Abstract
Background
Artesunate-amodiaquine (ASAQ) and artemether-lumefantrine (AL) are the currently recommended first- and second-line therapies for uncomplicated Plasmodium falciparum infections in Equatorial Guinea. This study was designed to evaluate the efficacy of these artemisinin-based combinations and detect mutations in P. falciparum kelch13-propeller domain gene (Pfkelch13).
Methods
A single-arm prospective study evaluating the efficacy of ASAQ and AL at three sites: Malabo, Bata and Ebebiyin was conducted between August 2017 and July 2018. Febrile children aged six months to 10 years with confirmed uncomplicated P. falciparum infection and other inclusion criteria were sequentially enrolled first in ASAQ and then in AL at each site, and followed up for 28 days. Clinical and parasitological parameters were assessed. The primary endpoint was PCR-adjusted adequate clinical and parasitological response (ACPR). Samples on day-0 were analysed for mutations in Pfkelch13 gene.
Results
A total 264 and 226 patients were enrolled in the ASAQ and AL treatment groups, respectively. Based on per-protocol analysis, PCR-adjusted cure rates of 98.6% to 100% and 92.4% to 100% were observed in patients treated with ASAQ and AL, respectively. All study children in both treatment groups were free of parasitaemia by day-3. Of the 476 samples with interpretable results, only three samples carried non-synonymous Pfkelch13 mutations (E433D and A578S), and none of them is the known markers associated with artemisinin resistance.
Conclusion
The study confirmed high efficacy of ASAQ and AL for the treatment of uncomplicated falciparum infections as well as the absence of delayed parasite clearance and Pfkelch13 mutations associated with artemisinin resistance. Continued monitoring of the efficacy of these artemisinin-based combinations, at least every two years, along with molecular markers associated with artemisinin and partner drug resistance is imperative to inform national malaria treatment policy and detect resistant parasites early.
Trial registration ACTRN12617000456358, Registered 28 March 2017; http://www.anzctr.org.au/trial/MyTrial.aspx
Similar content being viewed by others
Background
Countries in the World Health Organization (WHO) African Region bear most of the malaria burden and account for 94% of the estimated global malaria cases (213 million cases) and deaths (380,000) in 2019 [1]. Providing effective treatment to patients suffering from uncomplicated falciparum malaria prevents progression of the disease to a severe form or death and consequently reduces mortality and disease burden. Plasmodium falciparum resistance to anti-malarial drugs poses a constant threat to the successful treatment of malaria infections. The emergence and spread of chloroquine and sulfadoxine/pyrimethamine resistances have led the WHO to recommend artemisinin-based combination therapy (ACT) for the treatment of uncomplicated falciparum infection [2]. ACT combines potent and fast-acting artemisinin derivatives with a long and slow-acting partner drug able to clear residual parasitemias. The currently recommended artemisinin-based combinations are artemether-lumefantrine (AL), artesunate-amodiaquine (ASAQ), artesunate-sulfadoxine/pyrimethamine (ASSP), artesunate-mefloquine (ASMQ), and dihydroartemisinin-piperaquine (DHAPPQ) and artesunate-pyronaridine (ASPY) [3].
Improved access to effective antimalarial treatments has contributed significantly to a marked reduction in the burden of malaria in recent years [1]. AL followed by ASAQ are the most commonly recommended first and/or second line anti-malarial drugs in malaria endemic countries in Africa [1] and they remain efficacious after more than a decade of use [3]. However, the efficacy of ACT is threatened by the recent emergence of parasites resistant to artemisinin and partner drugs. Point mutations in the P. falciparum kelch 13 (Pfkelch13) propeller domain gene have been found to confer artemisinin partial resistance expressed by delayed parasite clearance [4, 5]. Since 2012, resistance to both artemisinin and partner drugs has been observed in several countries in Southeast Asia [5,6,7,8,9] leading to treatment failure with artemisinin-based combinations, such as DHA-PPQ [10,11,12,13]. Moreover, and more worryingly, mutants Pfkelch13 parasites (R561H) confering artemisinin partial resistance has been shown to emerge in 2014–2015 in one site in Rwanda followed by its expansion in another site located apart 100 km, several months later [14]. These findings pose a real threat to malaria case management, a fundamental component of current malaria intervention, and make regular monitoring of the efficacy of ACT, as recommended by the WHO [3], essential to support timely review of malaria treatment guidelines and ensure that malaria patients receive efficacious treatment [3].
