Abstract
Breast cancer is the most common cancer in women, and its high mortality has become one of the biggest health problems globally. Several studies have reported an association between breast cancer and ATM gene variants. This study aimed to demonstrate and analyze the relationship between ATM gene polymorphisms and breast cancer prevalence rate. A systematic literature review was undertaken using the following databases: Medline (PubMed), Web of sciences, Scopus, EMBASE, Cochrane, Ovid, and CINHAL to retrieve all cross-sectional studies between January 1990 and January 2020, which had reported the frequency of ATM variants in patients with breast cancer. A random-effects model was applied to calculate the pooled prevalence with a 95% confidence interval. The pooled prevalence of ATM variants in patients with breast cancer was 7% (95% CI: 5−8%). Also, the pooled estimate based on type of variants was 6% (95% CI: 4−8%; I square: 94%; P: 0.00) for total variants¸ 0% (95% CI: 0−1%; I square: 0%; P: 0.59) for deletion variants, 12% (95% CI: 7−18%; I square: 99%; P: 0.00) for substitution variants, and 2% (95% CI: 4−9%; I square: 67%; P: 0.08) for insertion variants. This meta-analysis showed that there is a significant relationship between ATM variants in breast cancer patients. Further studies are required to determine which of the variants of the ATM gene are associated with BRCA mutations.
Similar content being viewed by others
Introduction
Breast cancer is a global health problem and has the highest worldwide incidence among women. Approximately 1.7 million patients have diagnosed with breast cancer annually [1]. Furthermore, breast cancer is a significant cause of cancer-related death in women [2, 3]. The high rate of cancer progression and metastasis are two unsolved problems associated with this high mortality rate [4]. Breast cancer is a complex disorder; its etiology has not been entirely explained. Like other cancers, genetic factors have an essential role in the familial and sporadic forms of breast cancer [4]. Twin studies indicate that the heritability of breast cancer is between 27 and 31% [5, 6]. Three genetic factors are involved in breast cancer progression: high penetrance genetic mutations such as those in the BRCA1 and BRCA2 genes [7,8,9], intermediate penetrance variants such as those in ATM, BARD1, PALB2, and CHECK2 genes [10], and low-penetrance variants such as SNPs. The first and second mutation groups explain approximately 22% of breast cancer risk. Still, it seems that a complex interaction between low penetrance susceptibility genes and environmental factors is vital for the development and progression of breast cancer [10,11,12,13].
DNA repair systems protect the genome against mutagens and have an essential role in cell cycle regulation. Different gene mutations in these systems can increase the risk of cancer development [11, 14]. Ataxia telangiectasia mutated (ATM) is a protein kinase enzyme with a crucial role in the DNA repair system, especially in DNA double-strand repair. This gene is located on chromosome 11q 22–23 and includes 66 exons [15]. ATM acts as an intracellular sensor that becomes active in response to DNA double-strand break and then phosphorylates many downstream tumor suppressor genes such as BRCA1, p53, chk2, and chk1 [16]. Thus, it is a strong candidate for mutation in cancer multistage development [17]. ATM biallelic mutation causes an autosomal recessive disease known as Ataxia-Telangiectasia [18]. Ataxia Telangiectasia was described in 1926 and is characterized by telangiectasia, cerebellar Ataxia, neurological abnormalities, immunological deficiency, hypersensitivity to ionizing radiation (IR), and predisposition to cancer [19]. Truncated mutations in the ATM gene inhibit its expression and cause Ataxia Telangiectasia; however, missense mutations change its function and are common in cancers [20, 21]. It was reported that ATM gene expression decreased in breast cancer tissues and cells compared to controls [22]. Various studies show that classic A-T heterozygote women have a 4-fold increased risk for breast cancer [23–25]. Some studies have reported an association of ATM gene mutations with breast cancer, and some have evaluated the prevalence of this gene variant in breast cancer. Still,the exact prevalence of ATM mutations in breast cancer is unclear, and there is no systematic review on the prevalence of ATM variants in breast cancer. We aimed to undertake a systematic review and meta-analysis of the prevalence of ATM variants in breast cancer from different countries, the association of these variants with the BRCA status of patients, and the prevalence of other variants in the ATM gene.
