Abstract
Aim of the review
In the past decade, increasing research attention investigated the novel therapeutic potential of steroidal cardiac glycosides in cancer treatment. Huachansu and its main active constituent Bufalin have been studied in vitro, in vivo and clinical studies. This review aims to summarize the multi-target and multi-pathway pharmacological effects of Bufalin and Huachansu in the last decade, with the aim of providing a more comprehensive view and highlighting the recently discovered molecular mechanisms.
Results
Huachansu and its major derivative, Bufalin, had been found to possess anti-cancer effects in a variety of cancer cell lines both in vitro and in vivo. The underlying anti-cancer molecular mechanisms mainly involved anti-proliferation, apoptosis induction, anti-metastasis, anti-angiogenesis, epithelial–mesenchymal transition inhibition, anti-inflammation, Na+/K+-ATPase activity targeting, the steroid receptor coactivator family inhibitions, etc. Moreover, the potential side-effects and toxicities of the toad extract, Huachansu, and Bufalin, including hematological, gastrointestinal, mucocutaneous and cardiovascular adverse reactions, were reported in animal studies and clinic trails.
Conclusions
Further research is needed to elucidate the potential drug–drug interactions and multi-target interaction of Bufalin and Huachansu. Large-scale clinical trials are warranted to translate the knowledge of the anticancer actions of Bufalin and Huachansu into clinical applications as effective and safe treatment options for cancer patients in the future.
Similar content being viewed by others
Introduction
Traditional Chinese Medicine (TCM) is a medical practice with more than 2500 years of history in China. It has recently been recognized as a new type of chemotherapy adjuvant that can improve the efficacy of chemotherapy and ameliorate the side effects of cancer chemotherapies. Ever since the launch of chemotherapy for tumor treatment, natural products have become an important source of the development of novel cancer therapies. Although there is no pathological concept of cancer under TCM theory, it is easy to find the descriptions of cancer-like symptoms in the ancient medical documents described as lumps, bumps, and toxins, termed “Chuang, Yong, Zhong and Du (疮痈肿毒)” in Chinese [1]. Therefore, identifying drugs used to treat lumps and bumps, as well as other cancer-related symptoms, such as relief from fever, diarrhea, vomiting, and pain, may link the traditional use of the agent to the modern pathological concept of cancer and may strengthen the pharmacological relevance of TCM to contemporary anti-cancer treatments.
Chansu (CS, Senso in Japanese) is the dried secretion from the skin glands of Bufo bufo gargarizans Cantor or Bufo melanostictus Schneider [2]. According to the principles of TCM theory, CS is commonly used to counteract toxicity, alleviate pain, and induce resuscitation [3, 4]. It can be considered as an anti-infectious agent for pyogenic infection induced unconsciousness and may be related to its anti-inflammatory and anti-microbial effects [3, 5]. In TCM practice, CS is prescribed to patients with “heat and toxins” syndrome, which refers to the modern concepts including acute gastroenteritis, severe vomiting, diarrhea, abdominal pain, high fever, carbuncles, lumps, and bumps [6]. Huachansu (HCS) is an injectable form of the sterilized hot-water extract of CS [7]. It is manufactured by Anhui Jinchan Biochemistry Company Ltd., in Huaibei, China [Chinese Food and Drug Administration, FDA (ISO9002)] and is widely used for inflammatory diseases as well as for the treatment for various types of cancer, including liver, lung, pancreatic, and colorectal cancers in China [8,9,10,11,12].
The molecular basis for the anti-inflammatory effect of HCS is proposed to be the bioactive steroidal cardiac glycosides [13]. Indeed, glycosides isolated from HCS have been shown to possess blood pressure stimulation, respiratory excitation, anti-inflammatory, anesthetic, and anti-neoplastic activities [14]. HCS and its derived single compounds may achieve their anti-inflammatory effects by modulating nuclear factor-κB (NF-κB) signaling and down-regulating inflammatory-related genes such as cyclooxygenases, lipoxygenases, inducible nitric oxide synthase, and thereby decrease nitric oxide and prostaglandin E2 (PGE2) production [9, 11,12,13]. In cancerous cells, glycosides derived from HCS also exhibit cytostatic and cytotoxic activities, induce cellular apoptosis, inhibits angiogenesis, reverses chemotherapeutic drug resistance, and modulate immune responses. Previous studies suggest that Na+/K+ pump or sodium- and potassium-activated adenosine 5′-triphosphatase (Na+, K+-ATPase) is a potential drug target that contributes to the selective control role of cardiac glycosides in tumor proliferation, but does not affect normal cell growth [10, 15, 16]. Moreover, accumulating evidence reveals the anti-cancer effect of HCS and its derived single compounds in several tumor types in vitro and in vivo.
Furthermore, in the last decade, some studies have proposed new properties and effects of HCS, Chansu and their major active constitutes, bufalin, in the treatment of cancer (Fig. 1). Interestingly, there are an increasing number of studies investigating both in vitro and in vivo experiments in the recent 5 years, indicating an increased awareness of the translational potential of HCS and its derived steroidal cardiac glycosides in animal studies. In this review article, data on the anti-cancer effect of HCS and its major active constitutes bufalin published in the recent 10-years were retrieved from databases including PubMed, MEDLINE, CNKI, and clinicaltrial.gov. This review focuses on the anti-cancer pharmacological effects and mechanisms of action of HCS and bufalin, with emphasis on elaborating the translational potential and future clinical application. This review article also discusses the recent studies on drug delivery and its derivatives.
The bioactive constituents of Huachansu
The chemical composition and pharmacological activity of HSC have been investigated since the 1980s [7, 14, 15, 17, 18]. HSC contains two primary bioactive chemical components, indole alkaloids (bufotenine, bufotenidine, cinobufotenine, and serotonin), and steroidal cardiac glycosides [7, 14, 15, 18]. Their extraction rate is mainly determined by the extraction method. High performance liquid chromatography (HPLC) quantitative analysis confirmed that the aqueous extract of HSC yield around 20-fold higher serotonin than bufadienolides (75.7 ± 0.1 mg/g and 3.8 ± 0.0 mg/g, respectively), while methanol or ethanol extraction solution contains 5–26 times higher concentrations of bufadienolides, with only trace amounts of serotonin [14]. So far, there are more than 28 steroidal cardiac glycosides identified from HCS [19]. The investigation into the potential use of cardiac glycosides in cancer therapeutic was initiated more than 40 years ago, yet was abandoned due to the toxicities [20]. However, in 1999, Scandinavian oncologist Haux [21] reported that digoxin induced tumor cell apoptosis in a variety of human cancer cell lines at non-toxic concentration. Recent studies demonstrated that steroidal cardiac glycosides are the major anti-neoplasm component of HCS.
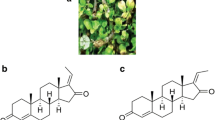
Bufalin (PubChem CID: 9547215, Chemical formula C24H34O4, Fig. 2) is a cardiac glycoside and the major active component is attributed to the anti-tumor activity of HCS [12]. Similar to other bufadienolides (such as resibufogenin, cinobufagin, and bufotalin), Bufalin is a cardioactive C-24 steroid characterized by an α-pyrone ring at C-17 [22, 23]. Bufalin exhibits a variety of biological activities. Its structural similarity with digitoxin accounts for both the therapeutic effect as well as the unwanted side-effects such as cardiotonic, blood pressure stimulatory and respiratory stimulatory effects in cancer treatment [20, 24, 25]. Recent studies emerge on purified compounds and cardiac glycosides. Bufalin may represent a promising form of targeted cancer chemotherapy for long-term applications without severe side effects.
Shows the structure of Bufalin (C24H34O4, Pubchem CID: 9547215). Retrieved from National Center for Biotechnology Information. PubChem Compound Database; CID = 9547215, from https://pubchem.ncbi.nlm.nih.gov/compound/9547215 (accessed 22 Aug 2018)
Cytostatic effects of Huachansu and Bufalin on tumors
It appears that the cytostatic effect of Bufalin and HCS is well demonstrated in a variety of tumors such as breast, colorectal, gastric, lung, liver and bone tumors. The 50% inhibition dose (IC50) among cancer cell lines after 1–3 days of treatment are summarized in Table 1. The in vitro effects of Bufalin and HCS in arresting cell cycle, inducing intrinsic and extrinsic apoptosis are reproducible across studies. Recent studies have confirmed that Bufalin can lead to cell cycle redistribution, at least in part by its role in the inhibition of Na+/K+-ATPase activity, in various types of human cancers [26, 27]. In cancer cells, Bufalin can arrest cell cycle at G0/G1 and G2/M phase, owing to the various dosage of treatment and cell lines [28,29,30]. The sensitivity to Bufalin treatment varies among cell lines. Currently, there is a lack of in-depth study to predict the exact effect of Bufalin on cell cycle. Moreover, it is worth noting that one article published in 2013 by Clifford and Kaplan suggested that Bufalin and other cardiotonic steroids, including Ouabain and Digitoxin, can inhibit membrane Na+/K+-ATPase activity in multiple cell lines regardless of metastatic potential [31, 32]. Also, human breast tumor cells are more resistant to growth inhibition and apoptosis induction of cardiotonic steroids than non-tumorigenic breast cells [31]. The 50% inhibitory dose with 24-h of Bufalin treatment in metastatic invasive ductal carcinoma MCF10CA1 cell and the invasive estrogen receptor (ER)-negative MDA-MD-231 breast cancer cell is two- and threefold, respectively, the dosage of human noncancerous mammary epithelial cells 184D and 184A1 [31]. Another study on the effect of HCS on normal human lymphocytes was marginal (with more than 60% viability at 48-h) compared with A-549, Jurkat, MCF-7 cancer cells (with less than 10% viability) at a dose of 0.16 mg/mL of CS extract [33]. However, a majority of studies investigating the cytostatic effect of HCS and Bufalin are carried out solely on tumorigenic cells instead of normal non-cancerous cells. Further investigation is warranted to elucidate the pharmacological action of Bufalin treatment.