In Equatorial Guinea, malaria is a major health problem for the entire population (1,355,982) with an estimated 321,438 (186,000–524,000) cases and 652 (440–1000) deaths in 2019 [1]. The majority of the disease burden occurs on the mainland, where children bear the brunt of the disease [15]. Equatorial Guinea recommended ASAQ as first-line drug in 2008 and AL as second-line drug in 2010 for the treatment of uncomplicated falciparum infection [16]. Since the introduction of these artemisinin-based combinations in the country, only one study (unpublished) has been conducted to evalute the efficacy of ASAQ, which showed PCR corrected cure rate > 96.6% [17]. Recently, PfK13 nonsynonimous mutation (M579I), associated with delayed parasite clearance and increased in vitro parasite survival rate as measured by the Ring-stage Survival Assay [18] has been detected in a Chinese traveller returning from Equatorial Guinea [19]. The current study evaluated the efficacy of ASAQ and AL as well as the detection of mutations in the Pfkelch13 gene to inform national malaria treatment policy.
Methods
Study area, design and population
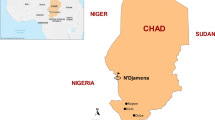
Equatorial Guinea is located in Central Africa and is divided into two regions: the mainland area, which lies between Cameroon and Gabon, and the island region (Bioko, Annobo'n and Corisco Bay). About 75% of the country's population lives in the mainland. The study was conducted in selected health facilities in three study sites: Malabo in Bioko Island and Bata and Ebebiyin in Litoral and Kié-Ntem provinces in the mainland, respectively (Fig. 1). The health facilities in the study were Regional Hospital in Malabo, Provincial Hospital and Angokong Health Centre in Ebebiyin, Regional Hospital, Maria Rafols Health Centre and Maria Gay Health Centre in Bata. The study was a single arm cohort study that investigated the clinical therapeutic efficacy of ASAQ and AL in the treatment of uncomplicated falciparum infection. Children with uncomplicated falciparum malaria who met the study inclusion criteria were enrolled sequentially, first to ASAQ until sample size was reached and then to AL in each site, and assessed clinically and parasitologically for 28 days, based on the 2009 WHO protocol [20].
Recruitment procedure and treatment
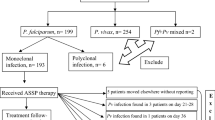
Potential study children attending study health facilities from August 2017 to July 2018 were screened and enrolled if they met the following eligibility criteria: age between 6 months and 10 years, axillary temperature ≥ 37.5 °C and/or history of fever in the past 24 h, P. falciparum monoinfection, parasitaemia of 500 to 200,000 asexual parasites/µl, willingness to comply with the study visit schedule, and informed consent from parents or guardians (Fig. 2). Children with exclusion criteria, including the presence of general danger signs or evidence of severe falciparum malaria, mixed or monoinfection with non-falciparum species, severe malnutrition, febrile conditions due to diseases other than malaria (measles, acute lower respiratory tract infection, severe diarrhea with dehydration), or known underlying chronic diseases (e.g., cardiac, renal, or liver disease, HIV/AIDS), received appropriate care and treatment according to national guidelines. In addition, children on regular medication that could have affected the pharmacokinetics of the study ACT and those with a history of hypersensitivity reactions to the medications were excluded.
Children in the ASAQ group received daily dose of ASAQ for three consecutive days based on recommended weight ranges: one tablet and two tablets of 25 mg artesunate/67.5 mg for children weighing ≥ 4.5 to < 9 kg and 9 to < 18 kg, respectively; one tablet and two tablets of 100 mg artesunate/270 mg amodiaquine for children weighing 18 to < 36 kg and ≥ 36 kg, respectively. For children treated et al. doses of AL were administered twice daily for 3 days according to recommended weight: one tablet for children weighing 5–14 kg, two tablets for 15–24 kg, and three tablets for 25–34 kg. All treatment doses were administered under direct observation by the study team and patients were observed for 30 min. If the first dose was vomited, the treatment dose was administered again. If vomited again, the patient received an artesunate injection according to national guidelines and the patient was withdrawn from the study. Study drugs were obtained from the WHO.
