Abstract
Acute myeloid leukemia (AML) is the most commonly diagnosed leukemia in adults (25%) and comprises 15–20% in children. It is a genetically heterogeneous aggressive disease characterized by the accumulation of somatically acquired genetic changes, altering self-renewal, proliferation, and differentiation of hematopoietic progenitor cells, resulting in uncontrolled clonal proliferation of malignant progenitor myeloid cells in the bone marrow, peripheral blood, and occasionally in other body tissues. Treatment with modern chemotherapy regimen (cytarabine and daunorubicin) usually achieves high remission rates, still majority of patients are found to relapse, resulting in only 40–45% overall 5 year survival in young patients and less than 10% in the elderly AML patients. The leukemia stem cells (LSCs) are characterized by their unlimited self-renewal, repopulating potential and long residence in a quiescent state of G0/G1 phase. LSCs are considered to have a pivotal role in the relapse and refractory of AML. Therefore, new therapeutic strategies to target LSCs with limited toxicity towards the normal hematopoietic population is critical for the ultimate curing of AML. Ongoing research works with natural products like parthenolide (a natural plant extract derived compound) and its derivatives, that have the ability to target multiple pathways that regulate the self-renewal, growth and survival of LSCs point to ways for a possible complete remission in AML. In this review article, we will update and discuss various natural products that can target LSCs in AML.
Similar content being viewed by others
Background
AML is a malignant, heterogeneous, clonal disease arising in hematopoietic stem or progenitor cells resulting from genetic and epigenetic alterations that perturb key processes like self-renewal, proliferation and differentiation. It is characterized by malignant clonal proliferation and/or differentiation of immature myeloid progenitor cells [1, 2]. In AML, the myeloid stem cells differentiate to malignant myeloblasts that cannot function as normal blood cells. Consequently bone marrow and peripheral blood gets accumulated with leukemic blasts resulting in significantly reduced production of healthy and functional white blood cells, red blood cells, platelets, and mature granulocytes.
AML is one of the most common hematological malignancy. The other common hematological malignancies includes Lymphoma (Hodgkin lymphoma and Non-Hodgkin lymphoma), Myeloma, Leukemia (acute lymphocytic leukemia, chronic lymphocytic leukemia and chronic myeloid leukemia), Myeloproliferative neoplasms (essential thrombocythemia, polycythemia vera and myelofibrosis) and Myelodysplastic syndromes (refractory anemia, refractory anemia with ring sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation and chronic myelomonocytic leukemia). In United States, as per American Cancer Society, an estimated 60,140 new cases of leukemia are expected in 2016 and 24,400 deaths resulting from leukemia. This includes 6,590 new cases of acute lymphocytic leukemia and 1,430 deaths, 18,960 new cases of chronic lymphocytic leukemia and 4,660 deaths, 19,950 new cases of acute myeloid leukemia and 10,430 deaths, and 8,220 new cases of chronic myeloid leukemia and 1,070 deaths [3].
AML is the most common form of acute leukemia in adults and constitutes approximately 80 percent of cases. The incidence of AML is about 1.3 per 100,000 for those under 65 and about 12.2 cases per 100,000 for those over 65 years [4]. There is significant patient-to-patient heterogeneity within the morphologic-genetic characteristics of leukemic blasts. Based on the morphologic heterogeneity, AML is classified into seven French–American–British (FAB) subtypes. A common feature of AML is the arrest of aberrant differentiation leading to accumulation of more than 20% blasts in the bone marrow [5]. Mutations in genes involved in regulation of cell proliferation (Fms-like tyrosine kinase 3, c-KIT, RAS etc.,) and apoptosis (nucleophosmin, p53 etc) are used as a prognostic factor of clinical outcome in AML. These genetic alterations are potential therapeutic targets, but targeting them have failed to show any sufficient activity against the various cell types present in AML [6].
The present chemotherapeutic approach for the management of the disease is based on the concept of targeting leukemic cells specifically to eradicate them while minimally affecting normal cells. The induction therapy with cytarabine in combination with new generation anthracycline antibiotics (daunorubicin/Epirubicin/Idarubicin/Mitoxantrone) is the back bone of AML treatment [7], and has significantly improved the rate of remission in AML over the years, but more than 50% relapse with resistant disease [8], causing the death of most patients, remains a major hindrance in a successful AML chemotherapy [9].
AML stem cells
Acute myeloid leukemia stem cells/leukemia initiating cells (LSCs) were defined as those cells capable of regenerating human AML cell populations in irradiated non-obese diabetic/severe combined immunodeficient (SCID) mice after transplantation [10, 11]. LSCs possessing this renewal property were found to display a CD34+CD38− phenotype, which is similar to normal human hematopoietic progenitors population [12, 13]. The existence of LSCs which are characterized by their self-renewal capability, unlimited repopulating potential and prolonged residence in the G0/G1 phase of cell cycle in a quiescent state was initially pointed out by Lapidot et al. [11]. Limitless self-renewal is an important property of cancer stem cells that is distinct from tumor cell proliferation. The self-renewing cell division of LSCs can result in one/both daughter cells that have the ability for further self-renewal and/or generate differentiated progenitor blast cell lineages, in a way that is highly analogous to normal hematopoietic stem cells [14]. A crucial aspect of AML is that the tumor population is heterogeneous and that LSCs are biologically distinct from the highly differentiated blast cells [15]. Further studies on the immunophenotype of LSCs have shown that these cells can be defined as expressing CD34, CD117, CD123, CD382, CD902 and HLA-DR2 [16]. Some of these markers are also expressed in hematopoietic stem cells (HSCs), but CD123, CD47, CD44, CLL-1, CD96, CD90, CD32, CD25, and TIM-3 is reported to be leukemia-specific [17].