Materials and methods
All approaches used in this study were in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA), and the protocol had been registered in the International Prospective Register of Systematic Reviews (PROSPERO), under the registration number of CRD42018114400.
Search terms and complex search syntax
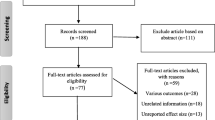
Medline (PubMed), Web of sciences, Scopus, EMBASE, Cochrane, Ovid, and CINHAL databases were searched to evaluate the prevalence of ataxia telangiectasia mutated (ATM) variants in breast cancer. In current study “breast carcinoma”, “breast tumor”, “breast neoplasm”, “breast neoplasms”, “breast cancer”, " breast cancers”, “breast tumors”, “mammary cancer”, “mammary cancers”, “breast carcinomas”, “mammary carcinoma”, " mammary carcinomas”, “ataxia telangiectasia mutated”, “ataxia telangiectasia mutated proteins”, “Ataxia Telangiectasia Mutated Proteins”, “ATM”, " mutation”, “mutations”, “variant” and “variants” keywords were searched in all mentioned databases. All reference lists of primary articles were manually reevaluated by two individuals (MM, ES) separately to avoid missing any papers. First, the authors reviewed the titles and abstracts to select the appropriate articles. The results of the primary search were reviewed, and some articles were eliminated in this step. After reviewing the entire text of the selected articles, the inclusion and exclusion criteria were set by two researchers separately (MM and ES) (Fig. 1).
Eligibility criteria
Articles were included in the current study using the following criteria: (1) evaluation of the epidemiological aspects of ATM variants in patients with breast cancer; (2) only full-text articles; (3) Cross-sectional studies. Case reports, reviews, animal studies, and cohort studies were excluded. The authors resolved all disputes during the data collection, compilation, and data analysis. The exclusion criteria for this study were unrelated studies, duplicate data, and the studies that have not answered the outcome questions, have not assessed the available data, and have not had a cross-sectional design.
Data extraction
The first ‘author’s name, publication date, sample size, country, type of variant, prevalence, BRCA status were extracted from all studies and statically analyzed. A data extraction form was created based on our group discussion and piloted according to 10 different studies types, then was modified and used by the data extractor. All processes from systematic search to final data extraction were undertaken independently by two researchers (Kappa statistic for agreement for quality assessment: 0.75). Both authors assessed any controversy,and, in case of dispute, the data was evaluated by the third author (YM).
Risk of bias
Two of the authors (MM and ES) performed a qualitative evaluation of the studies based on the Newcastle-Ottawa Quality Assessment Scale (NOS) was performed by two of the authors (MM and ES). This scale is designed to evaluate the qualitative evaluation of observational studies. According to NOS, each study was examined by six items in three groups; selection, comparability, and exposure. Stars were given to each item, and the maximum score is 9. The external discussion method was used in case of differences in the score given to the published reports. Finally, the papers were categorized as low, moderate, and high risk. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was also completed for all articles.
Statistical analysis
The DerSimonian-Lirad random-effect model was used to pool the prevalence of ATM in patients with breast cancer (and 95% confidence interval estimation) using the Metaprop command in Stata 14. Cochran Q and I2 tests were used to investigate the heterogeneity and variance between studies. The Q statistic tells us whether there is statistically significant heterogeneity among the studies or not. I2 determines the amount of heterogeneity quantitatively. The range of I2 is from 0 to 100. In this study, we categorized it into three levels; low (25%), moderate (50%), and high (75%). The funnel diagram, ' ‘Egger’s teste, and graphs were used to evaluate publication bias. In the Egger regression model, the ratio of the effect size on the standard error, which is the standard index (z-score), is taken as the dependent variable and predicts its value over the standard error inverse (1/SE). Subgroup analyses were performed to examine any confounding factors that may influence the prevalence of the disease. Subgroup analyses were performed for BRCA status, countries, and variants of ATM. We used P-value to decide on the statistical hypothesis test results. All two-way statistical tests were considered as α = 0.05.