In vivo evaluation of Huachansu and its derivative Bufalin on tumor inhibition
To evaluate the anticancer potential of Bufalin in vivo, various xenograft mice models are carried out throughout cancer types such as breast, cervical, colorectal, liver, gallbladder, lung, and pancreatic cancer. The results are very compelling that Bufalin can inhibit xenograft tumor growth, increase sensitivity to chemotherapy, and prolong the survival rate of mice. Most of the studies reported a dosage of 0.1–2 mg/kg intraperitoneally (i.p.) administered at a frequency of once per day 5 days per week to every 3 days for a period ranging from 12 days to 6 weeks in mice xenograft model without inducing significant weight loss or adverse effects, as summarized in Table 2. In the recent 10 years, although in vivo studies have been increasingly used to assess the effect of Bufalin. Most of the studies have used the xenografts models and reported the attenuation of tumor growth rate as well as the reduction of tumor weight. Yet, the lack of orthotropic models in the Bufalin research led to a relative shortage in the evaluation of the systemic effect of Bufalin contributing to not only the tumor itself but to the microenvironment or tumor metastasis. Further studies are warranted to elucidate the related effects.
The acute toxicity measured as 50% lethal dose values for Bufalin in mice when administered intraperitoneally was approximately 2.2 mg/kg, as first reported in1960 by Okada et al. [34] by Wang et al. in 2003 [35] and by Liu et al. in 2016 [36], consistently. Bufalin showed the lowest toxicity when injected intraperitoneally among principle active components, including cinobufagin and resibufogenin, isolated from CS [34]. When administered intravenously (i.v.) to rabbits, Bufalin induced an elevation of blood pressure accompanied by marked respiratory excitation. Yet, although Bufalin is structurally slimier to digitoxin that both of them possess the same aglycone except for the lactone ring in 17-position, digitoxin does not induce respiratory excitation induced by the i.v. administration of Bufalin was not observed with digitoxin intervention at a dosage as high as 0.2 mg/kg via i.v. injection [34]. A study in 1995 examined the toxicity and teratogenicity of CS using a single i.p. injection into maternal mice during the embryonic organogenesis [37]. At doses below 50 mg (dry weight)/kg body weight, no detectable changes were observed in both maternal mice and fetuses; however, at a dose of 50 mg/kg, adverse effects were found with a reduction in maternal mice body weight, liver, and kidney structural abnormalities, and an increased number of resorbed and dead fetuses [37].
It is worth noting that most studies have reported a significant reduction in tumor growth rate and tumor weight after i.p. injection of Bufalin. An article reported a contradictory result in a breast cancer xenograft model with an intra-tumoral injection of Bufalin in 2017 [38]. The results showed that intra-tumoral injection of 10 μL of 1 μM Bufalin, three times a week for 4 weeks, equivalent to 3.87 ng per mice, significantly enhanced tumor growth by promoting inflammatory response compared with intratumoral injection of saline via increasing expression of COX-2/IL-8, promoting p-65 NF-κB translocation and modulating mitogen-activated protein kinase (MAPK) pathways [38]. This contradictory result may be due to the difference in not only the route of administration but also the difference in the equivalent dose among studies.
As previously reported, for example, Han, et al. conducted a study in 2007 and reported an anti-tumor effect of Bufalin with i.p. injection of 0.5, 1.0 or 1.5 mg/kg/day for 10 days in mice hepatocellular carcinoma (HCC) xenograft model with a body weight around 18–20 g, which is equivalent to an i.p. injection of 10 to 30 mg/mice of Bufalin throughout the course of treatment [39]. Other studies, as summarized in Table 2, reported anti-tumor effect with i.p. administration of doses ranging from 0.5 to 1.5 mg/kg at a frequency of daily injection for 10 days, every other day injection for 20 days or once every 3 days for 4 weeks. There appears to be more than a 1000-fold difference between the anti-tumor effect reported by i.p. injection and the pro-inflammatory or tumor-promoting effects upon intra-tumoral injection. A variation in doses is also found. According to the material and safety data sheet, the median lethal dose of Bufalin administered to mice by i.v. injection was 0.74 mg/kg [38]. It is plausible that the contradictory results on the tumor suppressing or the tumor-promoting effects of Bufalin may be due to the variation among different cancer cell lines, different route of administration, or different doses used. In future research, more in-depth study using the same treatment protocol is needed to better assess the role of Bufalin in cancer treatment.
Molecular mechanisms of anti-tumor activity
The cell cycle arresting, intrinsic (also known as mitochondria-mediated) and extrinsic (also known as receptor-mediated) apoptosis-inducing effects of Bufalin at concentrations ranging from 1 nM to 10 μΜ, such as intracellular reactive oxygen species (ROS) production, caspase-dependent apoptosis, modulating MAPK signaling cascade, inhibiting NF-κB signaling, are well not only well established among various cancer cell lines [29, 40,41,42,43,44,45,46,47,48,49,50,51,52,53]. The steroid receptor coactivator (SRC) family, including SRC-1, SRC-2, and SRC-3, is involved in molecular and physiological processes across diseases by activating nuclear receptors and other transcriptional factors, such as NF-κB. SRCs are frequently overexpressed in malignancies and are associated with cancer cell proliferation, invasion, and metastasis [54,55,56]. Previous studies suggested that Bufalin promotes SRC-3 protein degradation in breast, gastric and lung cancer [36, 55,56,57,58]. Moreover, Bufalin suppressed triple negative breast cancer proliferation at a nanomolar concentration (~ 3–5 nM), which the concentration of which digoxin required to inhibit SRC-3/SRC-1 is greater than 200 nM [56]. The effective concentration of Bufalin to inhibit SRCs is within the on cardiac toxicity concentration reported in patients plasma (~ 9 nM) [12]. Furthermore, the water soluble prodrug of Bufalin, 3-phospho-bufalin, inhibits the growth of orthotopic triple negative breast cancer [55]. All in all, Bufalin is a potent and safe steroid receptor coactivators inhibitor.
Although the exact mechanism of the anti-cancer effect of Βufalin has hitherto been unknown. It is worth noting that several novel mechanisms contributing to the anti-cancer effects of Bufalin are proposed and increasingly studied in the recent 10 years, including sensitization of TRAIL-mediated apoptosis, autophagic cell death induction, reversing chemotherapy drug resistance, suppressing cellular invasion, migration, and anti-adhesion, inhibiting epithelial–mesenchymal transition, as shown in Table 3. A schematic summary of the molecular mechanism of Bufalin-mediated anti-cancer effect is shown in Fig. 3.
Induce cell death other than apoptosis
In contrast to the apoptosis-inducing activity of Bufalin, well-documented in various cancer cell lines, 100 nM of Bufalin does not induce caspase-dependent apoptosis in human colorectal caco-2 and HT-22 cell lines [59]. Instead, Bufalin induces cell death by triggering autophagy, possibly through a ROS- and JNK-dependent pathways in colorectal cancer cells, followed by increased expression of ATG5 and Beclin-1 without significant induction of apoptosis, PARP cleavage, and caspase-3 cleavage [59]. It may be plausible that Bufalin enhances the radiosensitivity of colorectal cancer through ROS-mediated autophagy, which deserves further investigation [59].
In addition to inducing apoptosis-dependent cell death, laboratory studies have suggested that Bufalin (0.1–1 μM) inhibits Na+/K+-ATPase activity, a crucial ion pump, and transducer ligand receptor. It suppresses cancer cell proliferation and possesses synergistic effect with Sorafenib against liver cancer [60, 61]. Changes in Na+/K+-ATPase activity play an important role in cell survival and function, and high expression of Na+/K+-ATPase subunit α is associated with poor overall survival in liver cancer patients [27]. Moreover, compared with adjacent tissues, the expression of Na+/K+-ATPase subunit α1 was significantly elevated in liver cancer tissues, suggesting that the specific targeting of Na+/K+-ATPase can have therapeutic effects on cancer cells without noticeable effect on normal cells [27]. The role of Na+/K+-ATPase in mediating cytotoxicity of Bufalin should be highlighted, and further studies are warranted.
Metastasis inhibition (angiogenesis, MMPs, EMT, others)
Angiogenesis
Hyperactive angiogenesis is a hallmark of cancer cells. It not only provides oxygen and extra nutrient, maintains the high proliferation rate of cancer cells, but also promotes local and distant metastasis of malignant cells [62]. Bufalin had been reported to inhibit cancer cell migration and invasion in liver cancer and lung cancer cell lines by down-regulating vascular endothelial growth factor (VEGF), which plays a major role in tumor angiogenesis [46, 63, 64]. Anti-angiogenic drugs, such as Sorafenib, may be complicated by relatively easy-acquired drug resistance, rapid onset of relapse after discontinuation, and the potential for tumor metastasis. A previous study revealed that Bufalin enhances the anti-angiogenic effect of Sorafenib via AKT/VEGF signaling [64]. The co-administration of Bufalin and Sorafenib provides a novel therapeutic option for patients with advanced HCC and is worthy for further in-depth investigations [63, 64].
MMPs
Matrix metalloproteinases (MMPs) are a class of enzymes that degrade extracellular matrix proteins and are thought to play important roles in cell proliferation, migration, angiogenesis, and etc. In cancer cells, especially those with high metastatic potentials, MMPs, in particular, MMP-2 and MMP-9, are highly expressed [65]. MMP-2 and MMP-9 primarily control cancer cell motility, and down-regulation of these proteins was reported across studies to reduce cancer metastasis [66, 67]. Bufalin was found to down-regulate MMPs in human liver cancer, lung cancer, osteosarcoma, and etc. [68,69,70]. In human HCC, Bufalin inhibits metastasis both in vitro and in vivo through pathways including the Akt/GSK3β signaling, E-cadherin/β-catenin nuclear translocation [66, 71,72,73]. In lung cancer cells, Bufalin (25–10 nM) was observed to suppress MMP-2 and MMP-9 expression, which were mediated through multiple pathways, including p38 MAPK, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase1/2 (Erk1/2), focal adhesion kinase (FAK), Rho-associated protein kinase 1 (ROCK1) and NF-κB [69, 70, 74]. Bufalin is a broad inhibitor of various inflammatory signaling pathways in cancer. It was noticed that in human hepatoma cells, the expression of the inflammatory protein COX-2 is down-regulated and is associated with the inhibition of cell invasion and migration [66].
