yousefi M. The insulin-like growth factor-I receptor (IGF-IR) in breast cancer: biology and treatment strategies. Tumor Biology. 2016;37(9):11711–21.
Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, Ye J, Wei Q, Wang J, Zhao L. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes & Diseases. 2015;2(1):13–25.
Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial–mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11(2):503–13.
Wang R, Li H, Guo X, Wang Z, Liang S, Dang C. IGF-I induces epithelial-to-mesenchymal transition via the IGF-IR‐Src‐MicroRNA-30a‐E-cadherin pathway in nasopharyngeal carcinoma cells. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. 2016;24(4):225–31.
Wang YH, Han XD, Qiu Y, Xiong J, Yu Y, Wang B, Zhu ZZ, Qian BP, Chen YX, Wang SF. Increased expression of insulin‐like growth factor‐1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J Surg Oncol. 2012;105(3):235–43.
Dale OT, Aleksic T, Shah KA, Han C, Mehanna H, Rapozo DC, Sheard JD, Goodyear P, Upile NS, Robinson M. IGF-1R expression is associated with HPV-negative status and adverse survival in head and neck squamous cell cancer. Carcinogenesis. 2015;36(6):648–55.
Numata K, Oshima T, Sakamaki K, Yoshihara K, Aoyama T, Hayashi T, Yamada T, Sato T, Cho H, Shiozawa M. Clinical significance of IGF1R gene expression in patients with stage II/III gastric cancer who receive curative surgery and adjuvant chemotherapy with S-1. J Cancer Res Clin Oncol. 2016;142(2):415–22.
Heskamp S, Boerman OC, Molkenboer-Kuenen JD, Wauters CA, Strobbe LJ, Mandigers CM, Bult P, Oyen WJ, van der Graaf WT, van Laarhoven HW. Upregulation of IGF-1R expression during neoadjuvant therapy predicts poor outcome in breast cancer patients. PLoS One. 2015;10(2), e0117745.
Yeo CD, Park KH, Park CK, Lee SH, Kim SJ, Yoon HK, Lee YS, Lee EJ, Lee KY, Kim T-J. Expression of insulin-like growth factor 1 receptor (IGF-1R) predicts poor responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer patients harboring activating EGFR mutations. Lung Cancer. 2015;87(3):311–7.
Robertson JF, Ferrero J-M, Bourgeois H, Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M, McCaffery I. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14(3):228–35.
Guha M. Anticancer IGF1R classes take more knocks. Nat Rev Drug Discov. 2013;12(4):250.
Sclafani F, Kim TY, Cunningham D, Kim TW, Tabernero J, Schmoll HJ, Roh JK, Kim SY, Park YS, Guren TK. A randomized phase II/III study of dalotuzumab in combination with cetuximab and irinotecan in chemorefractory, KRAS wild-type, metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(12):djv258.
Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–69.
Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28(1):20–47.
Foulstone E, Prince S, Zaccheo O, Burns J, Harper J, Jacobs C, Church D, Hassan A. Insulin‐like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205(2):145–53.
Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5(10):2503.
LeRoith D, Werner H, Beitner-Johnson D, Roberts Jr AT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16(2):143–63.
Braulke T. Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res. 1998;31(2-3):242–6.
Byrd JC, Devi GR, De Souza AT, Jirtle RL, MacDonald RG. Disruption of ligand binding to the insulin-like growth factor II/mannose 6-phosphate receptor by cancer-associated missense mutations. J Biol Chem. 1999;274(34):24408–16.
Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18(15):2471–9.
Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine I, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19(5):3278–88.
Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167(3):344–51.
Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions*. Endocr Rev. 1995;16(1):3–34.
Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily 1. Endocr Rev. 1999;20(6):761–87.
Doepfner K, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21(9):1921–30.
Kaleko M, Rutter WJ, Miller AD. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990;10(2):464–73.
Stiles C, Capone GT, Scher C, Antoniades H, Van Wyk J, Pledger W. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci. 1979;76(3):1279–83.