Enrolled children were followed for up to 28 days at scheduled visits on days 1, 2, 3, 7, 14, 21, and 28, and at unscheduled visits if symptoms worsened or recurred. Clinical and parasitological examinations were performed at each visit. If the parents/caregivers did not show up for the scheduled visit, a team member visited them at home.
Sample size estimation
A minimum sample of 73 children per drug per site was estimated, based on the assumption of a 5% treatment failure rate for each drug ASAQ and AL with a 95% confidence level and 5% precision. An additional 20% was added to allow for "lost to follow-up" and withdrawal during the 28-day follow-up period. The target sample size was 88 patients per drug per site.
Malaria microscopy
Thick and thin blood slides were stained with Giemsa and examined microscopically to detect malaria parasites and determine level of parasitaemia based on the WHO procedure [20]. The number of asexual parasites was counted against 200 white blood cells (or per 500 if the count was < 100 parasites/200 white blood cells). A blood slide was confirmed negative if no parasite was seen after counting 1000 white blood cells. Parasite density, defined as parasites per µl, was calculated assuming a leukocyte count of 6000/µl of blood. All blood slides were examined by two independent microscopists. A third microscopist re-examined the slides with discordant results in terms of species diagnosis, parasite density of > 50% or presence of parasites. Final parasite density was calculated by averaging the two closest counts.
Genotyping of malaria parasite
Filter paper blood samples were collected from each patient on day-0 and in case of recurrence, on the day of parasite recurrence (from day-7). Samples were dried and stored in individual plastic bags containing desiccant. Each dried blood spot was punched out with a sterile puncher, and the spots were placed in numerical order in a 96-well plate. Parasite DNA was extracted using QIAamp DNA Blood Mini Kit (Qiagen). The DNA samples (day-0 and day of recurrence) were analysed for genotyping of the highly polymorphic regions msp1, msp2 (merozoite surface proteins 1 and 2) and glurp (glutamate-rich protein) loci, as described elsewhere [21]. The genotypic profiles of the parasites at day-0 and day of recurrence were compared to determine whether the recurent infections were a recrudescence (same strain) or a new infection (different strain), according to the current WHO-recommended algorithm [22]. As an explanatory endpoint, reinfection and recrudescence were also determined by the newly proposed two out of three (2/3) algorithm [23]. In this strategy, the classification of recurrent failures is based on a consensus result of msp1 and msp2 and disparate results are resolved by glurp. Such an analysis demands concommittant results from at least two markers for classification of reinfection or recrudescence compared to three with the standard WHO methodology. If results for only 2 markers were available and results for a third marker were missing, the PCR correction was classified as undertermined. Samples were analysed at Institut Pasteur in Paris, France.
Molecular markers of artemisinin resistance
DNA extracted from day-0 dried blood spots were analysed to detect the presence of mutations in the propeller domain of Pfkelch13 gene (PF3D7_1343700) previously described to be associated with artemisinin resistance [4]. PCR amplification (codons 440–680, 720 bp) was performed using the method described by Ariey et al. [4]. For the inner round, five μl DNA was amplified with 0.25 μM each primer, 0.2 mM dNTP, 2.5 mM MgCl2, and 1.25 U Taq DNA polymerase (Solis Biodyne, Estonia), in 25 μl volume using the following cycling program: 15 min at 95 °C, then 35 cycles of 30 s at 95 °C, 2 min at 58 °C, 2 min at 72 °C, and final extension 10 min at 72 °C. For the outer PCR round, 5 μl of primary PCR products were amplified under the same conditions, except for annealing and extension (1 min). PCR products were detected using capillary gel electrophoresis (Fragment Analyzer, Agilent, France). Double strand sequencing of PCR products was performed by Eurofins (Germany). Sequence polymorphisms were identified with the CLC Main Workbench 20 software (Qiagen) by using the 3D7 strain of P. falciparum (PF3D7_1343700) as a reference sequence. Electropherograms with mixed alleles were considered as mutant for the purpose of mutation frequency estimation. The quality control was assessed by including three blinded quality-control samples (wild type, C580Y and R539T) in each 96-well sequencing plate.