For over 10 years, the backbone of AML therapy has remained same, with an initial remission induction therapy followed by several months of consolidation therapy. The initial remission induction uses a combination of nucleoside analogs drugs (eg, cytosine arabinoside) and anthracyclines antibiotics (eg, idarubicin, daunorubicin) that interfere with DNA replication to induce apoptosis primarily in replicating cells, while the consolidation therapy consists of cytosine arabinoside in multiple cycles [15, 18]. Since LSCs maintain a largely quiescent cell cycle status, conventional drugs are unlikely to affect the stem cell population, as the LSCs are primarily in G0 phase of cell cycle; LSCs population may also possess natural mechanisms of survival (drug efflux pumps, multi-drug resistance (MDR)) as they are developmentally primitive than tumor cells; and because the LSCs are biologically similar to normal cells as they have fewer oncogenic lesions, thus less susceptible to tumor-specific drugs [19].
Chemotherapeutic treatment of AML can result in complete remission in most cases, but relapses frequently occur [20], leading to an overall survival of only 30% to 40% at 4 years after diagnosis [21]. A high relapse rate suggests that current therapies spare the LSCs in AML, and point out the role of this compartment that forms the reservoir for subsequent relapse and resistance. These leukemic stem cells are also being considered to be the initiator of the malignancy, that is often resistant to standard chemotherapy drugs. Even a small number of LSC that survive the initial induction chemotherapy can either lead to drastic reduction in overall survival if they expand rapidly after the end of treatment cycle or lead to relapse if they remain dormant after cessation of therapy [22].
The abundance of LSCs has been associated with clinical relapse or refractory disease [21]. Evidence from recent studies points out that AML is maintained by a population of LSCs, which is insensitive to conventional chemotherapy and have a central role in the relapse of AML [23]. Stem cell frequency at diagnosis offers a new prognostic factor in AML, and a large CD34+CD38- population reflects a higher percentage of chemotherapy-resistant cells that will lead to the outgrowth of minimal residual disease, thereby affecting clinical outcome [21]. ATP-binding cassette (ABC) transporters are transmembrane proteins capable of exporting a wide variety of chemotherapeutic drugs from the cytosol and play a major role in conferring multidrug resistance to the host cells. Normal HSC are known to express high levels of surface membrane proteins involved in drug resistance such as MDR1 [24] and BCRP1/ABCG2 (ABC transporters associated with drug resistance) [25] that function to efflux certain molecules. Chemotherapeutic drugs such as anthracyclines are known to be substrates for these efflux pumps and is readily removed from these cells relatively fast. LSCs also express resistant-related proteins like MDR1 and BCRP1 for their multidrug-resistant characters [26].
LSCs share many characteristics with normal hematopoietic stem cells (HSCs), such as a hierarchical developmental pattern, a mostly quiescent state, display heterogeneity within the stem cell compartment and an immunophenotype similar to HSC (CD34+, CD38-, CD71-, and HLA-DR-) [16], but at the same time can be phenotypically distinguished from HSCs with of aberrant expression of several distinct (ex. CD123) and sporadically occurring markers among individual patients. Due to the presence of a large set of common features, it has been extremely difficult to elucidate strategies to differentially target the LSC population while sparing HSC. Despite that, recent publications show that LSC population still display some unique molecular properties such as constitutive activation of nuclear factor κB (NF-κB), expression of CD123, and potentially elevated levels of interferon regulatory factor 1 (IRF-1) and death-associated protein (DAP) kinase [27]. These features define LSC population as a critical target in AML therapy and suggests that LSC-selective therapies that spare the hematopoietic stem cell population should improve treatment outcomes in AML.
Maintenance of LSC population
The pro-inflammatory transcription factor, NF-κB, is known to have antiapoptotic activity and its regulated gene products play a critical role in the proliferation, survival and chemoresistance [28]. Many studies have shown that the NF-κB signaling pathway plays an important role in the drug resistance of tumor cells and many chemotherapeutic drugs and radiotherapy induce NF-κB expression in vitro and in vivo [29]. NF-κB has been found to be constitutively activated in human AML stem cells while normal human CD34+ progenitor cells do not express NF-κB [30]. Targeting this aberrant expression may induce apoptotic stimulus and/or sensitize LSC to a variety of other agents and thus can be used to target LSC without significant toxicity to normal hematopoietic stem cells (HSC) [31]. Strikingly, the commonly used AML chemotherapy agents (nucleoside analogues and anthracyclines) does not inhibit NF-κB, instead leads to further upregulation of NF-κB activity [32–34] .
The PI3K/Akt/mTOR pathway is a key signaling cascade in mammalian cells, which regulates mRNA translation of genes that encode for pro-oncogenic proteins, leading to malignant cell survival in various cancers [35]. Constitutive and cytokine-mediated activation of PI3K/Akt/mTOR signaling pathway is a common feature in AML patients, and inhibition of this pathway is a viable therapeutic strategy in the treatment of AML [36]. Xu et al. [37] and Zhao et al. [38] have reported that Akt, a critical substrate of PI3 kinase, is activated in AML blasts, and there is a dose-dependent decrease in survival of most AML patient samples after incubation with the PI3 kinase inhibitor LY294002, while normal hematopoietic progenitors were less affected, suggesting preferential targeting of leukemia cells. The downstream targets of PI3K-Akt include the proapoptotic protein BAD, caspase-9 and NF-κB. Under some circumstances, Ras/PI3K -mediated pathway is already known to activate NF-κB, which suggests a common survival pathway of LSCs triggered by both factors [39]. Birkenkamp et al. [40] have reported that NF-κB was constitutively activated in 73% of AML cases, and the activation status was associated with resistance to spontaneous apoptosis. Treatment of these primary AML cells with the PI3 kinase inhibitor LY294002 and Ras inhibitor L-744832 resulted in downregulation of NF-κB DNA binding activity.
The tumor suppressor gene, PTEN (phosphatase and tensin homologue) is the major negative regulators of the PI3 kinase pathway, which regulates diverse cellular processes, including growth, survival and proliferation of LSCs. PTEN modulates PI3 kinase pathway by dephosphorylating the intermediary PIP3, lipid-signaling molecule [41]. It is also one of the most frequently mutated proteins in human cancers, leading to constitutive activation of PI3 kinase signaling pathway [42]. Deletion of PTEN promotes HSC proliferation leading to HSC depletion through a cell-autonomous mechanism, and generation of transplantable leukaemia-initiating cells. As a result, HSCs is unable to maintain themselves without PTEN, while LSCs proliferation and self-renewal are enhanced by PTEN deletion. Treatment of LSCs with rapamycin was found to deplete leukaemia-initiating cells while restoring normal HSC function, proving that these effects were mostly mediated through mTOR [43].