Hartog H, Horlings HM, van der Vegt B, Kreike B, Ajouaou A, van de Vijver MJ, Boezen HM, de Bock GH, van der Graaf WT, Wesseling J. Divergent effects of insulin-like growth factor-1 receptor expression on prognosis of estrogen receptor positive versus triple negative invasive ductal breast carcinoma. Breast Cancer Res Treat. 2011;129(3):725–36.
Cappuzzo F, Toschi L, Tallini G, Ceresoli G, Domenichini I, Bartolini S, Finocchiaro G, Magrini E, Metro G, Cancellieri A. Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2006;17(7):1120–7.
Park E, Park SY, Kim H, Sun P-L, Jin Y, Cho SK, Kim K, Lee C-T, Chung J-H. Membranous insulin-like growth factor-1 receptor (IGF1R) expression is predictive of poor prognosis in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma. J Pathol Transl Med. 2015;49(5):382.
Lorenzatti G, Huang W, Pal A, Cabanillas AM, Kleer CG. CCN6 (WISP3) decreases ZEB1-mediated EMT and invasion by attenuation of IGF-1 receptor signaling in breast cancer. J Cell Sci. 2011;124(10):1752–8.
Li H, Xu L, Li C, Zhao L, Ma Y, Zheng H, Li Z, Zhang Y, Wang R, Liu Y. Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Mol Cancer. 2014;13(1):1.
Li H, Xu L, Zhao L, Ma Y, Zhu Z, Liu Y, Qu X. Insulin-like growth factor-I induces epithelial to mesenchymal transition via GSK-3β and ZEB2 in the BGC-823 gastric cancer cell line. Oncol Lett. 2015;9(1):143–8.
Zhou J, Wang J, Zeng Y, Zhang X, Hu Q, Zheng J, Chen B, Xie B, Zhang W-M. Implication of epithelial-mesenchymal transition in IGF1R-induced resistance to EGFR-TKIs in advanced non-small cell lung cancer. Oncotarget. 2015;6(42):44332.
Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Torres-Garcia VZ, Corominas-Faja B, Cuyàs E, Bonavia R, Visa J, Martin-Castillo B, Barrajón-Catalán E. IGF-1R/epithelial-to-mesenchymal transition (EMT) crosstalk suppresses the erlotinib-sensitizing effect of EGFR exon 19 deletion mutations. Sci Rep. 2013;3:2560.
Zhang Z, Wang J, Ji D, Wang C, Liu R, Wu Z, Liu L, Zhu D, Chang J, Geng R. Functional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancer. Clin Cancer Res. 2014;20(17):4559–73.
Muranen TE, Selfors L, Worster D, Iwanicki M, Song L, Morlaes F, Mills GB, Brugge JS. Inhibition of PI3K/mTOR leads to adaptive resistance in extracellular matrix attached cancer cells. Cancer Res. 2012;72(8 Supplement):4836.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90.
Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–84.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37.
Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51.
Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2(6):511–2.
Franco DL, Mainez J, Vega S, Sancho P, Murillo MM, de Frutos CA, del Castillo G, López-Blau C, Fabregat I, Nieto MA. Snail1 suppresses TGF-β-induced apoptosis and is sufficient to trigger EMT in hepatocytes. J Cell Sci. 2010;123(20):3467–77.
Nieto MA, Huang RY-J, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45.
Wijnhoven B, Dinjens W, Pignatelli M. E‐cadherin—catenin cell—cell adhesion complex and human cancer. Br J Surg. 2000;87(8):992–1005.
Goswami MT, Reka AK, Kurapati H, Kaza V, Chen J, Standiford TJ, Keshamouni VG. Regulation of complement-dependent cytotoxicity by TGF-β-induced epithelial–mesenchymal transition. Oncogene. 2016;35(15):1888–98.
Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial–mesenchymal transition in cancer. Eur J Cancer. 2015;51(12):1638–49.
Bui Q, Kang K. Abstract P1-05-06: essential role of notch-4/STAT3 signaling in epithelial-mesenchymal transition of tamoxifen-resistant human breast cancer. Cancer Res. 2016;76(4 Supplement):1. -05-06-P1-05-06.
Stewart TA, Azimi I, Davis FM, Thompson EW, Brooks AJ, Roberts-Thomson SJ, Monteith GR. Abstract P2-07-05: a potential role for Janus protein tyrosine kinases in the regulation of epithelial-mesenchymal transition in a model of epidermal growth factor induced breast cancer epithelial-mesenchymal transition. Cancer Res. 2015;75(9 Supplement):2. -07-05-P2-07-05.