EMT
The epithelial–mesenchymal transition (EMT) is a process in which epithelial cells malignant phenotypes to become mesenchymal stem cells that promote metastasis. Bufalin was found to inhibit EMT in cervical, colorectal, hepatic, lung cancer by downregulating mesenchymal markers and upregulating epithelial markers [66, 67, 71, 75,76,77,78,79,80]. Bufalin can attenuate transforming growth factor-β (TGF-β)-induced upregulation of N-cadherin, vimentin, and Snail while downregulating E-cadherin by targeting hypoxia-inducible factor-1α (HIF-1α) [76]. In addition, the regulating of motility and invasiveness of cancer cells may be related to the PI3K/AKT/mTOR pathway [76, 81], Wnt/β-catenin pathway [66, 77], Hedgehog signaling pathway [67, 82], and etc.
Others
Bufalin is reported to act as a natural anti-inflammatory small molecule that upregulates osteoprotegerin and down-regulates receptor activator of NF-κB ligand (RANKL), ameliorating cannabinoid 2 receptor (CB2)-mediated cancer-induced pain and bone destruction [83]. Bufalin also down-regulated several metastasis-related genes, such as Rho-associated (Rho A), integrins and FAK [70, 80, 84, 85]. A recent study revealed that bufalin combined with sorafenib synergistically inhibited liver cancer cell migration by targeting mTOR/Akt/VEGF signaling and affecting tumor vascular microenvironment [63, 64].
Omics approach in the study of antineoplastic effects of Bufalin and Huachansu
Few genomics and proteomics approaches are used to study the anti-cancer effects of Bufalin or HCS. Using a comparative proteomics approach, Xie et al. [86, 87] identified that Bufalin modifies 24 differentially expressed protein, particularly, the expression of proteins involve in cell metabolism, apoptosis, and cytoskeleton structure. Among them, the heat shock protein 27 (Hsp27) decreased remarkably, and its down-regulation played a critical role in bufalin-induced apoptosis in osteosarcoma cells [86, 87]. Another study used two quantitative proteomics methods, isobaric tags for relative and absolute quantification (iTRAQ)-based and label-free proteomic analysis, to study the target-related proteins of Bufalin in human A549 lung cancer cells [88]. The number of the differentially expressed protein commonly found in the two methods is 45 proteins, suggesting that the involvement of oxidative stress and the fibronectin-related pathways are important pathways for the anti-cancer effect of Bufalin [88]. Wu et al. [89] reported in 2014 that Bufalin modulates about 165-apoptosis-related genes in human lung cancer CNI-H460 cells using Affymetrix GeneChip. These results provide a deeper understanding of the anti-proliferative and cytotoxic mechanisms of Bufalin at the genetic level in gene assays [89]. Although a small number of proteomics studies and gene chip arrays have studied the target-related proteins or genes involved in the anti-cancer effect of Bufalin, which can serve as a paradigm for further studies of the molecular basis of Bufalin against various types of tumors, there is a lack of transcriptome analysis and other high-throughput screening in examining or predicting cell response to Bufalin treatment at both cytotoxic level and non-cytotoxic level. There is a need for high-throughput analysis to better associate the change of mRNA levels correlated with protein–protein interactions or protein–DNA interactions [90]. Further work is warranted on the molecular mechanism of Bufalin and HCS against various tumors.
Drug delivery and its derivatives
Bufalin participates in complex cell-signal transduction pathways, which contribute to its suppressive effect of tumor progression in various cancer types. However, its structural similarity to digitoxin, digoxin and other cardiac glycosides accounts for the toxic effects at a high dosage. Previous studies suggest that Na+/K+-ATPase is a potential drug target that contributes to the emerging role of cardiac glycosides in selectively controlling tumor proliferation, but does not affect normal cell growth [36]. In the recent years, emerging newly discovered HCS derivatives have been shown to process anti-tumor effects, such as BF211, have been shown to inhibit colorectal, gastric, and lung cancer [91, 92].
In the process of seeking to improve the anti-cancer properties of Bufalin, several drug carriers have been synthesized with Bufalin, such as carbon-based nanomaterials, including liposomes and polymeric microspheres, folate receptor-targeted supramolecules, or [18F]fluoroethyl conjugates [93,94,95,96,97,98,99,100,101]. Structurally modified and synthetic Bufalin-loaded nanoparticles are designed to promote tumor-specific drug release and cytotoxicity, increase cellular uptake, and improve bio-distribution at the tumor site [99, 102, 103]. Some studies have reported that internalization of the Bufalin nanoparticles may at least partially contribute to the increased intracellular uptake of Bufalin in cancer cells [85, 93, 95, 102, 104,105,106]. However, there is still a need for better therapeutic materials with good drug solubility, well binding affinity, high tumor-specific targeting, low systemic toxicity and rapid clearance.
Potential cardiotoxic property of Bufalin and the side-effects of toad extract and HCS in clinical studies
As a cardioactive steroid, Bufalin has a variety of biological activities, such as cardiotonic, blood pressure stimulation, and etc. At high dosages, cardioactive steroids can cause cardiac arrhythmias and exhibit cardiotoxicity. The concentration of CS (400 ng/mL) extract and Bufalin alone can induce myocardial cell arrest within a few seconds after administration by altering intracellular calcium storage in cardiomyocytes and possibly acts on sites other than the Na+/K+-ATPase [107]. Koh et al. [108] reported that zebrafish larvae responded to 100 µM Bufalin and showed a decrease in heart rates, early depolarization and polymorphic arrhythmia-like changes. Another study found that the addition of Bufalin to guinea pig papillary muscle (0.4 µmol/L) or atrium (225 nmol/L) preparations can lead to arrhythmias [109]. Further study on the effect of ethanol extract of Chinese toad venom (EET) on mice showed that 5 mg/kg of EET caused liver toxicity cardiomyocytes injuries [110,111,112]. In a Pilot study of HCS in patients with HCC, non-small cell lung cancer, or pancreatic cancer showed that side effects that may have been related to HCS were hematologic (thrombocytopenia and leukopenia), gastrointestinal (loss of appetite, constipation and diarrhea), mucocutaneous (dental ulcers and rashes), and cardiovascular (premature ventricular contraction and hypertension) in nature. Others include myalgia, dyspnea and dizziness [12]. However, when using HCS, no dose-limiting toxicity (DLT) was observed at doses up to 8-times higher than the usual dose used in China (conventional doses = 20–25 mL; highest dose = 162 mL). To the best of our knowledge, the typical therapeutic doses of HCS have not found significant side-effects in clinical studies. However, it has been reported that HCS can reduce the side-effects of chemotherapy and radiotherapy in gastric cancer, lung cancer, colorectal cancer, and etc. [9, 11, 113,114,115,116]. Due to the potential toxic effects of other cardiac glycosides, careful clinical evaluation should be performed prior to Bufalin administration.
Clinical trials
Huachansu injection is a sterile hot-aqueous extract of dried toad skin, is approved by the Chinese FDA for use at oncology clinics in China. Since the 1970s, various clinical studies in China have demonstrated the anti-cancer properties of HCS, with a total response rate of 10% and 16% for patients with advanced liver and lung cancer, respectively. In a previous clinical study, the quality and consistency of three separate lots of HCS were evaluated [12]. It was found that and levels of bufalin and resibufogenin in HCS were 18.0–19.5 ng/mL, and 17.7–19.0 ng/mL, respectively [12]. The variation between three lots of HCS was remarkably close, with less than 10% variation. In the detection of bufadienolides concentration after i.v. infusion of HCS in human plasma specimens, the levels of Bufalin, cinobufagin, cinobufotalin, and resibufogenin in the used in a phase I clinical trial was 14.3 ± 0.03, 3.35 ± 0.1, 21.5 ± 0.22, and 24.5 ± 2.18 ng/mL, respectively [12]. As for Bufalin, which studies have shown is the major bufadienolide with the most pronounced anti-cancer activity, was further evaluated its concentration in plasma by liquid chromatography with mass spectrometry (LC/MS/MS) and found a maximal plasma levels at the end of the 2-h infusion which is proportional to the amount of drug administered (0.81–3.38 ng/mL) [12]. In a clinical phase I study of HCS in patients with HCC, non-small cell lung cancer, or pancreatic cancer published in 2009 and found that HCS is partially effective on cancer patients [12]. Only mild adverse events were observed, with doses up to five times the conventional clinical dose, which is approximately 15 mL of drug per meter squared of body mass (mL/m2) [12]. Furthermore, a phase II randomized controlled trial (RCT) of patients with advanced pancreatic adenocarcinoma (PaCa) treated with HCS in 2012 reported no clinical benefit with the addition of HCS to gemcitabine, which is similar to over 30 previously published well-designed RCTs in patients with advanced PaCa evaluating gemcitabine in combination with other cytotoxic or biologic agents [117]. However, the lack of efficacy observed in these trials does not preclude the possible efficacy of HCS in other solid malignancies. There is an increase in related publications later (Fig. 1a), indicating an increasing research interest on the potential use of HCS and its derived steroidal cardiac glycosides in cancer therapy. However, the report on the outcome of any RCT published after the phase II clinical trial is not yet available.