The JAK-STAT signaling pathway regulates a variety of biological functions, including hematopoiesis, immune-regulation, fertility, lactation, growth and embryogenesis, throughout development [44]. STATs are constitutively activated in several solid tumors and hematological malignancies, including AML [45, 46]. Activation of STATs provides a growth advantage to tumor cells, allowing accumulation, and also confers resistance to conventional therapies that rely on apoptotic machinery to eliminate tumor cells [47]. An increase in STAT3 and STAT5 phosphorylation was reported in AML blasts suggesting vital role of JAK/STAT signaling pathway to support AML stem cell growth and survival [48].
The p53 gene is wild type in more than 90% of AML patient samples [49]. Primary AML cells treated with proteasome inhibitors and the anthracycline idarubicin was found to induce activation of p53 along with increase in the levels of p53 target genes GADD45, p21, and Bax, all of which are strongly implicated in p53-mediated apoptosis [50]. Hence, strategies involving activation of the p53 mediated pathway can be utilized in the majority of AML patients.
Wnt/beta-catenin, Hedgehog and Notch signaling pathways are all involved in the regulation of HSC self-renewal mechanisms and these pathways are dysregulated in leukemic stem cells. There are crosstalks between Wnt/beta-catenin, Hedgehog and Notch signaling, and the PI3K/Akt pathway. Glycogen synthase kinase-3β, involved in canonical Wnt signaling, regulates the molecules involved in Hh signaling, while the pathological response to oncogenic Hh signaling is dependent on Wnt signaling pathway. The PI3K/Akt survival signaling pathway regulates the stem/progenitor cells by promoting Wnt/beta-catenin pathway through phosphorylation of GSK-3β, thereby stabilizing β-catenin [51].
The Hedgehog (Hh) signaling pathway is important in the regulation of stem/progenitor cell expansion, cell differentiation, tissue polarity, cell proliferation and tissue repair [52]. Hh regulates hematopoietic stem/progenitor cells via stromal cells. Abnormal Hh pathway activation occurs in several human cancers, including AML, where Hh signaling promotes the maintenance of LSCs and enhance resistance to chemotherapeutic agents [53]. Aberrant activation of Hh signaling is involved in a variety of cancers including AML [54], and is required for the maintenance of the LSC population [55]. Hh signaling pathway is active in primary CD34+ LSCs and cytokine-responsive CD34+ cell lines (Kasumi-1, Kasumi-3 and TF-1) and contributes to the survival and drug resistance of CD34+ leukemia stem cells [56]. AML derived stromal cells had markedly lower expression of hedgehog-interacting protein than healthy donor-derived stromal cells and was found to support the proliferation of SMO+ leukemic cells [57].
The Wnt/beta-catenin (Wingless) pathway modulates self-renewal, proliferation, differentiation and apoptosis. Wnt proteins are a diverse family of lipid-modified glycoproteins that bind to Frizzled receptors and lipoprotein receptor-related protein -5/6 coreceptors. Aberrant activation of Wnt pathway by genetic and epigenetic changes is prominent in the initiation and progression of AML. Overexpression of beta-catenin is commonly found in AML samples and is an independent adverse prognostic factor [58]. The Wnt/β-catenin pathway is normally active in HSCs, but β-catenin is not essential for self-renewal of HSCs. Constitutively active β-catenin cooperates with HoxA9/M to induce AML from non-self renewing granulocyte/macrophage restricted progenitors cells, and pharmacological inhibition of β-catenin was found to impairs LSCs formation [59]. Hence targeting the Wnt/β-catenin pathway represents a viable therapeutic option in AML.
Notch signaling plays a crucial role in development, stem cell self-renewal and hematopoiesis. The target genes of Notch involved in cell cycle regulation (cyclin D1, cyclin A , p21, p27), cell proliferation and survival (c-myc, NF-κB2 , Akt, mTOR), embryonic development (Hes1, Hes6), angiopoiesis (VEGF, VEGFR-2), invasion and metastasis (MMP-9, MMP-2) [60]. Deregulation of Notch signaling has been reported in multiple human hematological malignancies, including AML [61]. Human AML samples have extremely low levels of activated Notch receptor and expression of downstream targets, even with a robust expression of Notch receptors, suggesting that Notch is not constitutively activated [62]. In AML initiating cells, activation of Notch was found to inhibit AML growth and survival in vitro and in vivo, which involved caspase-mediated apoptosis driven by Bcl-2 and p53 in response to induction of Notch signaling [63, 64].
Similarly, the polycomb group (PcG) repressor protein Bmi-1 has been shown to mediate self-renewal of both HSC [41] and leukemic stem cells. Bmi-1 is overexpressed in AML cells and is associated with an unfavorable prognosis [65]. Repression of Bmi-1 in normal and leukemic CD34+ AML cells by a lentiviral RNA interference approach impairs self-renewal and induces apoptosis [66]. Data from all these studies supports the concept that basic mechanisms of self-renewal are shared between HSCs and malignant LSCs.
MicroRNAs are naturally occurring 18 to 25 nucleotide RNAs that can hybridize to specific target messenger RNA and repress their translation into proteins by controlling endogenous mRNAs at the post-transcriptional level. Several recently publications demonstrated that microRNA levels are altered in AML and correlates with clinical outcome [67]. miR-126 was reported to regulate distinct self-renewal outcomes in normal hematopoietic stem cells and LSC population from AML patients. AML stem cell fraction generally expressed high levels of miR-126 and is associated with poor survival and higher chance of relapse. Overexpression of miR-126 was found to maintain LSCs in a more primitive state by increasing quiescent cell population and increase chemoresistance, while knockdown results in reduced cell growth by inducing apoptosis in vitro and in vivo [68, 69]. PI3K/Akt/mTOR pathway was found to be targeted by miR-126 [70]. miR-9 was reported to promote proliferation of AML stem cells via negative regulation of Hes1 expression by interacting with 3′-untranslated region of Hes1 mRNA. Knockdown of miR-9 can inhibit the AML stem cell proliferation in vitro and increase the survival in a xenotransplant mouse model [71].