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–28.
Wang Y, Shi J, Chai K, Ying X, P Zhou B. The role of Snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–72.
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83.
Tran DD, Corsa CAS, Biswas H, Aft RL, Longmore GD. Temporal and spatial cooperation of Snail1 and Twist1 during epithelial–mesenchymal transition predicts for human breast cancer recurrence. Mol Cancer Res. 2011;9(12):1644–57.
Sobrado VR, Moreno-Bueno G, Cubillo E, Holt LJ, Nieto MA, Portillo F, Cano A. The class I bHLH factors E2-2A and E2-2B regulate EMT. J Cell Sci. 2009;122(7):1014–24.
Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL. FOXC2 expression links epithelial–mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73(6):1981–92.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30.
Tsubaki M, Komai M, Fujimoto S-i, Itoh T, Imano M, Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K. Activation of NF-κB by the RANKL/RANK system up-regulates snail and twist expressions and induces epithelial-to-mesenchymal transition in mammary tumor cell lines. J Exp Clin Cancer Res. 2013;32(1):1.
Li C-W, Xia W, Huo L, Lim S-O, Wu Y, Hsu JL, Chao C-H, Yamaguchi H, Yang N-K, Ding Q. Epithelial–mesenchymal transition induced by TNF-α requires NF-κB–mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72(5):1290–300.
Grego-Bessa J, Díez J. Pompa JLdl: Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle. 2004;3(6):716–9.
Stemmer V, De Craene B, Berx G, Behrens J. Snail promotes Wnt target gene expression and interacts with β-catenin. Oncogene. 2008;27(37):5075–80.
Braun J, Hoang-Vu C, Dralle H, Hüttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29(29):4237–44.
Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–6.
Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. The Lancet. 1998;351(9113):1393–6.
Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62(4):1030–5.
Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94(13):972–80.
Travis RC, Appleby PN, Martin RM, Holly JM, Albanes D, Black A, Bueno-de-Mesquita HB, Chan JM, Chen C, Chirlaque M-D. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016;76(8):2288–300.
Tas F, Bilgin E, Tastekin D, Erturk K, Duranyildiz D. Serum IGF-1 and IGFBP-3 levels as clinical markers for patients with lung cancer. Biomedical reports. 2016;4(5):609–14.
Li Y, Zhang J, Zheng C, Zhu H, Yu H, Fan L. Circulating insulin-like growth factor-1 level and ovarian cancer risk. Cell Physiol Biochem. 2016;38(2):589–97.
Bolton KA, Avery-Kiejda KA, Holliday EG, Attia J, Bowden NA, Scott RJ. A polymorphic repeat in the IGF1 promoter influences the risk of endometrial cancer. Endocr Connect. 2016;5(3):115–22.
Sivakumar R, Koga H, Selvendiran K, Maeyama M, Ueno T, Sata M. Autocrine loop for IGF-I receptor signaling in SLUG-mediated epithelial-mesenchymal transition. Int J Oncol. 2009;34(2):329.
Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34(8):803–8.
Shimizu C, Hasegawa T, Tani Y, Takahashi F, Takeuchi M, Watanabe T, Ando M, Katsumata N, Fujiwara Y. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol. 2004;35(12):1537–42.
Taliaferro-Smith L, Oberlick E, Liu T, McGlothen T, Alcaide T, Tobin R, Donnelly S, Commander R, Kline E, Nagaraju GP. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget. 2015;6(7):4757.
Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, Wakasugi T, Funahashi H, Sato M, Takeyama H. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160(1):90–101.
Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16(11):1858–66.
Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13(7):663–9.
Dupont J, Fernandez AM, Glackin CA, Helman L, LeRoith D. Insulin-like growth factor 1 (IGF-1)-induced twist expression is involved in the anti-apoptotic effects of the IGF-1 receptor. J Biol Chem. 2001;276(28):26699–707.
Bachelder RE, Yoon S-O, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription implications for the epithelial–mesenchymal transition. J Cell Biol. 2005;168(1):29–33.