Results
Study characteristics
The searches retrieved a total of 1101 original-research articles, the titles of which were examined by two independent reviewers. Forty-eight articles were excluded because they were not related to the topic of study. Eight hundred and sixty-three studies were excluded because of the unavailability of the full text. After all, they did not evaluate the data available for the sample selection because they contained duplicate data from an included research or did not have a cross-sectional design (Fig. 1). Finally, the remaining 24 articles were retained for analysis (Table 1).
Quantities results
The overall pooled prevalence of ATM in patients with breast cancer was 7% (95% CI: 6−9%; I square: 93%; P: 0.00) (Fig. 2).
The prevalence of different ATM variants in studies
We classify ATM variants into four groups: All variants, deletion, insertion and substitution variants. The effect range of this study was between 0.05 and 0.08. The prevalence of substitution variants was higher than other variants. Deletion and insertion were seen rarely in Breast cancer according to this study. The pooled estimate based on type of variants was 0% (95% CI: 0−1%; I square: 0%; P: 0.59), 2% (95% CI: 4−9%; I square: 67%; P: 0.08), 12% (95% CI: 7−18%; I square: 99%; P: 0.00) and 6% (95% CI: 4−8%; I square: 94%; P: 0.00) for deletion, insertion, substitution variants and total, respectively (Table 2).
The prevalence of ATM in patients with breast cancer in the world by BRCA status
The studies were divided into positive, negative, and not determined groups based on BRCA mutations. Positive groups had BRACA1/2 mutations, and negative groups do not. Some studies did not determine the status of BRCA mutations, and we classify these data as an undetermined group. The pooled prevalence of ATM in patients with breast cancer in several BRCA status was 7% (95% CI: 5−8%; I square: 97%; P: 0.00). The pooled estimate based on type of variants was 3% (95% CI: 2−4%; I square: 85%; P: 0.00), 11% (95% CI: 7−15%; I square: 99%; P: 0.00) and 12% (95% CI: 6−18%; I square: 98%; P: 0.00) for negative, positive, and not determined, respectively (Table 2). It appears that the prevalence of ATM variants in the BRCA positive group was more than negative.
The prevalence of ATM in patients with breast cancer in the world by countries
The pooled prevalence of ATM in patients with breast cancer in several continent was 7% (95% CI: 5−8%; I square: 98%; P: 0.00), 5% (95% CI: 1−11%; I square: 80%; P: 0.01) and 9% (95% CI: 4−14%; I square: 96%; P: 0.00) for European, Asian and American population, respectively (Table 2).
Publication bias
The results of ‘Egger’s test showed no publication bias in the pooled prevalence of ATM in patients with breast cancer (coefficient = 0.098, P = 0.98). Also, the funnel plot is reported in Fig. 3.
Meta-regression
Meta-regression results on the heterogeneity of studies showed that the sample size and mean age of study participants had no significant effect on the prevalence of ATM in patients with breast cancer.
Discussion
ATM is an essential protein that protects the genome from the effects of genotoxic agents, such as ionizing radiation. This protein can detect DNA double-strand breaks and directly or indirectly activate many other proteins that are important in the DNA repair system [47]. According to these studies, there is an association between ATM variants and breast cancer risk. In the current study, the prevalence of ATM variants in breast cancer patients was evaluated. Our results showed significant differences among countries. ATM variants were highest in breast cancer patients from the USA,t but these variants were not involved in the prevalence of patients from northern Europe (Finland, Denmark, and Sweden.)