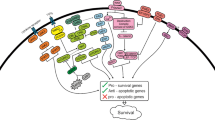
Relapse of the disease is thought to occur because of the failure of chemotherapy to eradicate LSCs. Targeting LSCs with novel agents will help to achieve a prolonged clinical remission. Development of new therapeutic strategies utilizing the survival (NF-kB, STAT, PI3 kinase, PTEN, p53) and self-renewal (Wnt, Hedgehog, Notch) pathways for the eradication of these dormant LSCs has critical therapeutic importance for the ultimate goal of cure for AML (Fig. 1).
Leukemia stem cells in AML. Transforming mutations in hematopoietic stem cells (HSC) and/or common myeloid progenitor (CMP) leads to the formation of leukemia stem cells (LSCs), which have mutations in survival signaling pathways and altered self renewal capacity. Chemotherapy can significantly abolish the AML blast cells population while the LSCs survive, and instigate relapse in the future
Natural Products for targeting LSCs
The pivotal obstacle for developing an efficient therapy targeting stem cell is to distinguish the apoptotic stimuli that can effectively target the LSCs while sparing normal hematopoietic stem cells in the milieu of an uncharacterized in vivo microenvironment. The highly proliferative bulk of AML population can be effectively targeted by conventional chemotherapy, while specific strategies designed to target this stem cell microenvironment may be effective. Along with genes involved in the control of stem cell self-renewal, leukemic stem cells are known to express high levels of genes involved in anti-apoptotic mechanisms. As standard chemotherapy approaches may not effectively target the LSC population, blocking molecular pathways involved in cell survival and chemoresistance that is unregulated in these populations can induce apoptosis in a selective manner. Various studies with leukemia progenitor cells show that drugs which can target multiple pathways deregulated in the LSCs, such as NF-κB, PI3 kinase and STAT can produce a significant apoptotic effect [72–75].
Natural products have been the mainstay of cancer chemotherapy for the past 50 years. Three quarters of currently available drugs are natural products or related to them [76]. More than 60% of the 140 anti-cancer agents approved since 1940 can be traced to a natural product. In 2000, 57% of all drugs in clinical trials for cancer were either natural products or their derivatives [77]. When compared with synthesized chemical compounds, natural products show a favorable profile in terms of their absorption and metabolism in the body with low toxicity. Natural products are also shown to possess multi-faceted mechanism that can hit multiple pathways that are de-regulated in cancer cells to achieve greater therapeutic efficacy.
Parthenolide
Parthenolide (Fig. 2a), a naturally occurring sesquiterpene lactone containing an α- methylene-γ-lactone ring and an epoxide, belong to the germacranolide class and is present in the medicinal plant Tanacetum parthenium (feverfew) [78]. Parthenolide was reported to induce robust apoptosis in total as well as more primitive CD34+ populations from primary human AML specimens representing different French-American-British (FAB) subtypes while sparing normal hematopoietic cells. A side-by-side with the standard chemotherapy drug (cytarabine) showed that cytarabine was more toxic than parthenolide for normal cells with reduced toxicity to AML stem (CD34+/CD38-) and progenitor cells. Parthenolide was also shown to preferentially target AML progenitors (in vitro colony assay) and stem cell population in SCID mice xenograft model through the inhibition of NF-κB, proapoptotic activation of tumor suppressor p53, and increased reactive oxygen species (ROS) production [27].
Even though parthenolide is very effective in inducing AML LSC-specific cell death, its poor pharmacologic properties limit its clinical application. According to a Phase I dose escalation study of feverfew with standardized doses of parthenolide in patients with cancer, doses up to 4 mg as a daily oral capsule resulted in parthenolide plasma level well below detection limit of 0.5 ng/ml [79]. Recent studies on chemically modified parthenolide analogue, dimethylamino-parthenolide (Fig. 2b), have shown an oral bioavailability of ~70% compared with intravenous administration in mouse and canine models with an improvement in selectively eradicating AML stem and progenitor cells [80]. Dimethylamino-parthenolide also showed potent in vivo biological activity in spontaneous canine acute leukemia models and is currently evaluated in a Phase II clinical trial in AML patients. Further refinement in the bioavailability and selective toxicity will lead the way to a promising therapeutic drug.
Recent study by Pei et al. [81] shows that a parthenolide-based drug regimen containing parthenolide, 2-deoxyglucose and temsirolimus is a potent method of targeting AML stem cells while having no adverse effect on normal stem cells.
Triptolide
Triptolide (Fig. 2c) is a bioactive diterpenoid triepoxide found in commonly used traditional Chinese medicinal plant, Tripterygium wilfordii (Thunder God Vine). Recent studies show that triptolide has a broad-spectrum anti-cancer activity against various hematological malignancies and solid tumors (IC50 of 2.6–50 nM in vitro and effective tumor inhibition in xenograft mice models at 0.15– 3 mg/kg in vivo), resulting in inhibition of tumor growth and inducing tumor cell apoptosis [82]. Due to toxicity and narrow therapeutic window, triptolide has been classified as an anticancer agent with limited therapeutic applications. Meanwhile, there are reports which suggest that triptolide might be useful as a promising chemotherapy sensitizer if it is used at low doses. It was used to enhance the cytotoxicity of conventional anticancer drugs (5-fluorouracil [83], cisplatin [84], dexamethasone [85], hydroxycamptothecin [86], etc) at low doses with limited adverse effects. Liu et al. [87] have reported that low-dose triptolide in combination with idarubicin induces apoptosis in LSC-like cells derived from KG1a cell line. KG1a cell line is derived from a male AML patient, in which most of the cells are still CD34+/CD38-, and they do not spontaneously differentiate into granulocytes and macrophage-like cells. The effects of triptolide on LSC-like cells is mediated through ROS generation, downregulation of Nrf2 pathway and HIF1α pathways.