Treatment response measure
Treatment response was classified as early treatment failure (ETF), late clinical failure (LCF), late parasitological failure (LPF) and adequate clinical and parasitological response (ACPR) before and after PCR correction using the WHO protocol [18]. The primary study endpoint was PCR-corrected adequate clinical and parasitological response. Secondary endpoints included parasitaemia at day-3 and PCR-uncorrected treatment failure.
Ethical considerations
The study was approved by the Ministry of Health and Social Welfare of Equatorial Guinea (No. 731–150), the Ethics Committee of Spanish National Health Institute, Carlos III (CEI PI 57_2016-v3) and the WHO Research Ethics Review Committee (ERC.000286). Parents or guardians were informed about the study procedure, its benefits and potential risks, and their consent to enroll their children was obtained in writing before enrollment. They gave their written consent before enrolling their children in the study. If a patient, parent, or guardian was illiterate, he or she chose a witness to co-sign the consent form.
Data analysis
Data were double entered, cleaned, and analysed using the software programme WHO excel (http://www.who.int/malaria/publications/atoz/9789241597531/en/). Enrolled patients who were lost to follow-up or withdrawn from the study, had recurrent parasitaemia with new infection, or unknown PCR (indeterminate or missing) were excluded from per-protocol analysis. However, these cases were included in the Kaplan–Meier analysis up to the day of loss or withdrawal from the study. Descriptive statistics including percentages, mean, standard deviation, and range were used. Student's t-test was used for analysis of continuous variables (parasite density and age) and Fisher's exact test was used for categorical data. A p-value of < 0.05 was considered significant.
Results
Baseline characteristics of enrolled children
Of the 1725 children screened, 490 were enrolled in the study: 264 were treated with ASAQ (88 in Malabo, 88 in Bata and 88 in Ebibeyin) and 226 with AL (50 in Malabo, 88 in Bata and 88 in Ebibeyin). The target sample size per drug and per site (n = 88) was achieved except for the group treated with AL in Malabo site due to low malaria transmission. Baseline characteristics (age, axillary temperature, parasitaemia) of the study children in the different sites and treatment groups were comparable (Table 1).
Treatment responses
Table 2 shows the treatment outcomes as per-protocol and Kaplan Meier analysis before PCR correction. For patients treated on ASAQ, per-protocol analysis of uncorrected PCR data showed ACPR of 100% (95% CI 94.6–100%), 96.3% (95% CI 89.6–99.2%) and 88.8% (95% CI 79.7–94.7%) in Malabo, Bata and Ebebiyin sites, respectively. Among patients treated with AL, per-protocol analysis of uncorrected PCR data revealed ACPR of 92.9% (95% CI 80.5–98.5%), 95.3% (95% CI 88.4–98.7%) and 73.5% (95% CI 62.7–82.6%) in Malabo, Bata and Ebebiyin, respectively. For the 41 paired samples, msp1, msp2 and glurp results were available for 95.1%, 93.9% and 95.1%, respectively. Per-protocol analysis of PCR corrected data (using the WHO protocol) showed ACPR of 100% (95% CI 94.6–100%), 100% (95% CI 95.4–100%) and 98.6% (95% CI 92.5–100%) in patients treated with ASAQ in Malabo, Bata and Ebebiyin, respectively (Table 3). In children treated with AL, PCR corrected ACPR of 95.1% (95% CI 83.5–99.4%), 100% (95% CI 95.5–100%) and 92.4% (95% CI 83.2–97.5%) were in Malabo, Bata and Ebebiyin, respectively. Based on Kaplan Meier survival analysis, PCR-corrected cumulative cure rates varied from 98.7% to 100% for ASAQ and from 93.7% to 100% for AL (Table 3). Study children at the Ebebiyin site had a higher rate of new infections (9.1% and 19.3% in the ASAQ and AL groups, respectively), compared to less than 5% at the other sites (Table 3). However, the difference was significant only for the Ebebiyin group AL (Fisher's exact test: p = 0.01). Children in both treatment groups were parasite free on day-2 except one case in the ASAQ group in Bata (1.2%), and all of them cleared parasitaemia by day-3.