Ding Q, Xia W, Liu J-C, Yang J-Y, Lee D-F, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol Cell. 2005;19(2):159–70.
Park B-C, Kido Y, Accili D. Differential signaling of insulin and IGF-1 receptors to glycogen synthesis in murine hepatocytes. Biochemistry. 1999;38(23):7517–23.
Kim H-J, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-κB and snail. Mol Cell Biol. 2007;27(8):3165–75.
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung M-C. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol. 2004;6(10):931–40.
Rota LM, Wood TL. Crosstalk of the insulin-like growth factor receptor with the Wnt signaling pathway in breast cancer. Front Endocrinol. 2015;6.
Rota LM, Albanito L, Shin ME, Goyeneche CL, Shushanov S, Gallagher EJ, LeRoith D, Lazzarino DA, Wood TL. IGF1R inhibition in mammary epithelia promotes canonical Wnt signaling and Wnt1-driven tumors. Cancer Res. 2014;74(19):5668–79.
Kotiyal S, Bhattacharya S. Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Commun. 2014;453(1):112–6.
Playford MP, Bicknell D, Bodmer WF, Macaulay VM. Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of β-catenin. Proc Natl Acad Sci. 2000;97(22):12103–8.
Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10(1):238.
Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel M-J, Bertrand F, Cherqui G, Perret C, Capeau J. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20(2):252–9.
Wang Z, Li Y, Kong D, H Sarkar F. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11(6):745–51.
Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;94(7):910–7.
Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, Aranda S, Palomo S, McCormick F, Izpisúa-Belmonte JC. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115.
Wang Z, Li Y, Banerjee S, Sarkar FH. Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008;28(6A):3621–30.
Wang Z, Kong D, Banerjee S, Li Y, Adsay NV, Abbruzzese J, Sarkar FH. Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-κB signaling. Cancer Res. 2007;67(23):11377–85.
Eliasz S, Liang S, Chen Y, De Marco MA, Machek O, Skucha S, Miele L, Bocchetta M. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene. 2010;29(17):2488–98.
Medyouf H, Gusscott S, Wang H, Tseng J-C, Wai C, Nemirovsky O, Trumpp A, Pflumio F, Carboni J, Gottardis M. High-level IGF1R expression is required for leukemia-initiating cell activity in T-ALL and is supported by Notch signaling. J Exp Med. 2011;208(9):1809–22.
Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol. 2014;5:10.
Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23(36):6156–62.
Ma’in YM, Ali S, Ahmad A, Gadgeel S, Sarkar FH. Up-regulation of sonic hedgehog contributes to TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS One. 2011;6(1), e16068.
Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials—early lessons. J Mammary Gland Biol Neoplasia. 2008;13(4):471–83.
Garcı́a-Echeverrı́a C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro H-G, Cozens R. In vivo antitumor activity of NVP-AEW541—a novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5(3):231–9.
Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28(34):3009–21.
Buck E, Mulvihill M. Small molecule inhibitors of the IGF-1R/IR axis for the treatment of cancer. Expert Opin Investig Drugs. 2011;20(5):605–21.
Gombos A, Metzger-Filho O, Dal Lago L, Awada-Hussein A. Clinical development of insulin-like growth factor receptor—1 (IGF-1R) inhibitors: At the crossroad? Invest New Drugs. 2012;30(6):2433–42.
Rodon J, DeSantos V, Ferry RJ, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7(9):2575–88.
Abou-Alfa GK, Capanu M, O’Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60(2):319–24.
Gradishar WJ, Yardley DA, Layman R, Sparano JA, Chuang E, Northfelt DW, Schwartz GN, Youssoufian H, Tang S, Novosiadly R. Clinical and Translational Results of a Phase II, Randomized Trial of an Anti–IGF-1R (Cixutumumab) in Women with Breast Cancer That Progressed on Endocrine Therapy. Clin Cancer Res. 2016;22(2):301–9.
Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti JK. Dual blockade of epidermal growth factor receptor and insulin‐like growth factor receptor–1 signaling in metastatic pancreatic cancer: Phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer. 2014;120(19):2980–5.