Cantharidin
Cantharidin (Fig. 2d), a natural toxin of terpenoid class secreted by many species of blister beetles, is used as a medicinal agent to remove warts. It is not a substrate for the multidrug resistant pumps in LSCs, hence it is a promising compound for selectively targeting LSCs. Cantharidin and its derivative Norcantharidin were found to inhibit hepatic leukemia factor, a gene implicated in the regulation of HSCs, and is also overexpressed in LSCs [88]. In vitro, Cantharidin and Norcantharidin were found to specifically target primary AML stem and progenitor cells by regulating the expression of genes involved in survival pathways such as SLUG, NFIL3 and c-myc, thereby inducing p53 and mitochondrial-caspase cascade to induce apoptosis. The dose-limiting toxicity of Cantharidin and Norcantharidin in vivo, limit their clinical application [89]. Nonetheless, potent in vitro LSC activity of cantharidin can be exploited clinically with the synthesis of new derivatives with reduced toxicity and in combination use with a suitable chemotherapeutic agent.
Cyclopamine
Cyclopamine (11-deoxojervine) (Fig. 2e), naturally occurring steroidal jerveratrum alkaloid, is a teratogen isolated from the Veratrum californicum (corn lily). In AML, primary CD34+ blasts and CD34+ cell lines had a greater degree of hedgehog signaling pathway activation when compared to CD34− blasts. Treatment with cyclopamine or monoclonal antibody to neutralize Hh ligands, result in Hh inhibition inducing apoptosis in CD34+ cell lines as well as sensitizing them against cytarabine. Cyclopamine treatments failed to affect growth or survival of AML cell lines without the G protein-coupled receptor, Smoothened (SMO), indicating the specificity of cyclopamine [56].
Salinomycin
Salinomycin (Fig. 2f), a monocarboxylic polyether antibiotic, coccidiostat and ionophore with a preference for potassium, is isolated from Streptomyces albus [90]. Gupta et al. [91] reported that salinomycin can selectively kill human breast cancer stem cells. Salinomycin induces apoptosis and overcomes chemoresistance in LSCs and other tumor cells with ABC transporter-mediated multidrug resistance [92]. Human AML cell line KG1a treated with phenylbutyrate (histone deacetylase inhibitor) resembles characteristics of LSCs and display resistance to various chemotherapeutic drugs. Salinomycin treatment overcomes ABC transporter-mediated multidrug resistance to apoptosis-inducing concentrations of bortezomib and doxorubicin in human leukemia stem cell-like KG1a cells [93]. Salinomycin did not permit long-term adaptation and development of resistance of KG1a AML stem-like cells, which is an indictor of its value in clinical application. The proposed mechanisms of action include ROS generation, activation of Wnt/beta-Catenin pathway, inhibition of oxidative phosphorylation, cytoplasmic and mitochondrial K+ efflux, interference with ABC transporters and inducing differentiation of stem cells.
17-N-allylamino-17-demethoxy geldanamycin (17-AAG)
17-AAG (Fig. 2g) is a derivative of the antibiotic geldanamycin. It interacts reversibly with the ATP binding domain of HSP90 that is critical for its chaperone function, ultimately inducing tumor cell death [94]. 17-AAG was reported to preferentially induce apoptosis and eliminated the colony formation capacity of human AML LSCs. Treatment with low concentrations of 17-AAG selectively eliminates AML stem cells in vitro and in vivo by disrupting HSP90 client protein, HIF1α, while it failed to eradicate highly proliferative non-LSC terminal blast cells having constitutively active Akt-GSK3 signaling pathway [95].
Kinetin riboside (6-Furfurylaminopurine riboside)
Kinetin riboside (Fig. 2h), a natural compound present in the coconut milk, is an anti-proliferative agent belonging to the ‘Cytokinins’ class of plant hormones. Kinetin riboside treatment results in CDKN1A upregulation, ATP depletion, cell cycle arrest at the G2/M phase, disruption of mitochondrial membrane potential, release of cytochrome c, caspase-3 activation, upregulation of Bad and down-regulation of Bcl-2 [96–98]. In vitro, Kinetin riboside induces apoptosis in the CD34+/CD38- AML stem cell fraction and prevents LSC engraftment in NOD/SCID mouse model while sparing HSC fractions [99].
Resveratrol
Resveratrol (Fig. 2i) (3,5,4′-trans-trihydroxystilbene) is a polyphenolic phytoalexin that has anti-oxidant, anti-inflammatory, cardioprotective, and anti-tumor activities [100]. It is structural similarities to estradiol and diethylstilbestrol and is present in the skin of red grapes, red wine, cranberries, blueberries and various other fruits. Resveratrol can inhibit growth and induce apoptosis in several human cancer cells, including mouse and human leukemia cell lines, through various mechanisms such as; modulating nitric oxide production, accumulation of p53 and p21, inhibition of ribonucleotide reductase and DNA polymerase, inducing arrest at the S and G2 phases of the cell cycle and inhibiting interleukin-1β–induced activation of NF-κB [101]. Hu et al. reported that resveratrol can selectively inhibit the growth of leukemia stem cell-like KG1a cells and sensitize cells to cytolysis by cytokine-induced killer cells through upregulation of NKG2D ligands (ULBP1, ULBP2 and ULBP3) and TNF-related apoptosis-inducing ligand receptors (DR4) [102].
Avocatin B
Avocatin B (Fig. 2j), an Avocado (Persea americana) derived lipid, is a combination of two 17-carbon lipids (16-Heptadecene-1,2,4-triol & 16-heptadecyne-1,2,4-triol; 1:1 ratio) isolated from the methanolic extract of peel and seed of unripe avocado fruit. Cytotoxic property towards lung carcinoma, mammary adenocarcinoma, kidney carcinoma and pancreatic carcinoma cell lines has been reported by Oberlies et al. [103], with selectivity towards PC-3 human prostate adenocarcinoma cells, as potent as Adriamycin. It is also reported as an effective insecticide against yellow fever mosquito larva. Avocatin B was found to reduce viability of human primary AML progenitor and stem cells, while having no significant effect on normal peripheral blood CD34+ stem cells. Avocatin B induces ROS-dependent, mitochondria-mediated, apoptosis in AML cells, characterized by the release of apoptosis-inducing factor and cytochrome c in to the cytosol. It also inhibited fatty acid oxidation and decreased NAD and NADPH levels [104]. Avocatin B was also found to synergize other chemotherapeutics (cytarabine and doxorunicin) to induce leukemia cell death [105].