In 1996, the association of Ataxia–telangiectasia gene (ATM) mutation heterozygosity with breast cancer risk was reported from New York. This study reported that 6.6% of breast cancer patients in the USA had this ATM variant [46]. So, it can be concluded that there is a high prevalence of ATM variants in the United States. This issue is consistent with our results. Different studies on the ATM variants and the prevalence of breast cancer have been undertaken in northern Europe. Most studies reported that there was no association between common variants in the ATM gene and breast cancer susceptibility in patients from Sweden, Finland, and Denmark [47,29,, 48]. Therefore, these studies are consistent with our findings.
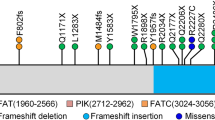
There are several variants of the ATM gene reported in different populations [49]. In our study, ATM variants were classified into three groups: deletion variants, insertion variants, and substitution variants. Deletions and insertions were reported in few cases. The incidence of substitution variants was more common than other variants. Most of the studies indicated the prevalence of the A-T substitution variant in the ATM gene [50, 51]. Another study reported that regardless of the common A-T mutation, substitution and especially missense mutations are the most common ATM variants in breast cancer patients.
Furthermore, a frameshift deletion in exon 28 of the ATM gene was reported in this article in one breast cancer patient (among 192 patients) that produce a truncated protein [52]. Another study reported one deletion 1563delAG and one insertion 2572insT in ATM gene among 190 breast cancer patients [53]. It seems that deletion and insertion in ATM genes are infrequent in breast cancer patients. There is little literature about the association of these variants,with breast cancer patients. There is little literature about the association of these variants withbreast cancer disease. We classified our data into BRCA positive and BRCA negative groups for identifying the association of ATM variants with BRCA1/2 mutation in breast cancer patients. The results showed that ATM variants in the BRCA positive group are more common. In some studies, the ATM mutation (causing Ataxia Telangiectasia) was reported as a risk factor for breast cancer patients who did not have a BRCA1/2 mutation. These studies suggested that ATM is as crucialas BRCA2 in breast cancer [54, 55]. Another study indicathat ATM mutations and BRCA1 mutations are associated with breast cancer patients [56].
Our results may support this latter study and suggest that there is a acumulative effect of ATM and BRCA1 mutations that increase the risk of breast cancer incidence. Overall it seems that the eprevalence of ATM variants ismore common in the USA than in other countries. Among different variants, isubstitutions are the most common, and there is an association between ATM variants and BRCA mutations in breast cancer incidence.
Availability of data and materials
Not applicable.
Abbreviations
- ATM:
-
Ataxia telangiectasia mutated
- IR:
-
Radiation
- PRISMA:
-
Reviews and meta-analyses statement
- PROSPERO:
-
Prospective register of systematic reviews
- ATM:
-
Ataxia telangiectasia mutated
References
da Costa Vieira RA, Biller G, Uemura G, Ruiz CA, Curado MP. Breast cancer screening in developing countries. Clinics. 2017;72(4):244–53.
Porter P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358(3):213–6.
Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. Cosponsored Am Soc Prevent Oncol. 2017;26(4):444–57.
Scimeca M, Urbano N, Bonfiglio R, Duggento A, Toschi N, Schillaci O, Bonanno E. Novel insights into breast cancer progression and metastasis: a multidisciplinary opportunity to transition from biology to clinical oncology. Biochim Biophys Acta Rev Cancer. 2019;1872(1):138–48.
Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, Graff RE, Holst K, Moller S, Unger RH, et al. Familial risk and heritability of cancer among twins in Nordic countries. Jama. 2016;315(1):68–76.
Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85.
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, Evans DG, Izatt L, Eeles RA, Adlard J, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–22.
Newman B, Mu H, Butler LM, Millikan RC, Moorman PG, King MC. Frequency of breast cancer attributable to BRCA1 in a population-based series of American women. Jama. 1998;279(12):915–21.