Using the 2/3 algorythm, PCR correction remained the same in 22/41 (53.7%) recurrences, changed to recrudescence in 12/41 (29.2%) recurrences, and was undertermined for 7/41 (17.1%) recurrences (Table 3). The failure rate increased significantly in Ebibeyin for both treatment arms (5.2% for ASAQ and 8.8% for AL). No significant changes were observed for the other two sites.
Artemisinin partial resistance marker
Among the 490 patients, three samples were missing and 11 gave non-interpretable results. Most of the samples with interpretable results (98.1%), carried Pfkelch13 wild type allele and only three (0.6%) had non-synonymous mutations (two carried A578S and one with E433D, Table 4). None were previously linked with artemisinin resistance. Of the 8 patients with recrudescent parasite, only four samples were successfully sequenced, and all carried Pfkelch13 wild type allele.
Discussion
Previous studies evaluating the efficacy of ASAQ in Equatorial Guinea reported a high cure rate with 97.3% ACPR in 2006 before the recommendation of ACT [24] and 96.6% ACPR four years later in 2010 [17]. The findings of the current study showed that patients treated with ASAQ achieved a high cure rate (PCR-adjusted ACPR), ranging from 98.6 to 100% across sites, demonstrating that it has maintained its efficacy after more than a decade of use in the country. The current study assessed the efficacy of AL for the first time in the country and showed similar high cure rate with a PCR-adjusted ACPR between 92 and 100%. Although, the cure rate of AL (92.4%) in Ebebiyin was above the threshold (< 90%) requiring for changing treatment policy [3], this data calls for close monitoring of the efficacy of this artemisinin-based combination. Overall, the findings are in line with reports from recent studies confirming that ASAQ and AL, the most commonly recommended ACTs for the treatment of uncomplicated falciparum infection, remain effective in Africa and support their continued use [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
It is worth noting that malaria transmission varies in the study sites, with a low level in Malabo (Bioko Island) and a moderate to high level in the mainland where Bata and Ebebiyin sites are located [15]. A survey conducted in Bata district reported higher malaria prevalence (58.9%) in the rural setting compared to 33.9% in the urban area, where the study patients were recruited, [45]. Health facility based rapid assessment of malaria indicators in 2016 revealed a test positivity rate of 70.9% (2071/2872) and 34.9% (1635/4687) among consultations with suspected malaria at Angokong Health Centre and Provincial Hospital, respectively, in Ebiyein district (Riloha Rivas, pers. commun.). The ongoing high malaria transmission observed in Ebibeyin compared to the other sites may explain the higher rate of new infections detected using the standard WHO PCR correction method [22] and the higher treatment failure rate using the 2/3 algorithm [23], especially for AL, for which the partner medicines has a shorter half-life. In the absence of a gold standard tool to distinguish between reinfection and recrudescence, it is difficult to interpret this increase in treatment failure rate using the 2/3 algorithm. Nevertheless, it is surprising that two medicines with opposite mechanism of resistance could fail at the same site and at the same time [46].
All study patients cleared their parasitaemia by day 3, indicating the absence of delayed parasite clearance, and together with lack of the known Pfkelch13 mutations associated with artemisinin resistance in South East Asia, may indicate the absence of partial artemisinin resistance in Equatorial Guinea. Ih addition, the data based on 476 clinical samples of P. falciparum, support that the indigenous M579I mutant, claimed to be associated with delayed parasite clearance and increased in vitro parasite survival rate [19], did not expand and spread across the country.
Conclusion
The study confirmed that ASAQ and AL remain highly effective in treating uncomplicated falciparum infections more than a decade after their use in Equatorial Guinea and that there are no known Pfkelch13 mutant parasites associated with artemisinin resistance. Continued monitoring of the efficacy of these artemisinin-based combinations, at least every 2 years, is imperative to inform national malaria treatment policy. In addition, recent evidence of the de novo emergence of the Pfkelch13 mutation (R561H) associated with artemisinin resistance in Africa calls for monitoring molecular markers associated with artemisinin and partner drug resistance to detect resistant parasites early. In this study, the 2/3 algorithm increased the failure rate at high transmission site compared to the standard WHO methodology. Further comparison and validation in different transmission setting are needed before this new suggested algorithm can be systematically implemented for PCR correction.