Conclusion and perspectives
AML is a lethal form of hematologic malignancy, typically of stem or progenitor cell origin. The main hurdle to treat and cure AML is the inability to efficiently target and eliminate leukemia stem cells. Importantly, LSC stands apart from more differentiated blast cells with distinct set of unique biological properties and in most cases are not effectively targeted by standard chemotherapy agents, which can effectively kill leukemic blast cells in majority of patients. Due to the pivotal role of stem cells in the genesis, perpetuation and clinical relapse of AML, recent studies were focused on characterizing the molecular properties of LSC population that could be used for selective induction of apoptosis. Molecular analysis of AML LSC population shows that survival signaling mediated by NF-κB, STAT, PI3 kinase pathways and self-renewal regulatory pathways such as Wnt/beta-catenin, Hedgehog and Notch signaling pathways represent potential targets for therapeutic intervention. Similarly, re-activation of p53-mediated apoptosis pathways have shown to induce apoptosis in the LSC population. These findings demonstrate that more durable remissions in AML can be accomplished using a combination of selective inhibitors of above-mentioned survival pathways in LSC along with traditional regimens. Recent research advancement in the understanding of LSC bestows an expanding list of strategies to target LSC and some of the natural products summarized above (Table 1) have already been tried and proven to be effective. Moreover, combinations of natural products with chemotherapeutic drugs have been shown to preferentially induce apoptosis in human LSC, which is promising. The dandelion phenomenon predicts that treatments, which selectively attack stem cells, will not immediately eliminate the differentiated tumor cells and require a longer treatment period to obtain clinical responses compared to conventional therapies targeting the bulk population [106]. Therefore, LSC directed therapies utilizing natural compounds alone, may need longer treatment duration to be effective while use in combination with standard drugs like cytarabine and daunorubicin produce an early response to reduce the tumor cell population. A rational design of parthenolide-based drug regimen based on proteomic, genomic, and metabolomic methods by Pei et al. [81] is a perfect example of comprehensive approach to develop natural products based anticancer drug regimens. Similar studies based on the LSC validated natural products can offer numerous possibilities for eradication of AML stem cells, which can be translated to the clinical system. Also more natural products need to be screened for their ability to selectively target the LSCs. Natural products may also serve as models for the preparation of more efficacious analogues using chemical methodology such as total or combinatorial synthesis, or manipulation of biosynthetic pathways.
Abbreviations
- AML:
-
Acute myeloid leukemia
- CD:
-
Cluster of differentiation
- HSC:
-
Hematopoietic stem cells
- LSC:
-
Leukemia stem cells
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PI3K:
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- ROS:
-
Reactive oxygen species
- STAT:
-
Signal Transducers and Activators of Transcription
References
Jordan C. Unique molecular and cellular features of acute myelogenous leukemia stem cells. Leukemia. 2002;16:559–62.
Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–107.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Warner JK, Wang JC, Hope KJ, Jin L, Dick JE. Concepts of human leukemic development. Oncogene. 2004;23:7164–77.
Basak NP, Banerjee S. Mitochondrial dependency in progression of acute myeloid leukemia. Mitochondrion. 2015;21:41–8.
Mint MA, Balsat M, Thenoz M, Koering C, Payen-Gay L, Cheok M, et al. Oncogene-and drug resistance-associated alternative exon usage in acute myeloid leukemia (AML). Oncotarget. 2015;7:2889–909.
Naito K, Ohnishi K. Current and new therapeutic strategies in acute myeloid leukemia. Gan To Kagaku Ryoho. 2005;32:292–6.
Bydlowski SP, Janz FdL. Hematopoietic Stem Cell in Acute Myeloid Leukemia Development. In: Pelayo R, editors. Advances in Hematopoietic Stem Cell Research. Rijeka: InTech; 2012. p. 261-276.
Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice. J Clin Invest. 2011;121:384–95.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8.
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44.
Horton SJ, Huntly BJ. Recent advances in acute myeloid leukemia stem cell biology. Haematologica. 2012;97:966–74.
Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–43.
Jordan CT, Guzman ML. Mechanisms controlling pathogenesis and survival of leukemic stem cells. Oncogene. 2004;23:7178–87.
Blair A, Hogge D, Sutherland H. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34+/CD71−/HLA-DR−. Blood. 1998;92:4325–35.
Zhou J, Chng WJ. Identification and targeting leukemia stem cells: The path to the cure for acute myeloid leukemia. World J Stem Cells. 2014;6:473.
Wiernik PH, Banks P, Case DJ, Arlin ZA, Periman PO, Todd MB, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79:313–9.
Chan WI, Huntly BJ. Leukemia stem cells in acute myeloid leukemia. Semin Oncol. 2008;35:326–35.
Damiani D, Tiribelli M, Raspadori D, Sirianni S, Meneghel A, Cavalllin M, et al. Clinical impact of CD200 expression in patients with acute myeloid leukemia and correlation with other molecular prognostic factors. Oncotarget. 2015;6:30212–21.
van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11:6520–7.
Stiehl T, Baran N, Ho AD, Marciniak-Czochra A. Cell division patterns in acute myeloid leukemia stem-like cells determine clinical course: a model to predict patient survival. Cancer Res. 2015;75:940–9.
Pollyea DA, Gutman JA, Gore L, Smith CA, Jordan CT. Targeting acute myeloid leukemia stem cells: a review and principles for the development of clinical trials. Haematologica. 2014;99:1277–84.
Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94.
Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34.
Ho MM, Hogge DE, Ling V. MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Exp Hematol. 2008;36:433–42.
Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–9.
Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
Siveen KS, Mustafa N, Li F, Kannaiyan R, Ahn KS, Kumar AP, et al. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget. 2014;5:634–48.
Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–7.
Zhou J, Ching YQ, Chng WJ. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: from molecular pathogenesis to therapeutic target. Oncotarget. 2015;6:5490–500.
Brach MA, Kharbanda SM, Herrmann F, Kufe DW. Activation of the transcription factor kappa B in human KG-1 myeloid leukemia cells treated with 1-beta-D-arabinofuranosylcytosine. Mol Pharmacol. 1992;41:60–3.