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–92.
Zavala VA, Serrano-Gomez SJ, Dutil J, Fejerman L. Genetic epidemiology of breast cancer in Latin America. Genes 2019;10(2).
Rudolph A, Chang-Claude J, Schmidt MK. Gene-environment interaction and risk of breast cancer. Br J Cancer. 2016;114(2):125–33.
Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447(7148):1087–1093.
Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, Masson G, Jakobsdottir M, Thorlacius S, Helgason A, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007;39(7):865–9.
Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer. 2016;16(1):35–42.
Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211.
Prokopcova J, Kleibl Z, Banwell CM, Pohlreich P. The role of ATM in breast cancer development. Breast Cancer Res Treatment. 2007;104(2):121–8.
Bartkova J, Bakkenist CJ, Rajpert-De Meyts E, Skakkebaek NE, Sehested M, Lukas J, Kastan MB, Bartek J. ATM activation in normal human tissues and testicular cancer. Cell cycle (Georgetown Tex). 2005;4(6):838–45.
Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, North B, Jayatilake H, Barfoot R, Spanova K et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nature Genet. 2006;38(8):873–875.
Yuille MA, Coignet LJ. The ataxia telangiectasia gene in familial and sporadic cancer. Recent Results in Cancer Research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 1998;154:156–73.
Teraoka SN, Malone KE, Doody DR, Suter NM, Ostrander EA, Daling JR, Concannon P. Increased frequency of ATM mutations in breast carcinoma patients with early onset disease and positive family history. Cancer. 2001;92(3):479–87.
Bretsky P, Haiman CA, Gilad S, Yahalom J, Grossman A, Paglin S, Van Den Berg D, Kolonel LN, Skaliter R, Henderson BE. The relationship between twenty missense ATM variants and breast cancer risk: the Multiethnic Cohort. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. Cosponsored Am Soc Prev Oncol. 2003;12(8):733–8.
Angele S, Taniere P, Hall J. [What do we know about ATM protein expression in breast tissue?]. Bulletin du cancer. 2001;88(7):671–5.
Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987;316(21):1289–94.
Olsen JH, Hahnemann JM, Borresen-Dale AL, Brondum-Nielsen K, Hammarstrom L, Kleinerman R, Kaariainen H, Lonnqvist T, Sankila R, Seersholm N, et al. Cancer in patients with ataxia-telangiectasia and in their relatives in the nordic countries. J Natl Cancer Inst. 2001;93(2):121–7.
Angèle S, Treilleux I, Tanière P, Martel-Planche G, Vuillaume M, Bailly C, Brémond A, Montesano R, Hall J. Abnormal expression of the ATM and TP53 genes in sporadic breast carcinomas. Clin Cancer Res. 2000;6(9):3536–44.
de Souza Timoteo AR, Gonçalves AÉMM, Sales LAP, Albuquerque BM, de Souza JES, de Moura PCP, et al. A portrait of germline mutation in Brazilian at-risk for hereditary breast cancer. Breast Cancer Res. 2018;172(3):637-46.
Prodosmo A, Buffone A, Mattioni M, Barnabei A, Persichetti A, De Leo A, et al. Detection of ATM germline variants by the p53 mitotic centrosomal localization test in BRCA1/2-negative patients with early-onset breast cancer. J Exp Clin Cancer Res. 2016;35(1):135.
Broeks A, Urbanus JHM, Floore AN, Dahler EC, Klijn JGM, Rutgers EJT, Devilee P, Russell NS, van Leeuwen FE, van’t Veer LJ. ATM-Heterozygous Germline Mutations Contribute to Breast Cancer–Susceptibility. Am J Hum Genet. 2000;66(2):494–500.
Broeks A, Braaf LM, Huseinovic A, Schmidt MK, Russell NS, van Leeuwen FE, et al. The spectrum of ATM missense variants and their contribution to contralateral breast cancer. Breast Cancer Res Treat. 2008;107(2):243-8.