Availability of data and materials
The dataset used in this study is available and can be shared upon reasonable request to NMCP through the corresponding author.
Abbreviations
- ACPR:
-
Adequate clinical and parasitological response
- ACTs:
-
Artemisinin-based combination therapies
- AL:
-
Artemether–lumefantrine
- ASAQ:
-
Artesunate-amodiaquine
- ASMQ:
-
Artesunate-mefloquine
- ASPY:
-
Artesunate-pyronaridine
- ASSP:
-
Artesunate-sulfadoxine/pyrimethamine
- DHAPPQ:
-
Dihydroartemisinin-piperaquine
- ETF:
-
Early treatment failure
- LCF:
-
Late clinical failure
- LPF:
-
Late parasitological failure
- msp1:
-
Merozoite surface proteins 1
- msp2:
-
Merozoite surface proteins 2
- glurp:
-
Glutamate-rich protein
- NMCP:
-
National Malaria Control Programme
- PCR:
-
Polymerase chain reaction
- WHO:
-
World Health Organization
References
WHO. World Malaria Report 2020. Geneva: World Health Organization; 2020. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020. Accessed 17 Feb 2021.
WHO. Antimalarial drug combination therapy: report of a WHO Technical Consultation. Geneva: World Health Organization; 2001.
WHO. Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance (2010–2019). Geneva: World Health Organization; 2020. https://www.who.int/publications/i/item/9789240012813.
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5.
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23.
Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–64.
Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211:670–9.
Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15:415–21.
Imwong M, Suwannasin K, Kunasol C, Sutawong K, Mayxay M, Rekol H, et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis. 2017;17:491–7.
Thanh NV, Thuy-Nhien N, Tuyen NT, Tong NT, Nha-Ca NT, Dong LT, et al. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin-piperaquine in the south of Vietnam. Malar J. 2017;16:27.
Phuc BQ, Rasmussen C, Duong TT, Dong LT, Loi MA, Ménard D, et al. Treatment failure of dihydroartemisinin/piperaquine for Plasmodium falciparum malaria. Vietnam Emerg Infect Dis. 2017;23:715–7.
Phyo AP, Ashley EA, Anderson TJC, Bozdech Z, Carrara VI, Sriprawat K, et al. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai-Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis. 2016;63:784–91.
van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019;19:952–61.
Uwimana A, Legrand E, Stokes BH, Ndikumana JM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8.
Ministry of Health and Social Welfare, Equatorial Guinea. National Strategic Plan for Malaria Control Equatorial Guinea 2016–2020. National Malaria Control Programme, Ministry of Health and Social Welfare, Equatorial Guinea; 2016.
Ministry of Health and Social Welfare, Equatorial Guinea. National Theraputical Guide of Malaria. National Malaria Control Programme, Ministry of Health and Social Welfare, Equatorial Guinea; 2019.
Ministry of Health and Social Welfare, Equatorial Guinea. Evaluation of the efficacy of artesunate+amodiaquine in uncomplicated Plasmodium falciparum malaria (2010). National Programme of Malaria (Equatorial Guinea)/National Centre of Tropical Medicine (ISCIII) (Spain), 2011.
Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–9.
Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidlein L, Zhou H, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017;376:991–3.
WHO. Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organisation; 2009. https://www.who.int/malaria/publications/atoz/9789241597531/en/
Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp. Am J Trop Med Hyg. 2003;68:133–9.
Medicines for Malaria Venture & WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations: informal consultation organized by the Medicines for Malaria Venture and cosponsored by the World Health Organization, 29–31 May 2007, Amsterdam, The Netherlands. Geneva: World Health Organization; 2008. https://apps.who.int/iris/handle/10665/43824.
Jones S, Kay K, Hodel EM, Chy S, Mbituyumuremyi A, Uwimana A, et al. Improving methods for analyzing antimalarial drug efficacy trials: molecular correction based on length-polymorphic markers msp-1, msp-2, and glurp. Antimicrob Agents Chemother. 2019;63:e00590-e619.