Laurent G, Jaffrézou JP. Signaling pathways activated by daunorubicin. Blood. 2001;98:913–24.
Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFκB activation: a role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503.
Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–7.
Reikvam H, Nepstad I, Bruserud Ø, Hatfield KJ. Pharmacologic targeting of the PI3K/mTOR pathway controls release of angioregulators from primary human acute myeloid leukemia cells and their neighboring stromal cells. Oncotarget. 2013;4:830–43.
Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–80.
Zhao S, Konopleva M, Cabreira-Hansen M, Xie Z, Hu W, Milella M, et al. Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD and promotes apoptosis in myeloid leukemias. Leukemia. 2004;18:267–75.
Martelli AM, Chiarini F, Evangelisti C, Cappellini A, Buontempo F, Bressanin D, et al. Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget. 2012;3:371–94.
Birkenkamp KU, Geugien M, Schepers H, Westra J, Lemmink HH, Vellenga E. Constitutive NF-κB DNA-binding activity in AML is frequently mediated by a Ras/PI3-K/PKB-dependent pathway. Leukemia. 2004;18:103–12.
Park I, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–5.
Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50.
Yilmaz ÖH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–82.
Furqan M, Mukhi N, Lee B, Liu D. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res. 2013;1:5–15.
Gouilleux-Gruart V, Gouilleux F, Desaint C, Claisse J, Capiod JC, Delobel J, et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87:1692–7.
Spiekermann K, Biethahn S, Wilde S, Hiddemann W, Alves F. Constitutive activation of STAT transcription factors in acute myelogenous leukemia. Eur J Haematol. 2001;67:63–71.
Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000;95:19–29.
Cook AM, Li L, Ho Y, Lin A, Li L, Stein A, et al. Role of altered growth factor receptor-mediated JAK2 signaling in growth and maintenance of human acute myeloid leukemia stem cells. Blood. 2014;123:2826–37.
Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–95.
Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci USA. 2002;99:16220–5.
Hsieh CH, Chao K, Liao HF, Chen YJ. Norcantharidin, derivative of cantharidin, for cancer stem cells. Evid Based Complement Alternat Med. 2013;2013:838651–62.
Irvine DA, Copland M. Targeting hedgehog in hematologic malignancy. Blood. 2012;119:2196–204.
Ok CY, Singh RR, Vega F. Aberrant activation of the hedgehog signaling pathway in malignant hematological neoplasms. Am J Pathol. 2012;180:2–11.
Wellbrock J, Latuske E, Köhler J, Wagner K, Stamm H, Vettorazzi E, et al. Expression of Hedgehog pathway mediator GLI represents a negative prognostic marker in human acute myeloid leukemia and its inhibition exerts antileukemic effects. Clin Cancer Res. 2015;21:2388–98.
Minami Y, Hayakawa F, Kiyoi H, Sadarangani A, Jamieson CH, Naoe T. Treatment with hedgehog inhibitor, PF-04449913, attenuates leukemia-initiation potential in acute myeloid leukemia cells. Blood. 2013;122:1649.
Kobune M, Takimoto R, Murase K, Iyama S, Sato T, Kikuchi S, et al. Drug resistance is dramatically restored by hedgehog inhibitors in CD34+ leukemic cells. Cancer Sci. 2009;100:948–55.
Kobune M, Iyama S, Kikuchi S, Horiguchi H, Sato T, Murase K, et al. Stromal cells expressing hedgehog-interacting protein regulate the proliferation of myeloid neoplasms. Blood Cancer J. 2012;2, e87.
Kim Y, Thanendrarajan S, Schmidt-Wolf I. Wnt/ß-catenin: a new therapeutic approach to acute myeloid leukemia. Leuk Res Treatment. 2011;2011:428960–63.
Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/β-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–3.
Liu N, Zhang J, Ji C. The emerging roles of Notch signaling in leukemia and stem cells. Biomark Res. 2013;1:1–23.
Tohda S. NOTCH Signaling Roles in Acute Myeloid Leukemia Cell Growth and Interaction with other Stemness-related Signals. Anticancer Res. 2014;34:6259–64.
Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, et al. Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med. 2013;210:321–37.
Lobry C, Ntziachristos P, Ndiaye-Lobry D, Oh P, Cimmino L, Zhu N, et al. Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J Exp Med. 2013;210:301–19.
Tse MT. Cancer: Activating Notch ameliorates AML. Nat Rev Drug Discovery. 2013;12.
Saudy NS, Fawzy IM, Azmy E, Goda EF, Eneen A, Salam EMA. BMI1 gene expression in myeloid leukemias and its impact on prognosis. Blood Cells Mol Dis. 2014;53:194–8.
Rizo A, Olthof S, Han L, Vellenga E, de Haan G, Schuringa JJ. Repression of BMI1 in normal and leukemic human CD34+ cells impairs self-renewal and induces apoptosis. Blood. 2009;114:1498–505.
Marcucci G, Mrózek K, Radmacher MD, Garzon R, Bloomfield CD. The prognostic and functional role of microRNAs in acute myeloid leukemia. Blood. 2011;117:1121–9.
Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105:3945–50.
de Leeuw DC, Denkers F, Olthof MC, Rutten AP, Pouwels W, Schuurhuis GJ, et al. Attenuation of microRNA-126 expression that drives CD34+ 38− stem/progenitor cells in acute myeloid leukemia leads to tumor eradication. Cancer Res. 2014;74:2094–105.
Lechman ER, Gentner B, Ng SW, Schoof EM, van Galen P, Kennedy JA, et al. miR-126 regulates distinct self-renewal outcomes in normal and malignant hematopoietic stem cells. Cancer Cell. 2016;29:214–28.
Tian C, You MJ, Yu Y, Zhu L, Zheng G, Zhang Y. MicroRNA-9 promotes proliferation of leukemia cells in adult CD34-positive acute myeloid leukemia with normal karyotype by downregulation of Hes1. Tumor Biol. 2015;1–11.
Rinkenbaugh AL, Baldwin AS. The NF-κB Pathway and cancer stem cells. Cells. 2016;5:16.
Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–42.
Ravandi F, Talpaz M, Kantarjian H, Estrov Z. Cellular signalling pathways: new targets in leukaemia therapy. Br J Haematol. 2002;116:57–77.