Discussion and future perspectives
At present, TCM is practiced all over the world. Natural products have long been an important source of cancer treatment. CS has been used for thousands of years in the practice of TCM in aqueous extract form for the treatment of cancer. HCS has been used either alone or in combination with chemotherapeutic agents. Since the phase I study of HCS in patients with liver, lung, or pancreatic cancer in 2009, there is an increase in related publications, indicating a great interest is brought on the potential use of HCS and Bufalin in human. HCS and Bufalin exhibit a wide range of biological effects in cancer, including inhibition of cell proliferation, induction of cell apoptosis, disruption of the cell cycle, inhibition of metastasis, reversing multi-drug resistance to chemotherapeutic agents, and regulation of the immune response. HCS and Bufalin also have multiple other effects such as respiratory excitation, anti-inflammation and analgesics [83, 118].
Cancer is characterized by inflammation, and the chronic inflammatory microenvironment is often associated with malignant progression. The anti-inflammatory effects of Bufalin may at least in part contribute to its anti-cancer effect targeting the tumor microenvironment. The involvement of various inflammatory mediators such as COX-2 and PGE2 may be produced not only by cancer cells, but also by the surrounding immune cells, fibroblasts, and endothelial cells. The production of inflammatory mediators may create a positive feedback loop to further promote cancer proliferation, angiogenesis, invasion, and metastasis. Although most studies reported the anti-inflammatory effect of Bufalin, in breast cancer, Bufalin was reported to promote inflammatory response, accompanied by increased COX-2/IL-8 expression and enhanced tumor growth in vivo [38]. The role of Bufalin on NF-κB signaling and pro-inflammatory mediators, such as COX-2, inducible nitric oxide synthase (iNOS), tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6) are studied across diseases, including rheumatoid arthritis [119, 120], asthma [118], bacterial infection [121], and chronic inflammatory disease [122]. It is worth highlighting that Bufalin may also possess chemo-preventive potential in skin cancer when administered topically in a rodent model [123]. Future research may extend to other types of human diseases. Research on the interaction between stromal cells and tumor cells may not only broaden the potential use of Bufalin and HCS, but also helps to understand the tumor immune microenvironment, thereby providing long-lasting repression against cancer growth for future cancer treatment.
Previous studies found that the expression of Na+/K+-ATPase subunit α1 is prognostic in liver cancer patients and is significantly elevated in cancer tissue compared with adjacent tissues [27, 60]. Recent studies of cardiac glycosides suggest Bufalin may potentially represent a promising form of targeted cancer chemotherapy on Na+/K+-ATPase subunit α1 that can be safely used for long periods without severe side effects [27, 60]. Furthermore, the results indicated that the down-regulation of Na+/K+-ATPase α1 can inhibit cell proliferation, migration, and invasion in vitro, and inhibit tumorigenesis in vivo [26, 27, 124]. These increase the likelihood of selecting patients with high Na+/K+-ATPase subunit α1, may improve the therapeutic efficacy of Bufalin and HCS. It is worth noting that since June 2015, another Na+/K+-ATPase inhibitor, RX108, is structurally similar to Bufalin and is undergoing phase I clinical trials to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamic properties in patients with locally advanced or metastatic solid tumors in Australia. However, as of July 2018, there were no recent reports for phase-I development. This study, if successfully completed, may provide valuable information about the safety of targeting Na+/K+-ATPase.
In the process of identifying active anti-cancer ingredients in Traditional Chinese Medicine, some encouraging results have been achieved and we deepened our understanding of the underlying mechanism of malignant behavior. With the growing awareness of the tumor microenvironment and the wholistic immunomodulation, it is possible that agents with systemic effects may be a double-edged sword. In the identification of novel therapeutic agents, agents that target multiple signaling pathways may have a stronger anti-cancer effect, but inevitably carry more adverse effects, such as gastrointestinal burden and hematological toxicities. Studies combining conventional cancer treatments (such as chemotherapy and radiotherapy) with TCM or derived components should be interpreted with caution, as drug interactions caused by combination therapies may have a negative impact on conventional cancer treatments, reducing their effectiveness. It is also essential to further study the underlying pathophysiological mechanisms of anti-cancer effects and adverse reactions. Inevitably, there is a great need to discover long-term effects, drug use sequences, drug–drug interactions, and individual choices further for different drugs, aimed at improving patients’ quality of life and prolonging survival.
Concluding remarks
Vigorous studies and discoveries have revealed the anti-tumor properties of Bufalin and HCS as potentially multi-targeted agents for cancer treatment. The results indicated that Bufalin inhibits tumor progression by inhibiting cancer cell proliferation via both apoptosis dependent or independent pathways, as well as inhibiting metastasis via repressing cell motility and angiogenesis. Many animal studies and clinical studies conducted over the past decade have revealed their clinical potentials, however, in the current study, long-term toxicological studies on different individuals, pharmacodynamics studies on various dosages and administration routes have been relatively lacking. Further research is needed to elucidate potential drug-drug interactions and multi-target interactions of Bufalin and HCS. Large-scale clinical trials are warranted to translate the knowledge for anti-cancer effects of Bufalin and HCS into clinical applications as effective and safe treatment options for future cancer patients. Overall, this review highlights the advances in the study of Bufalin and HCS as emerging anticancer agents over the past decade and may shed light on the future direction of Bufalin and HCS as novel anticancer agents for clinical applications.
Highlights
-
Huachansu and Bufalin possess anti-cancer effects both in vitro and in vivo.
-
The multi-target and multi-pathway pharmacological actions are promising.
-
Potential drug–drug interactions and multi-target interaction lacks studies.
-
Further large-scale clinical trials are warranted.
Abbreviations
- TCM:
-
Traditional Chinese Medicine
- CS:
-
Chansu
- HCS:
-
Huachansu
- NF-B:
-
nuclear Factor-κB
- PGE2 :
-
prostaglandin E2
- Na+, K+-ATPase:
-
Na+/K+ pump or sodium- and potassium-activated adenosine 5′-triphosphatase
- HPLC:
-
high-pressure liquid chromatography
- IC50:
-
the 50% inhibition dose
- ER:
-
estrogen receptor
- i.p.:
-
intraperitoneal
- i.v.:
-
intravenous
- COX-2:
-
cyclooxygenase-2
- IL-8:
-
interleukin-8
- MAPK:
-
mitogen-activated protein kinase
- HCC:
-
hepatocellular carcinoma
- ROS:
-
reactive oxygen species
- SRC:
-
steroid receptor coactivator
- TRAIL:
-
tumor necrosis factor related apoptosis-inducing ligand
- JNK:
-
c-Jun N-terminal kinase
- ATG5:
-
autophagy-related gene 5
- PARP:
-
poly-(ADP-ribose) polymerase
- MMPs:
-
matrix metalloproteinases
- EMT:
-
epithelial–mesenchymal transition
- VEGF:
-
vascular endothelial growth factor
- AKT:
-
protein kinase B
- GSK3β:
-
glycogen synthase kinase 3 beta
- Erk1/2:
-
extracellular signal-regulated kinase1/2
- FAK:
-
focal adhesion kinase
- ROCK1:
-
Rho-associated protein kinase 1
- TGF-β:
-
transforming growth factor-β
- HIF-1α:
-
hypoxia-inducible factor-1α
- PI3K:
-
phosphoinositide 3-kinase
- mTOR:
-
mammalian target of rapamycin
- RANKL:
-
receptor activator of nuclear factor-κ B ligand
- CB2:
-
cannabinoid 2 receptor
- Rho A:
-
Rho-associated
- Hsp27:
-
heat shock protein 27
- iTRAQ:
-
isobaric tags for relative and absolute quantification
- EET:
-
ethanol extract of Chinese toad venom
- DLT:
-
dose-limiting toxicity
- LC/MS:
-
liquid chromatography with mass spectrometry
- RCT:
-
randomized controlled trial
- iNOS:
-
inducible nitric oxide synthase
- TNF-α:
-
tumor necrosis factor alpha
- IL-6:
-
interleukin 6
- PaCa:
-
pancreatic adenocarcinoma
- RCT:
-
randomized controlled trial
References
Cho WC. Evidence-based anticancer materia medica. Hong Kong SAR: Springer; 2011. p. 390–406.
Huang K. Anesthetic and muscle-relaxing herbs: Chan Su. In: The pharmacology of Chinese herbs. 2nd edn. Boca Raton: CRC Press; 1999: 544.
Xu R, Xie HQ, Deng LL, Zhang JX, Yang FM, Liu JH, Hao XJ, Zhang YH. A new bufadienolide with cytotoxic activity from the Chinese traditional drug Ch’an Su. Chin J Nat Med. 2014;12(8):623–7.
Sakurai K, Yoshii E, Hashimoto H, Kubo K. Gas liquid chromatography of steroids of Ch’an Su. II. Reinvestigation on the determination of bufadienolides. Chem Pharm Bull. 1968;16(6):1140–3.
Shimizu Y, Morishita S. Metabolism and disposition of kyushin, a drug containing senso (ch’an su). Am J Chin Med. 1996;24(3–4):289–303.
Chang ZF, Jia DX, Bare J. Chinese materia medica. Beijing: Peoples Medical Publishing House; 2014.
Su YH, Huang XQ, Zhang DZ, Zhang YN, Xie JM, Linh CQ. HPLC separation and determination of bufadienolide in cinobufacini injection. Chin Tradit Pat Med. 2003;25:24–7.
Qin TJ, Zhao XH, Yun J, Zhang LX, Ruan ZP, Pan BR. Efficacy and safety of gemcitabine-oxaliplatin combined with Huachansu in patients with advanced gallbladder carcinoma. World J Gastroenterol. 2008;14(33):5210–6.
Xie X, Huang X, Li J, Lv X, Huang J, Tang S, Sun Y. Efficacy and safety of Huachansu combined with chemotherapy in advanced gastric cancer: a meta-analysis. Med Hypotheses. 2013;81(2):243–50.
Yin JH, Zhu XY, Shi WD, Liu LM. Huachansu injection inhibits metastasis of pancreatic cancer in mice model of human tumor xenograft. BMC Complement Altern Med. 2014;14:483.
Zhou B, Wu F, Yuan L, Miao Z, Zhu S. Is huachansu beneficial in treating advanced non-small-cell lung cancer? Evidence from a meta-analysis of its efficacy combined with chemotherapy. Evid Based Complement Alternat Med. 2015;2015:408145.