Szabo CI, Schutte M, Broeks A, Houwing-Duistermaat JJ, Thorstenson YR, Durocher F, et al. Are ATM mutations 7271T→ G and IVS10-6T→ G really high-risk breast cancer-susceptibility alleles? Cancer Res. 2004;64(3):840-3.
Atencio DP, Iannuzzi CM, Green S, Stock RG, Bernstein JL, Rosenstein BS. Screening breast cancer patients for ATM mutations and polymorphisms by using denaturing high‐performance liquid chromatography. Environ Mol Mutagen. 2001;38(2‐3):200-8.
Aloraifi F, McDevitt T, Martiniano R, McGreevy J, McLaughlin R, Egan CM, et al. Detection of novel germline mutations for breast cancer in non‐BRCA1/2 families. FEBS J. 2015;282(17):3424-37.
Fostira F, Saloustros E, Apostolou P, Vagena A, Kalfakakou D, Mauri D, et al. Germline deleterious mutations in genes other than BRCA2 are infrequent in male breast cancer. Breast Cancer Res Treat. 2018;169(1):105-13.
Birrell GW, Kneebone K, Nefedov M, Nefedova E, Jartsev M, Mitsui M, et al. ATM mutations, haplotype analysis, and immunological status of Russian patients with ataxia telangiectasia. Hum Mutat. 2005;25(6):593.
Lindeman GJ, Hiew M, Visvader JE, Leary J, Field M, Gaff CL, et al. Frequency of the ATM IVS10-6T→ G variant in Australian multiple-case breast cancer families. Breast Cancer Res. 2004;6(4):R401.
Brunet J, Gutiérrez‐Enríquez S, Torres A, Bérez V, Sanjosé S, Galceran J, et al. ATM germline mutations in Spanish early‐onset breast cancer patients negative for BRCA1/BRCA2 mutations. Clin Genet. 2008;73(5):465–73.
Soukupova J, Dundr P, Kleibl Z, Pohlreich P. Contribution of mutations in ATM to breast cancer development in the Czech population. Oncol Rep. 2008;19(6):1505–10.
La Paglia L, Laugé A, Weber J, Champ J, Cavaciuti E, Russo A, et al. ATM germline mutations in women with familial breast cancer and a relative with haematological malignancy. Breast Cancer Res Treat. 2010;119(2):443–52.
Minion LE, Dolinsky JS, Chase DM, Dunlop CL, Chao EC, Monk BJ. Hereditary predisposition to ovarian cancer, looking beyond BRCA1/BRCA2. Gynecol Oncol. 2015;137(1):86–92.
Pinto P, Paulo P, Santos C, Rocha P, Pinto C, Veiga I, et al. Implementation of next-generation sequencing for molecular diagnosis of hereditary breast and ovarian cancer highlights its genetic heterogeneity. Breast Cancer Res Treat. 2016;159(2):245–56.
Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL, et al. A study of over 35,000 women with breast cancer tested with a 25‐gene panel of hereditary cancer genes. Cancer. 2017;123(10):1721–30.
Ohnami S, Ohshima K, Nagashima T, Urakami K, Shimoda Y, Saito J, et al. Comprehensive characterization of genes associated with the TP53 signal transduction pathway in various tumors. Mol Cell Biochem. 2017;431(1):75–85.
Bozhanov SS, Angelova SG, Krasteva ME, Markov TL, Christova SL, Gavrilov IG, et al. Alterations in p53, BRCA1, ATM, PIK3CA, and HER2 genes and their effect in modifying clinicopathological characteristics and overall survival of Bulgarian patients with breast cancer. J Cancer Res Clin Oncol. 2010;136(11):1657–69.
Dörk T, Bendix R, Bremer M, Rades D, Klöpper K, Nicke M, Skawran B, Hector A, Yamini P, Steinmann D et al. Spectrum of ATM gene mutations in a hospital-based series of unselected breast cancer patients. Cancer Res. 2001;61(20):7608.