Charle P, Berzosa P, de Lucio A, Raso J, Nseng Nchama G, Benito A. Artesunate/amodiaquine malaria treatment for Equatorial Guinea (Central Africa). Am J Trop Med Hyg. 2013;88:1087–92.
Davlantes E, Dimbu PR, Ferreira CM, Florinda Joao M, Pode D, Félix J, et al. Efficacy and safety of artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in three provinces in Angola, 2017. Malar J. 2018;17:144.
Djallé D, Njuimo SP, Manirakiza A, Laganier R, Le Faou A, Rogier C. Efficacy and safety of artemether + lumefantrine, artesunate + sulphamethoxypyrazine-pyrimethamine and artesunate + amodiaquine and sulphadoxine-pyrimethamine + amodiaquine in the treatment of uncomplicated falciparum malaria in Bangui, Central African Republic: a randomized trial. Malar J. 2014;13:9.
Ndounga M, Pembe Issamou Mayengue, Casimiro PN, Koukouikila-Koussounda F, Bitemo M, Diassivy Matondo B, et al. Artesunate-amodiaquine versus artemether-lumefantrine for the treatment of acute uncomplicated malaria in Congolese children under 10 years old living in a suburban area: a randomized study. Malar J. 2015;14:423.
de Wit M, Funk AL, Moussally K, Nkuba DA, Siddiqui R, Bil K, et al. In vivo efficacy of artesunate-amodiaquine and artemether-lumefantrine for the treatment of uncomplicated falciparum malaria: an open-randomized, non-inferiority clinical trial in South Kivu. Democratic Republic of Congo Malar J. 2016;15:455.
Adegbite BR, Edoa JR, Honkpehedji YJ, Zinsou FJ, Dejon-Agobe JC, Mbong-Ngwese M, et al. Monitoring of efficacy, tolerability and safety of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Lambaréné, Gabon: an open-label clinical trial. Malar J. 2019;18:424.
Souleymane I, Clément KH, Denis MM, Berenger A, Baba C, Offianan TA, et al. Therapeutic efficacy of artesunate-amodiaquine and polymorphism of Plasmodium falciparum k13-propeller gene in Pala (Tchad). Int J Open Access Trials. 2017;1:1–6.
Abuaku B, Duah-Quashie NO, Quaye L, Matrevi SA, Quashie N, Gyasi A, et al. Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine combinations for uncomplicated malaria in 10 sentinel sites across Ghana: 2015–2017. Malar J. 2019;18:206.
Konaté A, Barro-Kiki PCM, Angora KE, Bédia-Tanoh AV, Djohan V, Kassi KF, et al. Efficacy and tolerability of artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria at two sentinel sites across Côte d’Ivoire. Ann Parasitol. 2018;64:49–57.
Ebenebe JC, Ntadom G, Ambe J, Wammanda R, Jiya N, Finomo F, et al. Efficacy of artemisinin-based combination treatments of uncomplicated falciparum malaria in under-five-year-old Nigerian children ten years following adoption as first-line antimalarials. Am J Trop Med Hyg. 2018;99:649–64.
Lingani M, Bonkian LN, Yerbanga I, Kazienga A, Valéa I, Sorgho H, et al. In vivo/ex vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine as first-line treatment for uncomplicated falciparum malaria in children: an open label randomized controlled trial in Burkina Faso. Malar J. 2020;19:8.
Grandesso F, Guindo O, Woi Messe L, Makarimi R, Traore A, Dama S, et al. Efficacy of artesunate-amodiaquine, dihydroartemisinin-piperaquine and artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Maradi. Niger Malar J. 2018;17:52.
Diallo MA, Yade MS, Ndiaye YD, Diallo I, Diongue K, Sy SA, et al. Efficacy and safety of artemisinin-based combination therapy and the implications of Pfkelch13 and Pfcoronin molecular markers in treatment failure in Senegal. Sci Rep. 2020;10:8907.
Dama S, Niangaly H, Djimde M, Sagara I, Guindo CO, Zeguime A, et al. A randomized trial of dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Malar J. 2018;17:347.