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge Y, Newman RA, Cohen L, Liu L, Thornton B, et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer. 2009;115(22):5309–18.
Wang L, Raju U, Milas L, Molkentine D, Zhang Z, Yang P, Cohen L, Meng Z, Liao Z. Huachansu, containing cardiac glycosides, enhances radiosensitivity of human lung cancer cells. Anticancer Res. 2011;31(6):2141–8.
Lee HJ, Koung FP, Kwon KR, Kang DI, Cohen L, Yang PY, Yoo HS. Comparative analysis of the bufonis venenum by using TLC, HPLC, and LC–MS for different extraction methods. J Pharmacopunct. 2012;15(4):52–65.
Dai LP, Wang ZM, Gao HM, Jiang X, Ding GZ. Determination of bufothionine in skin of Bufo bufo gargarizans and Huachansu injection. Zhongguo Zhong Yao Za Zhi. 2007;32(3):224–6.
Yang T, Shi R, Chang L, Tang K, Chen K, Yu G, Tian Y, Guo Y, He W, Song X, et al. Huachansu suppresses human bladder cancer cell growth through the Fas/Fasl and TNF-alpha/TNFR1 pathway in vitro and in vivo. J Exp Clin Cancer Res. 2015;34:21.
Toma S, Hirai Y, Sugimoto C, Shoji M, Oguni Y, Morishita S, Ito C, Horie M. Metabolic fate of bufalin and cinobufagin. Yakugaku Zasshi. 1991;111(11):687–94.
Yang Z, Luo H, Wang H, Hou H. Preparative isolation of bufalin and cinobufagin from Chinese traditional medicine ChanSu. J Chromatogr Sci. 2008;46(1):81–5.
Su YH, Nu X. Evaluation of pharmacodynamic effect of pharmaceutical agents of Chan Su. J Beijing Univ of TCM. 2001;24:51–4.
Calderon-Montano JM, Burgos-Moron E, Orta ML, Maldonado-Navas D, Garcia-Dominguez I, Lopez-Lazaro M. Evaluating the cancer therapeutic potential of cardiac glycosides. Biomed Res Int. 2014;2014:794930.
Haux J. Digitoxin is a potential anticancer agent for several types of cancer. Med Hypotheses. 1999;53(6):543–8.
Kamano Y, Nogawa T, Yamashita A, Hayashi M, Inoue M, Drasar P, Pettit GR. Isolation and structure of a 20,21-epoxybufenolide series from “Ch’an Su”. J Nat Prod. 2002;65(7):1001–5.
Takai N, Kira N, Ishii T, Yoshida T, Nishida M, Nishida Y, Nasu K, Narahara H. Bufalin, a traditional oriental medicine, induces apoptosis in human cancer cells. Asian Pac J Cancer Prev. 2012;13(1):399–402.
Datta P, Dasgupta A. Interactions between drugs and Asian medicine: displacement of digitoxin from protein binding site by bufalin, the constituent of Chinese medicines Chan Su and Lu-Shen-Wan. Ther Drug Monit. 2000;22(2):155–9.
Dasgupta A, Emerson L. Neutralization of cardiac toxins oleandrin, oleandrigenin, bufalin, and cinobufotalin by digibind: monitoring the effect by measuring free digitoxin concentrations. Life Sci. 1998;63(9):781–8.
Huang H, Zhang W. Bufalin induced apoptosis of bladder carcinoma cells through the inactivation of Na+K+-ATPase. Oncol Lett. 2018;16(3):3826–32.
Li H, Wang P, Gao Y, Zhu X, Liu L, Cohen L, Meng Z, Yang P. Na+/K+-ATPase alpha3 mediates sensitivity of hepatocellular carcinoma cells to bufalin. Oncol Rep. 2011;25(3):825–30.
Nasu K, Nishida M, Ueda T, Takai N, Bing S, Narahara H, Miyakawa I. Bufalin induces apoptosis and the G0/G1 cell cycle arrest of endometriotic stromal cells: a promising agent for the treatment of endometriosis. Mol Hum Reprod. 2005;11(11):817–23.
Huang WW, Yang JS, Pai SJ, Wu PP, Chang SJ, Chueh FS, Fan MJ, Chiou SM, Kuo HM, Yeh CC, et al. Bufalin induces G0/G1 phase arrest through inhibiting the levels of cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via mitochondrial signaling pathway in T24 human bladder cancer cells. Mutat Res. 2012;732(1–2):26–33.
Hsu CM, Tsai Y, Wan L, Tsai FJ. Bufalin induces G2/M phase arrest and triggers autophagy via the TNF, JNK, BECN-1 and ATG8 pathway in human hepatoma cells. Int J Oncol. 2013;43(1):338–48.
Clifford RJ, Kaplan JH. Human breast tumor cells are more resistant to cardiac glycoside toxicity than non-tumorigenic breast cells. PLoS ONE. 2013;8(12):e84306.
Laursen M, Gregersen JL, Yatime L, Nissen P, Fedosova NU. Structures and characterization of digoxin- and bufalin-bound Na+, K+-ATPase compared with the ouabain-bound complex. Proc Natl Acad Sci USA. 2015;112(6):1755–60.
Lee S, Lee Y, Choi YJ, Han KS, Chung HW. Cyto-/genotoxic effects of the ethanol extract of Chan Su, a traditional Chinese medicine, in human cancer cell lines. J Ethnopharmacol. 2014;152(2):372–6.
Okada M, Suga T, et al. Pharmacology of the principles isolated from Senso (Ch’an Su) the dried venom of the Chinese toad (IV). Asian Med J. 1960;3(4):155–60.
Wang FZ, Gong H. Pharmacological study and clinical use of ChanSu. Forum Tradit Chin Med. 2003;18:50–2.
Liu M, Feng LX, Sun P, Liu W, Wu WY, Jiang BH, Yang M, Hu LH, Guo DA, Liu X. A novel bufalin derivative exhibited stronger apoptosis-inducing effect than bufalin in A549 lung cancer cells and lower acute toxicity in mice. PLoS ONE. 2016;11(7):e0159789.
Chan WY, Ng TB, Yeung HW. Examination for toxicity of a Chinese drug, the toad glandular secretory product Chan su, in pregnant mice and embryos. Biol Neonate. 1995;67(5):376–80.
Chen HT, Sun D, Peng YC, Kao PH, Wu YL. Novel augmentation by bufalin of protein kinase C-induced cyclooxygenase-2 and IL-8 production in human breast cancer cells. Innate Immun. 2017;23(1):54–66.
Han KQ, Huang G, Gu W, Su YH, Huang XQ, Ling CQ. Anti-tumor activities and apoptosis-regulated mechanisms of bufalin on the orthotopic transplantation tumor model of human hepatocellular carcinoma in nude mice. World J Gastroenterol. 2007;13(24):3374–9.
Chen Y, Li M, Li Z, Gao P, Zhou X, Zhang J. Bufalin induces apoptosis in the U2OS human osteosarcoma cell line via triggering the mitochondrial pathway. Mol Med Rep. 2016;13(1):817–22.
Ding DW, Zhang YH, Huang XE, An Q, Zhang X. Bufalin induces mitochondrial pathway-mediated apoptosis in lung adenocarcinoma cells. Asian Pac J Cancer Prev. 2014;15(23):10495–500.
Efuet ET, Ding XP, Cartwright C, Pan Y, Cohen L, Yang P. Huachansu mediates cell death in non-Hodgkin’s lymphoma by induction of caspase-3 and inhibition of MAP kinase. Int J Oncol. 2015;47(2):592–600.
Hong SH, Choi YH. Bufalin induces apoptosis through activation of both the intrinsic and extrinsic pathways in human bladder cancer cells. Oncol Rep. 2012;27(1):114–20.
Hsiao YP, Yu CS, Yu CC, Yang JS, Chiang JH, Lu CC, Huang HY, Tang NY, Yang JH, Huang AC, et al. Triggering apoptotic death of human malignant melanoma a375.s2 cells by bufalin: involvement of caspase cascade-dependent and independent mitochondrial signaling pathways. Evid Based Complement Alternat Med. 2012;2012:591241.
Jiang L, Zhao MN, Liu TY, Wu XS, Weng H, Ding Q, Shu YJ, Bao RF, Li ML, Mu JS, et al. Bufalin induces cell cycle arrest and apoptosis in gallbladder carcinoma cells. Tumour Biol. 2014;35(11):10931–41.
Jiang Y, Zhang Y, Luan J, Duan H, Zhang F, Yagasaki K, Zhang G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology. 2010;62(6):573–83.
Li M, Yu X, Guo H, Sun L, Wang A, Liu Q, Wang X, Li J. Bufalin exerts antitumor effects by inducing cell cycle arrest and triggering apoptosis in pancreatic cancer cells. Tumour Biol. 2014;35(3):2461–71.
Masuda Y, Kawazoe N, Nakajo S, Yoshida T, Kuroiwa Y, Nakaya K. Bufalin induces apoptosis and influences the expression of apoptosis-related genes in human leukemia cells. Leuk Res. 1995;19(8):549–56.
Miao Q, Bi LL, Li X, Miao S, Zhang J, Zhang S, Yang Q, Xie YH, Zhang J, Wang SW. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 cells: roles of apoptosis and autophagy. Int J Mol Sci. 2013;14(1):1370–82.
Sun L, Chen T, Wang X, Chen Y, Wei X. Bufalin induces reactive oxygen species dependent bax translocation and apoptosis in ASTC-a-1 cells. Evid Based Complement Alternat Med. 2011;2011:249090.
Wang D, Bi Z. Bufalin inhibited the growth of human osteosarcoma MG-63 cells via down-regulation of Bcl-2/Bax and triggering of the mitochondrial pathway. Tumour Biol. 2014;35(5):4885–90.