Buchholz TA, Weil MM, Ashorn CL, Strom EA, Sigurdson A, Bondy M, et al. A Ser49Cys variant in the ataxia telangiectasia, mutated, gene that is more common in patients with breast carcinoma compared with population controls. Cancer Interdisc Int J Am Cancer Soc. 2004;100(7):1345–51.
Savitsky KB-SA, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995.
Prasanna Athma RR, Michael S. Molecular genotyping shows that ataxia-telangiectasia heterozygotes are predisposed to breast cancer. 1996.
Milne RL. Variants in the ATMgene and breast cancer susceptibility. Genome Med. 2009;1(1):12.
Tommiska J, Jansen L, Kilpivaara O, Edvardsen H, Kristensen V, Tamminen A, Aittomäki K, Blomqvist C, Børresen-Dale A-L, Nevanlinna H. ATM variants and cancer risk in breast cancer patients from Southern Finland. BMC Cancer. 2006;6:209.
Bernstein JL, Bernstein L, Thompson WD, Lynch CF, Malone KE, Teitelbaum SL, Olsen JH, Anton-Culver H, Boice JD, Rosenstein BS, et al. ATM variants 7271T > G and IVS10-6T > G among women with unilateral and bilateral breast cancer. Br J Cancer. 2003;89(8):1513–6.
Fukunaga H, Taki Y, Prise KM. Diversity of ATM gene variants: a population-based genome data analysis for precision medicine. Hum Genomics. 2019;13(1):38.
Goldgar DE, Healey S, Dowty JG, Da Silva L, Chen X, Spurdle AB, Terry MB, Daly MJ, Buys SM, Southey MC, et al. Rare variants in the ATMgene and risk of breast cancer. Breast Cancer Res. 2011;13(4):R73.
Annegien Broeks LMB, Angelina Huseinovic MK, Schmidt, Nicola S, Russell FE, van Leeuwen F, Hogervorst LJ van. ‘t Veer: The spectrum of ATM missense variants and their contribution to contralateral breast cancer. Breast Cancer Res Treatment. 2007;107.
Thorstenson YR, Roxas A, Kroiss R, Jenkins MA, Yu KM, Bachrich T, Muhr D, Wayne TL, Chu G, Davis RW et al. Contributions of ATM mutations to familial breast and ovarian cancer. Cancer Res 2003;63(12):3325.
Moran O, Nikitina D, Royer R, Poll A, Metcalfe K, Narod SA, Akbari MR, Kotsopoulos J. Revisiting breast cancer patients who previously tested negative for BRCA mutations using a 12-gene panel. Breast Cancer Res Treat. 2017;161(1):135–42.
Hamdi Y, Soucy P, Kuchenbaeker KB, Pastinen T, Droit A, Lemaçon A, Adlard J, Aittomäki K, Andrulis IL, Arason A, et al. Association of breast cancer risk in BRCA1 and BRCA2 mutation carriers with genetic variants showing differential allelic expression: identification of a modifier of breast cancer risk at locus 11q2.23. Breast Cancer Res Treatment. 2017;161(1):117–134.
Acknowledgements
We would like to express our gratitude to Gordon A. Ferns at Brighton and Sussex Medical School for reviewing and improving the text.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: YM, ES, and MM. Data curation: YM and MS. Investigation: YM, ES, and MM. Writing-original draft: YM, PK, and MV. Writing-review and editing: MaM, MM, and RN. Analysis and/or interpretation of data: YM. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
For this type of study, formal consent is not required.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moslemi, M., Vafaei, M., Khani, P. et al. The prevalence of ataxia telangiectasia mutated (ATM) variants in patients with breast cancer patients: a systematic review and meta-analysis. Cancer Cell Int 21, 474 (2021). https://doi.org/10.1186/s12935-021-02172-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02172-8