Yeka A, Kigozi R, Conrad MD, Lugemwa M, Okui P, Katureebe C, et al. Artesunate/amodiaquine versus artemether/lumefantrine for the treatment of uncomplicated malaria in Uganda: a randomized trial. J Infect Dis. 2016;213:1134–42.
Roth JM, Sawa P, Makio N, Omweri G, Osoti V, Okach S, et al. Pyronaridine-artesunate and artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a randomized controlled non-inferiority trial. Malar J. 2018;17:199.
Yeka A, Wallender E, Mulebeke R, Kibuuka A, Kigozi R, Bosco A, et al. Comparative efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria in Ugandan children. J Infect Dis. 2019;219:1112–20.
Uwimana A, Penkunas MJ, Nisingizwe MP, Warsame M, Umulisa N, Uyizeye D, et al. Efficacy of artemether-lumefantrine versus dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria among children in Rwanda: an open-label, randomized controlled trial. Trans R Soc Trop Med Hyg. 2019;113:312–9.
Ishengoma DS, Mandara CI, Francis F, Talundzic E, Lucchi NW, Ngasala B, et al. Efficacy and safety of artemether-lumefantrine for the treatment of uncomplicated malaria and prevalence of Pfk13 and Pfmdr1 polymorphisms after a decade of using artemisinin-based combination therapy in mainland Tanzania. Malar J. 2019;18:88.
Teklemariam M, Assefa A, Kassa M, Mohammed H, Mamo H. Therapeutic efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria in a high-transmission area in northwest Ethiopia. PLoS One. 2017;12:e0176004.
Warsame M, Hassan AM, Hassan AH, Jibril AM, Khim N, Arale AM, et al. High therapeutic efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Somalia. Malar J. 2019;18:231.
Ncogo P, Herrador Z, Romay-Barja M, García-Carrasco E, Nseng G, Berzosa P, et al. Malaria prevalence in Bata district, Equatorial Guinea: a cross-sectional study. Malar J. 2015;14:456.
Venkatesan M, Gadalla NB, Stepniewska K, Dahal P, Nsanzabana C, Moriera C, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am J Trop Med Hyg. 2014;91:833–843.
Acknowledgements
We thank all parents or guardians of the study children who participated in the study. We also thank the health workers at the study health facilities (Regional Hospital in Malabo, Provincial Hospital and Angokong Health Centre in Ebebiyin, Regional Hospital, Maria Rafols Health Centre and Maria Gay Health Centre in Bata). We also thank the Bill and Melinda Gates Foundation for their financial support.
Disclaimer
Angela K. Lao Seoane, Spes Caritas and Pascal Ringwald are employees of the World Health Organization and they alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policies or views of the World Health Organization.
Funding
Open access funding provided by University of Gothenburg. Funding was obtained from the Bill and Melinda Gates Foundation through WHO (grant no. OPP1140599). This work was also supported by the French Government (Agence Nationale de la Recherche) Investissement d’Avenir programme, Laboratoire d’Exceellence (LabEx) “Frech Parasitology Alliance For Health Care” (ANR-11-LABX-0024-PARAFRAP).
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by MRR, PB, LG, PR and MW. MRR, PB, RMA, SNE, PRN, WPP, AKLS and LG implemented the study, supervised data collection and ensured quality control of the study. MW, PR and SCN provided technical support and ensured data validation and analysis. DM and EL performed molecular analysis to distinguish recrudescence from reinfection and mutations in Pfkelch13 propeller domain gene. MW lead the writing of the manuscript with contributions from the other co-authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Ministry of Health and Social Welfare of Equatorial Guineas (No. 731–150), the Ethics Committee of the Spanish National Health Institute, Carlos III (CEI PI 57_2016-v3), and the WHO Research Ethics Review Committee (ERC.000286). The parents or guardians of the study children provided written informed consent before enrolling their children in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Riloha Rivas, M., Warsame, M., Mbá Andeme, R. et al. Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine and polymorphism in Plasmodium falciparum kelch13-propeller gene in Equatorial Guinea. Malar J 20, 275 (2021). https://doi.org/10.1186/s12936-021-03807-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03807-x