Watabe M, Kawazoe N, Masuda Y, Nakajo S, Nakaya K. Bcl-2 protein inhibits bufalin-induced apoptosis through inhibition of mitogen-activated protein kinase activation in human leukemia U937 cells. Cancer Res. 1997;57(15):3097–100.
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu HR, Li Q. Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: new hope for cancer patients. Asian Pac J Cancer Prev. 2012;13(11):5339–43.
Arnaud-Batista FJ, Costa GT, Oliveira IM, Costa PP, Santos CF, Fonteles MC, Uchoa DE, Silveira ER, Cardi BA, Carvalho KM, et al. Natriuretic effect of bufalin in isolated rat kidneys involves activation of the Na+–K+-ATPase-Src kinase pathway. Am J Physiol Renal Physiol. 2012;302(8):F959–66.
Song X, Zhang C, Zhao M, Chen H, Liu X, Chen J, Lonard DM, Qin L, Xu J, Wang X, et al. Steroid receptor coactivator-3 (SRC-3/AIB1) as a novel therapeutic target in triple negative breast cancer and its inhibition with a phospho-bufalin prodrug. PLoS ONE. 2015;10(10):e0140011.
Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, Wang J, Qi R, Matzuk AJ, Song X, Madoux F, et al. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 2014;74(5):1506–17.
Calderon-Montano JM, Burgos-Moron E, Orta ML, Garcia-Dominguez I, Maldonado-Navas D, Lopez-Lazaro M. Bufalin is a steroid receptor coactivator inhibitor-letter. Cancer Res. 2015;75(6):1156.
Lonard DM, Xu J, O’Malley BW. Bufalin is a steroid receptor coactivator inhibitor-response. Cancer Res. 2015;75(6):1157.
Xie CM, Chan WY, Yu S, Zhao J, Cheng CH. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic Biol Med. 2011;51(7):1365–75.
Gao Y, Li HX, Xu LT, Wang P, Xu LY, Cohen L, Yang PY, Gu K, Meng ZQ. Bufalin enhances the anti-proliferative effect of sorafenib on human hepatocellular carcinoma cells through downregulation of ERK. Mol Biol Rep. 2012;39(2):1683–9.
Wang H, Zhang C, Chi H, Meng Z. Synergistic anticancer effects of bufalin and sorafenib by regulating apoptosis associated proteins. Mol Med Rep. 2018;17(6):8101–10.
Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213–9.
Wang H, Zhang C, Chi H, Meng Z. Synergistic anti-hepatoma effect of bufalin combined with sorafenib via mediating the tumor vascular microenvironment by targeting mTOR/VEGF signaling. Int J Oncol. 2018;52(6):2051–60.
Wang H, Zhang C, Ning Z, Xu L, Zhu X, Meng Z. Bufalin enhances anti-angiogenic effect of sorafenib via AKT/VEGF signaling. Int J Oncol. 2016;48(3):1229–41.
Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34.
Gai JQ, Sheng X, Qin JM, Sun K, Zhao W, Ni L. The effect and mechanism of bufalin on regulating hepatocellular carcinoma cell invasion and metastasis via Wnt/beta-catenin signaling pathway. Int J Oncol. 2016;48(1):338–48.
Sheng X, Sun X, Sun K, Sui H, Qin J, Li Q. Inhibitory effect of bufalin combined with Hedgehog signaling pathway inhibitors on proliferation and invasion and metastasis of liver cancer cells. Int J Oncol. 2016;49(4):1513–24.
Chen YY, Lu HF, Hsu SC, Kuo CL, Chang SJ, Lin JJ, Wu PP, Liu JY, Lee CH, Chung JG, et al. Bufalin inhibits migration and invasion in human hepatocellular carcinoma SK-Hep1 cells through the inhibitions of NF-kB and matrix metalloproteinase-2/-9-signaling pathways. Environ Toxicol. 2015;30(1):74–82.
Chueh FS, Chen YY, Huang AC, Ho HC, Liao CL, Yang JS, Kuo CL, Chung JG. Bufalin-inhibited migration and invasion in human osteosarcoma U-2 OS cells is carried out by suppression of the matrix metalloproteinase-2, ERK, and JNK signaling pathways. Environ Toxicol. 2014;29(1):21–9.
Wu SH, Hsiao YT, Kuo CL, Yu FS, Hsu SC, Wu PP, Chen JC, Hsia TC, Liu HC, Hsu WH, et al. Bufalin inhibits NCI-H460 human lung cancer cell metastasis in vitro by inhibiting MAPKs, MMPs, and NF-kappaB pathways. Am J Chin Med. 2015;43(6):1247–64.
Qiu DZ, Zhang ZJ, Wu WZ, Yang YK. Bufalin, a component in Chansu, inhibits proliferation and invasion of hepatocellular carcinoma cells. BMC Complement Altern Med. 2013;13:185.
Zhang ZJ, Yang YK, Wu WZ. Bufalin attenuates the stage and metastatic potential of hepatocellular carcinoma in nude mice. J Transl Med. 2014;12:57.
Yu Z, Feng H, Sun X, Zhuo Y, Li M, Zhou Z, Huang L, Jiang Y, Zhu X, Zhang X, et al. Bufalin suppresses hepatocarcinogenesis by targeting beta-catenin/TCF signaling via cell cycle-related kinase. Sci Rep. 2018;8(1):3891.
Huang AC, Yang MD, Hsiao YT, Lin TS, Ma YS, Peng SF, Hsia TC, Cheng YD, Kuo CL, Chung JG. Bufalin inhibits gefitinib resistant NCI-H460 human lung cancer cell migration and invasion in vitro. J Ethnopharmacol. 2016;194:1043–50.
Kang X, Lu P, Cui Y, Wang Y, Zhang Q, Gong Y, Xu Z. Bufalin reverses hepatocyte growth factor-induced resistance to afatinib in H1975 lung cancer cells. Zhonghua Zhong Liu Za Zhi. 2015;37(7):490–6.
Wang H, Zhang C, Xu L, Zang K, Ning Z, Jiang F, Chi H, Zhu X, Meng Z. Bufalin suppresses hepatocellular carcinoma invasion and metastasis by targeting HIF-1alpha via the PI3K/AKT/mTOR pathway. Oncotarget. 2016;7(15):20193–208.
Wang J, Cai H, Xia Y, Wang S, Xing L, Chen C, Zhang Y, Xu J, Yin P, Jiang Y, et al. Bufalin inhibits gastric cancer invasion and metastasis by down-regulating Wnt/ASCL2 expression. Oncotarget. 2018;9(34):23320–33.
Wang J, Xia Y, Zuo Q, Chen T. Molecular mechanisms underlying the antimetastatic activity of bufalin. Mol Clin Oncol. 2018;8(5):631–6.
Zhao L, Liu S, Che X, Hou K, Ma Y, Li C, Wen T, Fan Y, Hu X, Liu Y, et al. Bufalin inhibits TGF-beta-induced epithelial-to-mesenchymal transition and migration in human lung cancer A549 cells by downregulating TGF-beta receptors. Int J Mol Med. 2015;36(3):645–52.
Liu F, Tong D, Li H, Liu M, Li J, Wang Z, Cheng X. Bufalin enhances antitumor effect of paclitaxel on cervical tumorigenesis via inhibiting the integrin alpha2/beta5/FAK signaling pathway. Oncotarget. 2016;7(8):8896–907.
Xie J, Lin W, Huang L, Xu N, Xu A, Chen B, Watanabe M, Liu C, Huang P. Bufalin suppresses the proliferation and metastasis of renal cell carcinoma by inhibiting the PI3K/Akt/mTOR signaling pathway. Oncol Lett. 2018;16(3):3867–73.
Wang H, Ning Z, Li Y, Zhu X, Meng Z. Bufalin suppresses cancer stem-like cells in gemcitabine-resistant pancreatic cancer cells via Hedgehog signaling. Mol Med Rep. 2016;14(3):1907–14.
Ji D, Liang Z, Liu G, Zhao G, Fang J. Bufalin attenuates cancer-induced pain and bone destruction in a model of bone cancer. Naunyn Schmiedebergs Arch Pharmacol. 2017;390(12):1211–9.
Ozdemir A, Simay YD, Ibisoglu B, Yaren B, Bulbul D, Ark M. Cardiac glycoside-induced cell death and Rho/Rho kinase pathway: implication of different regulation in cancer cell lines. Steroids. 2016;109:29–43.
Li H, Hu S, Pang Y, Li M, Chen L, Liu F, Liu M, Wang Z, Cheng X. Bufalin inhibits glycolysis-induced cell growth and proliferation through the suppression of Integrin beta2/FAK signaling pathway in ovarian cancer. Am J Cancer Res. 2018;8(7):1288–96.
Xie XB, Yin JQ, Wen LL, Gao ZH, Zou CY, Wang J, Huang G, Tang QL, Colombo C, He WL, et al. Critical role of heat shock protein 27 in bufalin-induced apoptosis in human osteosarcomas: a proteomic-based research. PLoS ONE. 2012;7(10):e47375.
Xie XB, Wen LL, Yin JQ, Liao HY, Zou CY, Wang B, Huang G, Shen JN. Proteomics research of bufalin-induced apoptosis in osteosarcoma cell lines. Zhongguo Zhong Yao Za Zhi. 2014;39(14):2739–43.
Zhang DM, Feng LX, Liu M, Jin WH, Luo J, Nie AY, Zhou Y, Li Y, Wu WY, Jiang BH, et al. Possible target-related proteins and signal network of bufalin in A549 cells suggested by both iTRAQ-based and label-free proteomic analysis. Proteomics. 2016;16(6):935–45.
Wu SH, Hsiao YT, Chen JC, Lin JH, Hsu SC, Hsia TC, Yang ST, Hsu WH, Chung JG. Bufalin alters gene expressions associated DNA damage, cell cycle, and apoptosis in human lung cancer NCI-H460 cells in vitro. Molecules. 2014;19(5):6047–57.
Fell DA. Beyond genomics. Trends Genet. 2001;17(12):680–2.