Jassem J, Langer C, Karp D, Mok T, Benner R, Green S, Park K, Novello S, Strausz J, Gualberto A. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC). In: ASCO annual meeting proceedings. 2010. p. 7500.
Higano CS, Berlin J, Gordon M, LoRusso P, Tang S, Dontabhaktuni A, Schwartz J, Cosaert J, Mehnert J. Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors. Invest New Drugs. 2015;33(2):450–62.
Langer CJ, Novello S, Park K, Krzakowski M, Karp DD, Mok T, Benner RJ, Scranton JR, Olszanski AJ, Jassem J. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non–small-cell lung cancer. J Clin Oncol. 2014;32(19):2059–66.
Garofalo C, Manara M, Nicoletti G, Marino M, Lollini P, Astolfi A, Pandini G, Lopez-Guerrero J, Schaefer K, Belfiore A. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene. 2011;30(24):2730–40.
Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci. 2010;107(24):10791–8.
Křížková K, Chrudinová M, Povalová A, Selicharová I, Collinsová M, Vanek V, Brzozowski AM, Jiracek J, Zakova L. The insulin-IGF hybrids as molecular probes of hormone: receptor binding specificity. Biochemistry. 2016.
Vigneri R, Goldfine I, Frittitta L. Insulin, insulin receptors, and cancer. J Endocrinol Invest. 2016;39(12):1365–76.
Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65(23):11118–28.
Haluska P, Carboni JM, TenEyck C, Attar RM, Hou X, Yu C, Sagar M, Wong TW, Gottardis MM, Erlichman C. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther. 2008;7(9):2589–98.
Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM, Reeves K, Chen J, Robinson D, Li A. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69(1):161–70.
Barnes CJ, Ohshiro K, Rayala SK, El-Naggar AK, Kumar R. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin Cancer Res. 2007;13(14):4291–9.
Buck E, Eyzaguirre A, Thomson S, Mulvihill M, Barr S, Brown E, O’Connor M, Yao Y, Pachter J, Miglarese M. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68(20):8322–32.
Atzori F, Tabernero J, Cervantes A, Prudkin L, Andreu J, Rodríguez-Braun E, Domingo A, Guijarro J, Gamez C, Rodon J. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17(19):6304–12.
Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, Eckhardt SG, Eid JE, Greig G, Habben K. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16(8):2458–65.
Schwartz GK, Tap WD, Qin L-X, Livingston MB, Undevia SD, Chmielowski B, Agulnik M, Schuetze SM, Reed DR, Okuno SH. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2013;14(4):371–82.
Beckwith H, Yee D. Minireview: were the IGF signaling inhibitors All Bad? Mol Endocrinol. 2015;29(11):1549–57.
Cao Y, Roth M, Piperdi S, Montoya K, Sowers R, Rao P, Geller D, Houghton P, Kolb EA, Gill J. Insulin-like growth factor 1 receptor and response to anti-IGF1R antibody therapy in osteosarcoma. PLoS One. 2014;9(8), e106249.
Fuchs CS, Azevedo S, Okusaka T, Van Laethem J-L, Lipton L, Riess H, Szczylik C, Moore M, Peeters M, Bodoky G. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26(5):921–7.
Watkins DJ, Ayers M, Cunningham D, Tabernero J, Tejpar S, Kim T-Y, Kim TW, Kim SY, Roh JK, Beale PJ. Molecular analysis of the randomized phase II/III study of the anti-IGF-1R antibody dalotuzumab (MK-0646) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory KRAS wild-type metastatic colorectal cancer (mCRC). In: ASCO annual meeting proceedings. 2012. p. 3531.
Mohapatra B, Ahmad G, Nadeau S, Zutshi N, An W, Scheffe S, Dong L, Feng D, Goetz B, Arya P. Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases. Biochimica et Biophysica Acta (BBA)-Molecular. Cell Res. 2013;1833(1):122–39.
Fang D, Wang H-Y, Fang N, Altman Y, Elly C, Liu Y-C. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J Biol Chem. 2001;276(7):4872–8.
Caligiuri MA, Briesewitz R, Yu J, Wang L, Wei M, Arnoczky KJ, Marburger TB, Wen J, Perrotti D, Bloomfield CD. Novel c-CBL and CBL-b ubiquitin ligase mutations in human acute myeloid leukemia. Blood. 2007;110(3):1022–4.