Steelman L, Pohnert S, Shelton J, Franklin R, Bertrand F, McCubrey J. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218.
Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–8.
Demain AL, Vaishnav P. Natural products for cancer chemotherapy. Microb Biotechnol. 2011;4:687–99.
Pajak B, Gajkowska B, Orzechowski A. Molecular basis of parthenolide-dependent proapoptotic activity in cancer cells. Folia Histochem Cytobiol. 2008;46:129–35.
Curry Iii EA, Murry DJ, Yoder C, Fife K, Armstrong V, Nakshatri H, et al. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest New Drugs. 2004;22:299–305.
Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–35.
Pei S, Minhajuddin M, D'Alessandro A, Nemkov T, Stevens BM, Adane B, et al. Rational Design of a Parthenolide-based Drug Regimen That Selectively Eradicates Acute Myelogenous Leukemia Stem Cells. J Biol Chem. 2016;291:21984–2000.
Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH. Triptolide: structural modifications, structure–activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep. 2012;29:457–75.
Li Y, Hu S. Triptolide sensitizes liver cancer cell lines to chemotherapy in vitro and in vivo. Panminerva Med. 2014;56:211–20.
Wang G, Wang X, Xu X. Triptolide potentiates lung cancer cells to cisplatin-induced apoptosis by selectively inhibiting the NER activity. Biomark Res. 2015;3:1–10.
Huang X, Yang M, Jin J. Triptolide enhances the sensitivity of multiple myeloma cells to dexamethasone via microRNAs. Leuk Lymphoma. 2012;53:1188–95.
Yang SW, Wang W, Xie XY, Zhu WP, Li FQ. In vitro synergistic cytotoxic effect of triptolide combined with hydroxycamptothecin on pancreatic cancer cells. Am J Chin Med. 2011;39:121–34.
Liu Y, Chen F, Wang S, Guo X, Shi P, Wang W, et al. Low-dose triptolide in combination with idarubicin induces apoptosis in AML leukemic stem-like KG1a cell line by modulation of the intrinsic and extrinsic factors. Cell Death Dis. 2013;4, e948.
Moore MA, Dorn DC, Schuringa JJ, Chung KY, Morrone G. Constitutive activation of Flt3 and STAT5A enhances self-renewal and alters differentiation of hematopoietic stem cells. Exp Hematol. 2007;35:105–16.
Dorn DC, Kou CA, Png KJ, Moore MA. The effect of cantharidins on leukemic stem cells. Int J Cancer. 2009;124:2186–99.
Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. 2012;2012:1–17.
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59.
Fuchs D, Heinold A, Opelz G, Daniel V, Naujokat C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390:743–9.
Fuchs D, Daniel V, Sadeghi M, Opelz G, Naujokat C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun. 2010;394:1098–104.
Powers MV, Valenti M, Miranda S, Maloney A, Eccles SA, Thomas G, et al. Mode of cell death induced by the HSP90 inhibitor 17-AAG (tanespimycin) is dependent on the expression of pro-apoptotic BAX. Oncotarget. 2013;4:1963–75.
Newman B, Liu Y, Lee HF, Sun D, Wang Y. HSP90 inhibitor 17-AAG selectively eradicates lymphoma stem cells. Cancer Res. 2012;72:4551–61.
Dudzik P, Dulińska Litewka J, Wyszko E, Jędrychowska P, Opałka M, Barciszewski J, et al. Effects of kinetin riboside on proliferation and proapoptotic activities in human normal and cancer cell lines. J Cell Biochem. 2011;112:2115–24.
Choi BH, Kim W, Wang QC, Kim DC, Tan SN, Yong JWH, et al. Kinetin riboside preferentially induces apoptosis by modulating Bcl-2 family proteins and caspase-3 in cancer cells. Cancer Lett. 2008;261:37–45.
Rajabi M, Gorincioi E, Santaniello E. Antiproliferative activity of kinetin riboside on HCT-15 colon cancer cell line. Nucleosides Nucleotides Nucleic Acids. 2012;31:474–81.
McDermott SP, Eppert K, Notta F, Isaac M, Datti A, Al-awar R, et al. A small molecule screening strategy with validation on human leukemia stem cells uncovers the therapeutic efficacy of kinetin riboside. Blood. 2012;119:1200–7.
Cakir Z, Saydam G, Sahin F, Baran Y. The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60 acute myeloid leukemia cells. J Cancer Res Clin Oncol. 2011;137:279–86.
Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, et al. Resveratrol blocks interleukin-1β–induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–95.
Hu L, Cao D, Li Y, He Y, Guo K. Resveratrol sensitized leukemia stem cell-like KG-1a cells to cytokine-induced killer cells-mediated cytolysis through NKG2D ligands and TRAIL receptors. Cancer Biol Ther. 2012;13:516–26.
Oberlies NH, Rogers LL, Martin JM, McLaughlin JL. Cytotoxic and Insecticidal Constituents of the Unripe Fruit of Persea americana. J Nat Prod. 1998;61:781–5.
Lee EA, Angka L, Rota S-G, Hanlon T, Mitchell A, Hurren R, et al. Targeting Mitochondria with Avocatin B Induces Selective Leukemia Cell Death. Cancer Res. 2015;75:2478.
Tcheng M, Samudio I, Lee EA, Minden MD, Spagnuolo PA. The mitochondria target drug avocatin B synergizes with induction chemotherapeutics to induce leukemia cell death. Leuk Lymphoma. 2016;1–3.
Huff CA, Matsui WH, Smith BD, Jones RJ. Strategies to eliminate cancer stem cells: clinical implications. Eur J Cancer. 2006;42:1293–7.
Acknowledgements
Not applicable.
Funding
The authors were supported by grant from the Medical Research Center (to KSS) (Grant # 16115/16), Hamad Medical Corporation, Doha, State of Qatar.
Availability of data and materials
Please contact author for data requests.
Authors’ contributions
KSS was the major contributor in writing the manuscript. SU and RMM made extensive contributions to the design of the manuscript and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Siveen, K.S., Uddin, S. & Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol Cancer 16, 13 (2017). https://doi.org/10.1186/s12943-016-0571-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-016-0571-x