Wu XY, Tian F, Su MH, Wu M, Huang Y, Hu LH, Jin L, Zhu XJ. BF211, a derivative of bufalin, enhances the cytocidal effects in multiple myeloma cells by inhibiting the IL-6/JAK2/STAT3 pathway. Int Immunopharmacol. 2018;64:24–32.
Sun P, Feng LX, Zhang DM, Liu M, Liu W, Mi T, Wu WY, Jiang BH, Yang M, Hu LH, et al. Bufalin derivative BF211 inhibits proteasome activity in human lung cancer cells in vitro by inhibiting beta1 subunit expression and disrupting proteasome assembly. Acta Pharmacol Sin. 2016;37(7):908–18.
Hu Q, Liang B, Sun Y, Guo XL, Bao YJ, Xie DH, Zhou M, Duan YR, Yin PH, Peng ZH. Preparation of bufalin-loaded pluronic polyetherimide nanoparticles, cellular uptake, distribution, and effect on colorectal cancer. Int J Nanomed. 2014;9:4035–41.
Yang Z, Liu J, Huang Q, Zhang Z, Zhang J, Pan Y, Yang Y, Cheng D. Radiosynthesis and pharmacokinetics of [(18)F]fluoroethyl bufalin in hepatocellular carcinoma-bearing mice. Onco Targets Ther. 2017;10:329–38.
Zou A, Zhao X, Handge UA, Garamus VM, Willumeit-Romer R, Yin P. Folate receptor targeted bufalin/beta-cyclodextrin supramolecular inclusion complex for enhanced solubility and anti-tumor efficiency of bufalin. Mater Sci Eng C Mater Biol Appl. 2017;78:609–18.
Chen Q, Liu J. Transferrin and folic acid co-modified bufalin-loaded nanoliposomes: preparation, characterization, and application in anticancer activity. Int J Nanomed. 2018;13:6009–18.
Yuan J, Zhou X, Cao W, Bi L, Zhang Y, Yang Q, Wang S. Improved antitumor efficacy and pharmacokinetics of bufalin via PEGylated liposomes. Nanoscale Res Lett. 2017;12(1):585.
Li Y, Yuan J, Yang Q, Cao W, Zhou X, Xie Y, Tu H, Zhang Y, Wang S. Immunoliposome co-delivery of bufalin and anti-CD40 antibody adjuvant induces synergetic therapeutic efficacy against melanoma. Int J Nanomed. 2014;9:5683–700.
Shi XJ, Qiu YY, Yu H, Liu C, Yuan YX, Yin PH, Liu T. Increasing the anticancer performance of bufalin (BUF) by introducing an endosome-escaping polymer and tumor-targeting peptide in the design of a polymeric prodrug. Colloids Surf B Biointerfaces. 2018;166:224–34.
Liu T, Jia T, Yuan X, Liu C, Sun J, Ni Z, Xu J, Wang X, Yuan Y. Development of octreotide-conjugated polymeric prodrug of bufalin for targeted delivery to somatostatin receptor 2 overexpressing breast cancer in vitro and in vivo. Int J Nanomed. 2016;11:2235–50.
Liu T, Yuan X, Jia T, Liu C, Ni Z, Qin Z, Yuan Y. Polymeric prodrug of bufalin for increasing solubility and stability: synthesis and anticancer study in vitro and in vivo. Int J Pharm. 2016;506(1–2):382–93.
Zhang H, Huang N, Yang G, Lin Q, Su Y. Bufalin-loaded bovine serum albumin nanoparticles demonstrated improved anti-tumor activity against hepatocellular carcinoma: preparation, characterization, pharmacokinetics and tissue distribution. Oncotarget. 2017;8(38):63311–23.
Liu Y, Wang P, Sun C, Zhao J, Du Y, Shi F, Feng N. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. Int J Pharm. 2011;419(1–2):260–5.
Tian X, Yin H, Zhang S, Luo Y, Xu K, Ma P, Sui C, Meng F, Liu Y, Jiang Y, et al. Bufalin loaded biotinylated chitosan nanoparticles: an efficient drug delivery system for targeted chemotherapy against breast carcinoma. Eur J Pharm Biopharm. 2014;87(3):445–53.
Yin P, Wang Y, Qiu Y, Hou L, Liu X, Qin J, Duan Y, Liu P, Qiu M, Li Q. Bufalin-loaded mPEG-PLGA-PLL-cRGD nanoparticles: preparation, cellular uptake, tissue distribution, and anticancer activity. Int J Nanomed. 2012;7:3961–9.
Zhang HQ, Yin ZF, Sheng JY, Jiang ZQ, Wu BY, Su YH. A comparison study of pharmacokinetics between bufalin-loaded bovine serum albumin nanoparticles and bufalin in rats. Zhong Xi Yi Jie He Xue Bao. 2012;10(6):674–80.
Bick RJ, Poindexter BJ, Sweney RR, Dasgupta A. Effects of Chan Su, a traditional Chinese medicine, on the calcium transients of isolated cardiomyocytes: cardiotoxicity due to more than Na, K-ATPase blocking. Life Sci. 2002;72(6):699–709.
Koh CH, Wu J, Chung YY, Liu Z, Zhang RR, Chong K, Korzh V, Ting S, Oh S, Shim W, et al. Electronic supplementary material Identification of a Na(+)/K(+)-ATPase inhibition-independent proarrhythmic ionic mechanisms of cardiac glycosides. Sci Rep. 2017;7(1):2465.
Nesher M, Shpolansky U, Viola N, Dvela M, Buzaglo N, Cohen Ben-Ami H, Rosen H, Lichtstein D. Ouabain attenuates cardiotoxicity induced by other cardiac steroids. Br J Pharmacol. 2010;160(2):346–54.
Zhao M, Liu Y, Jin B, Hou K. Inhibition effect of ethanol extract of Chinese toad venom on H22 ascites and solid tumors and its toxicity in mice. J Qilu Oncol. 2005;22:1705–9.
Zhao M, Liu Y, Hou K, Zhu Z, Tian X. Inhibition effect of ethanol extract of toad venom on S180 ascites and solid tumors and its toxicity in mice. Shanxi Med J. 2006;03:189–92.
Zhao M, Liu Y, Hou K. Inhibition effect of ethanol extract of toad venom on Ehrlich’s ascites carcinomas and its toxicity in mice. Shanxi Med J. 2006;06:479–82.
Zhu LB, Liu QY. Clinical observation of cinobufacin combined with cisplatin through intrapericardial infusion in treatment of malignant pericardial effusion. Chin J Med Guide. 2009;09:1525–6.
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo N, Li XK, Tang W. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends. 2010;4(6):297–307.
Wang Z, Qi F, Cui Y, Zhao L, Sun X, Tang W, Cai P. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics. Biosci Trends. 2018;12(3):220–39.
Lin Y, Wang Z, Wang Z, Gan P. Clinical observation on cinobufotalin tablets combined with XELOX regimen in chemotherapy after colorectal cancer surgery. Chin Med Mod Distance Educ China. 2017;22:107–9.
Meng Z, Liu F, Shen Y, Yang P, Cohen L, Huo Y, Zhao Q, Ng CS, Chang DZ, Garrett CR. A randomized phase II study of gemcitabine (G) plus the cardiac glycoside huachansu (H) in the treatment of patients with locally advanced (LAPC) or metastatic pancreatic cancer (MPC). J Clin Oncol. 2011;29:4127.
Zhakeer Z, Hadeer M, Tuerxun Z, Tuerxun K. Bufalin inhibits the inflammatory effects in asthmatic mice through the suppression of nuclear factor-kappa B activity. Pharmacology. 2017;99(3–4):179–87.
Chang YW, Zhao YF, Cao YL, Gu W, Pang J, Zhan HS. Bufalin exerts inhibitory effects on IL-1beta-mediated proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2014;37(5):1552–9.
Rong X, Ni W, Liu Y, Wen J, Qian C, Sun L, Wang J. Bufalin, a bioactive component of the Chinese medicine chansu, inhibits inflammation and invasion of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2014;37(4):1050–8.
Xie S, Spelmink L, Codemo M, Subramanian K, Putsep K, Henriques-Normark B, Olliver M. Cinobufagin modulates human innate immune responses and triggers antibacterial activity. PLoS ONE. 2016;11(8):e0160734.
Wen L, Huang Y, Xie X, Huang W, Yin J, Lin W, Jia Q, Zeng W. Anti-inflammatory and antinociceptive activities of bufalin in rodents. Mediat Inflamm. 2014;2014:171839.
Tongkao-On W, Gordon-Thomson C, Dixon KM, Song EJ, Luu T, Carter SE, Sequeira VB, Reeve VE, Mason RS. Novel vitamin D compounds and skin cancer prevention. Dermatoendocrinol. 2013;5(1):20–33.
Lan YL, Wang X, Lou JC, Xing JS, Yu ZL, Wang H, Zou S, Ma X, Zhang B. Bufalin inhibits glioblastoma growth by promoting proteasomal degradation of the Na(+)/K(+)-ATPase alpha1 subunit. Biomed Pharmacother. 2018;103:204–15.
Yan S, Qu X, Xu C, Zhu Z, Zhang L, Xu L, Song N, Teng Y, Liu Y. Down-regulation of Cbl-b by bufalin results in up-regulation of DR4/DR5 and sensitization of TRAIL-induced apoptosis in breast cancer cells. J Cancer Res Clin Oncol. 2012;138(8):1279–89.
Wang Q, Li C, Zhu Z, Teng Y, Che X, Wang Y, Ma Y, Wang Y, Zheng H, Liu Y, et al. miR-155-5p antagonizes the apoptotic effect of bufalin in triple-negative breast cancer cells. Anticancer Drugs. 2016;27(1):9–16.
Pan S, Wang Y, Feng L, Fan C, Guo D, Liu X, Fan J. Study on proteomics of Hela cell apoptosis in bufalin-induced human cervical carcinoma. Zhongguo Zhong Yao Za Zhi. 2012;37(13):1998–2004.