Xu L, Zhang Y, Liu J, Qu J, Hu X, Zhang F, Zheng H, Qu X, Liu Y. TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in lipid rafts antagonises TRAIL-induced apoptosis in gastric cancer cells. Eur J Cancer. 2012;48(17):3288–99.
Zhang L, Teng Y, Fan Y, Wang Y, Li W, Shi J, Ma Y, Li C, Shi X, Qu X. The E3 ubiquitin ligase Cbl-b improves the prognosis of RANK positive breast cancer patients by inhibiting RANKL-induced cell migration and metastasis. Oncotarget. 2015;6(26):22918.
Sorokin A, Chen J. MEMO1, a new IRS1-interacting protein, induces epithelial–mesenchymal transition in mammary epithelial cells. Oncogene. 2013;32(26):3130–8.
Zhang Y, Pan Q, Zhong H, Merajver SD, Kleer CG. Inhibition of CCN6 (WISP3) expression promotes neoplastic progression and enhances the effects of insulin-like growth factor-1 on breast epithelial cells. Breast Cancer Res. 2005;7(6):1.
Kleer CG, Zhang Y, Merajver SD. CCN6 (WISP3) as a new regulator of the epithelial phenotype in breast cancer. Cells Tissues Organs. 2007;185(1-3):95–9.
Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, Lerner L, Chiu MI, Wild R, Epstein D. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9(10):2652–64.
Liao G, Wang M, Ou Y, Zhao Y. IGF-1-induced epithelial–mesenchymal transition in MCF-7 cells is mediated by MUC1. Cell Signal. 2014;26(10):2131–7.
Li C, Li J, Wu D, Han G. The involvement of survivin in insulin-like growth factor 1-induced epithelial-mesenchymal transition in gastric cancer. Tumor Biology. 2016;37(1):1091–6.
Zhao X, Dou W, He L, Liang S, Tie J, Liu C, Li T, Lu Y, Mo P, Shi Y. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32(11):1363–72.
Schwartz GK, Dickson MA, LoRusso PM, Sausville EA, Maekawa Y, Watanabe Y, Kashima N, Nakashima D, Akinaga S. Preclinical and first‐in‐human phase I studies of KW‐2450, an oral tyrosine kinase inhibitor with insulin‐like growth factor receptor‐1/insulin receptor selectivity. Cancer Sci. 2016;107(4):499–506.
Huang F, Chang H, Greer A, Hillerman S, Reeves KA, Hurlburt W, Cogswell J, Patel D, Qi Z, Fairchild C. IRS2 copy number gain, KRAS and BRAF mutation status as predictive biomarkers for response to the IGF-1R/IR inhibitor BMS-754807 in colorectal cancer cell lines. Mol Cancer Ther. 2015;14(2):620–30.
Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–35.
Siddle K. Molecular basis of signaling specificity of insulin and IGF receptors: neglected corners and recent advances. Front Endocrinol. 2012;3:34.
Argetsinger LS, Hsu GW, Myers MG, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem. 1995;270(24):14685–92.
Geng Y, Ju Y, Ren F, Qiu Y, Tomita Y, Tomoeda M, Kishida M, Wang Y, Jin L, Su F. Insulin receptor substrate 1/2 (IRS1/2) regulates Wnt/β-catenin signaling through blocking autophagic degradation of dishevelled2. J Biol Chem. 2014;289(16):11230–41.
Zha J, O’Brien C, Savage H, Huw L-Y, Zhong F, Berry L, Phillips GDL, Luis E, Cavet G, Hu X. Molecular predictors of response to a humanized anti–insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009;8(8):2110–21.
Mukohara T, Shimada H, Ogasawara N, Wanikawa R, Shimomura M, Nakatsura T, Ishii G, Park JO, Jänne PA, Saijo N. Sensitivity of breast cancer cell lines to the novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor NVP-AEW541 is dependent on the level of IRS-1 expression. Cancer Lett. 2009;282(1):14–24.
de Lint K, Poell JB, Soueidan H, Jastrzebski K, Rodriguez JV, Lieftink C, Wessels LF, Beijersbergen RL. Sensitizing triple-negative breast cancer to PI3K inhibition by co-targeting IGF1R. Mol Cancer Ther. 2016;15(7):1545–56.
Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4(10):1533–40.
Naing A, Lorusso P, Fu S, Hong D, Chen H, Doyle L, Phan AT, Habra M, Kurzrock R. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108(4):826–30.
Cosaceanu D, Budiu R, Carapancea M, Castro J, Lewensohn R, Dricu A. Ionizing radiation activates IGF-1R triggering a cytoprotective signaling by interfering with Ku-DNA binding and by modulating Ku86 expression via a p38 kinase-dependent mechanism. Oncogene. 2007;26(17):2423–34.
Iwasa T, Okamoto I, Suzuki M, Hatashita E, Yamada Y, Fukuoka M, Ono K, Nakagawa K. Inhibition of insulin-like growth factor 1 receptor by CP-751,871 radiosensitizes non–small cell lung cancer cells. Clin Cancer Res. 2009;15(16):5117–25.
Wang Y-H, Wang Z-X, Qiu Y, Xiong J, Chen Y-X, Miao D-S, De W. Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits growth, reduces invasion, and enhances radiosensitivity in human osteosarcoma cells. Mol Cell Biochem. 2009;327(1-2):257–66.
Ramcharan RN, Aleksic T, Gao S, Tanner J, Darvill N, Bridges E, Asher R, Watson AJ, Margison GP, Repapi E. Inhibition of type 1 insulin-like growth factor receptor (IGF-1R) influences processing of replication-associated DNA double-strand breaks (DSBs) and induces schedule-dependent sensitization of human melanoma to temozolomide (TMZ). Cancer Res. 2014;74(19 Supplement):1741.
Singh RK, Gaikwad SM, Jinager A, Chaudhury S, Maheshwari A, Ray P. IGF-1R inhibition potentiates cytotoxic effects of chemotherapeutic agents in early stages of chemoresistant ovarian cancer cells. Cancer Lett. 2014;354(2):254–62.
Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73.
Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang Z, Yang C, Yuan L, Ouyang G. Twist2 contributes to breast cancer progression by promoting an epithelial–mesenchymal transition and cancer stem-like cell self-renewal. Oncogene. 2011;30(47):4707–20.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100(7):3983–8.
Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53‐mediated apoptosis and acquiring a stem‐like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–68.
Hwang WL, Yang MH, Tsai ML, Lan HY, Su SH, Chang SC, Teng HW, Yang SH, Lan YT, Chiou SH. SNAIL regulates interleukin-8 expression, stem cell–like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology. 2011;141(1):279–91. e5.
Chang W-W, Lin R-J, Yu J, Chang W-Y, Fu C-H, Lai AC-Y, Yu J-C, Alice LY. The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors. Breast Cancer Res. 2013;15(3):1.
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, Van Buren G, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69(5):1951–7.
Yao C, Su L, Shan J, Zhu C, Liu L, Liu C, Xu Y, Yang Z, Bian X, Shao J. IGF/STAT3/NANOG/Slug signaling axis simultaneously controls epithelial‐mesenchymal transition and stemness maintenance in colorectal cancer. STEM CELLS. 2016.
Shan J, Shen J, Liu L, Xia F, Xu C, Duan G, Xu Y, Ma Q, Yang Z, Zhang Q. Nanog regulates self‐renewal of cancer stem cells through the insulin‐like growth factor pathway in human hepatocellular carcinoma. Hepatology. 2012;56(3):1004–14.
Acknowledgments
We would like to thank our lab colleague for critical reading of the manuscript. We apologize to several scientists whose outstanding work could not be cited.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors’ contributions
YL and RW designed the outline of the article. YL supervised the manuscript. HL wrote the manuscript. ISB revised and expanded the manuscript. XQ, LX and NS gave useful suggestions. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Authors listed have approved to submit this manuscript to the journal.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, H., Batth, I.S., Qu, X. et al. IGF-IR signaling in epithelial to mesenchymal transition and targeting IGF-IR therapy: overview and new insights. Mol Cancer 16, 6 (2017). https://doi.org/10.1186/s12943-016-0576-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-016-0576-5