Zhu Z, Li E, Liu Y, Gao Y, Sun H, Ma G, Wang Z, Liu X, Wang Q, Qu X, et al. Inhibition of Jak-STAT3 pathway enhances bufalin-induced apoptosis in colon cancer SW620 cells. World J Surg Oncol. 2012;10:228.
Zhang N, Xie Y, Tai Y, Gao Y, Guo W, Yu W, Li J, Feng X, Hao J, Gao Y, et al. Bufalin inhibits hTERT expression and colorectal cancer cell growth by targeting CPSF4. Cell Physiol Biochem. 2016;40(6):1559–69.
Qiu YY, Hu Q, Tang QF, Feng W, Hu SJ, Liang B, Peng W, Yin PH. MicroRNA-497 and bufalin act synergistically to inhibit colorectal cancer metastasis. Tumour Biol. 2014;35(3):2599–606.
Wang J, Chen C, Wang S, Zhang Y, Yin P, Gao Z, Xu J, Feng D, Zuo Q, Zhao R, et al. Bufalin inhibits HCT116 colon cancer cells and its orthotopic xenograft tumor in mice model through genes related to apoptotic and PTEN/AKT pathways. Gastroenterol Res Pract. 2015;2015:457193.
Takai N, Ueda T, Nishida M, Nasu K, Narahara H. Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int J Mol Med. 2008;21(5):637–43.
Lv J, Lin S, Peng P, Cai C, Deng J, Wang M, Li X, Lin R, Lin Y, Fang A, et al. Arenobufagin activates p53 to trigger esophageal squamous cell carcinoma cell apoptosis in vitro and in vivo. Onco Targets Ther. 2017;10:1261–7.
Li A, Qu X, Li Z, Qu J, Song N, Ma Y, Liu Y. Secreted protein acidic and rich in cysteine antagonizes bufalin-induced apoptosis in gastric cancer cells. Mol Med Rep. 2015;12(2):2926–32.
Tsai SC, Yang JS, Peng SF, Lu CC, Chiang JH, Chung JG, Lin MW, Lin JK, Amagaya S, Wai-Shan Chung C, et al. Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell death in SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol. 2012;41(4):1431–42.
Zhai X, Lu J, Wang Y, Fang F, Li B, Gu W. Reversal effect of bufalin on multidrug resistance in K562/VCR vincristine-resistant leukemia cell line. J Tradit Chin Med. 2014;34(6):678–83.
Wang LP, Zhao YN, Sun X, Gao RL. Effects of bufalin on up-regulating methylation of Wilm’s tumor 1 gene in human erythroid leukemic cells. Chin J Integr Med. 2017;23(4):288–94.
Zhu Z, Sun H, Ma G, Wang Z, Li E, Liu Y, Liu Y. Bufalin induces lung cancer cell apoptosis via the inhibition of PI3K/Akt pathway. Int J Mol Sci. 2012;13(2):2025–35.
Tsai SC, Lu CC, Lee CY, Lin YC, Chung JG, Kuo SC, Amagaya S, Chen FN, Chen MY, Chan SF, et al. AKT serine/threonine protein kinase modulates bufalin-triggered intrinsic pathway of apoptosis in CAL 27 human oral cancer cells. Int J Oncol. 2012;41(5):1683–92.
Tian X, Dai S, Sun J, Jiang S, Sui C, Meng F, Li Y, Fu L, Jiang T, Wang Y, et al. Bufalin induces mitochondria-dependent apoptosis in pancreatic and oral cancer cells by downregulating hTERT expression via activation of the JNK/p38 pathway. Evid Based Complement Alternat Med. 2015;2015:546210.
Zhang J, Sha J, Zhou Y, Han K, Wang Y, Su Y, Yin X, Hu H, Yao Y. Bufalin inhibits proliferation and induces apoptosis in osteosarcoma cells by downregulating MicroRNA-221. Evid Based Complement Alternat Med. 2016;2016:7319464.
Yin JQ, Shen JN, Su WW, Wang J, Huang G, Jin S, Guo QC, Zou CY, Li HM, Li FB. Bufalin induces apoptosis in human osteosarcoma U-2OS and U-2OS methotrexate300-resistant cell lines. Acta Pharmacol Sin. 2007;28(5):712–20.
Chou HY, Chueh FS, Ma YS, Wu RS, Liao CL, Chu YL, Fan MJ, Huang WW, Chung JG. Bufalin induced apoptosis in SCC4 human tongue cancer cells by decreasing Bcl2 and increasing Bax expression via the mitochondria dependent pathway. Mol Med Rep. 2017;16(6):7959–66.
Yuan B, He J, Kisoh K, Hayashi H, Tanaka S, Si N, Zhao HY, Hirano T, Bian B, Takagi N. Effects of active bufadienolide compounds on human cancer cells and CD4+ CD25+ Foxp3+ regulatory T cells in mitogen-activated human peripheral blood mononuclear cells. Oncol Rep. 2016;36(3):1377–84.
Yuan ZT, Shi XJ, Yuan YX, Qiu YY, Zou Y, Liu C, Yu H, He X, Xu K, Yin PH. Bufalin reverses ABCB1-mediated drug resistance in colorectal cancer. Oncotarget. 2017;8(29):48012–26.
Sun J, Xu K, Qiu Y, Gao H, Xu J, Tang Q, Yin P. Bufalin reverses acquired drug resistance by inhibiting stemness in colorectal cancer cells. Oncol Rep. 2017;38(3):1420–30.
Xiang RF, Wang Y, Zhang N, Xu WB, Cao Y, Tong J, Li JM, Wu YL, Yan H. MK2206 enhances the cytocidal effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR pathway. Cell Death Dis. 2017;8(5):e2776.
Chen Y, Guo Q, Zhang B, Kang M, Xie Q, Wu Y. Bufalin enhances the antitumor effect of gemcitabine in pancreatic cancer. Oncol Lett. 2012;4(4):792–8.
Liu X, Xiao XY, Shou QY, Yan JF, Chen L, Fu HY, Wang JC. Bufalin inhibits pancreatic cancer by inducing cell cycle arrest via the c-Myc/NF-kappaB pathway. J Ethnopharmacol. 2016;193:538–45.
Wu SH, Bau DT, Hsiao YT, Lu KW, Hsia TC, Lien JC, Ko YC, Hsu WH, Yang ST, Huang YP, et al. Bufalin induces apoptosis in vitro and has antitumor activity against human lung cancer xenografts in vivo. Environ Toxicol. 2017;32(4):1305–17.
Hong SH, Kim GY, Chang YC, Moon SK, Kim WJ, Choi YH. Bufalin prevents the migration and invasion of T24 bladder carcinoma cells through the inactivation of matrix metalloproteinases and modulation of tight junctions. Int J Oncol. 2013;42(1):277–86.
Kang KH, Han MH, Jeong JW, Park C, Lee SH, Lee HW, Hong SH, Choi YH, Hong SH. Bufalin sensitizes human bladder carcinoma cells to TRAIL-mediated apoptosis. Oncol Lett. 2017;14(1):853–9.
Dong Y, Yin S, Li J, Jiang C, Ye M, Hu H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis. 2011;16(4):394–403.
Yan S, Qu X, Xu L, Che X, Ma Y, Zhang L, Teng Y, Zou H, Liu Y. Bufalin enhances TRAIL-induced apoptosis by redistributing death receptors in lipid rafts in breast cancer cells. Anticancer Drugs. 2014;25(6):683–9.
Zou Z, Luo X, Nie P, Wu B, Zhang T, Wei Y, Wang W, Geng G, Jiang J, Mi Y. Inhibition of SRC-3 enhances sensitivity of human cancer cells to histone deacetylase inhibitors. Biochem Biophys Res Commun. 2016;478(1):227–33.
Xie CM, Liu XY, Yu S, Cheng CH. Cardiac glycosides block cancer growth through HIF-1alpha- and NF-kappaB-mediated Plk1. Carcinogenesis. 2013;34(8):1870–80.
Zhao H, Zhao D, Tan G, Liu Y, Zhuang L, Liu T. Bufalin promotes apoptosis of gastric cancer by down-regulation of miR-298 targeting bax. Int J Clin Exp Med. 2015;8(3):3420–8.
Zhao H, Zhao D, Jin H, Li H, Yang X, Zhuang L, Liu T. Bufalin reverses intrinsic and acquired drug resistance to cisplatin through the AKT signaling pathway in gastric cancer cells. Mol Med Rep. 2016;14(2):1817–22.
Zhao H, Li Q, Pang J, Jin H, Li H, Yang X. Blocking autophagy enhances the pro-apoptotic effect of bufalin on human gastric cancer cells through endoplasmic reticulum stress. Biol Open. 2017;6(10):1416–22.
Shen S, Zhang Y, Wang Z, Liu R, Gong X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int J Biol Sci. 2014;10(2):212–24.
Liu T, Wu C, Weng G, Zhao Z, He X, Fu C, Sui Z, Huang SX. Bufalin inhibits cellular proliferation and cancer stem cell-like phenotypes via upregulation of MiR-203 in glioma. Cell Physiol Biochem. 2017;44(2):671–81.
Qi F, Inagaki Y, Gao B, Cui X, Xu H, Kokudo N, Li A, Tang W. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011;102(5):951–8.
Hu F, Han J, Zhai B, Ming X, Zhuang L, Liu Y, Pan S, Liu T. Blocking autophagy enhances the apoptosis effect of bufalin on human hepatocellular carcinoma cells through endoplasmic reticulum stress and JNK activation. Apoptosis. 2014;19(1):210–23.
Gu W, Liu L, Fang FF, Huang F, Cheng BB, Li B. Reversal effect of bufalin on multidrug resistance in human hepatocellular carcinoma BEL-7402/5-FU cells. Oncol Rep. 2014;31(1):216–22.
Zhai B, Hu F, Yan H, Zhao D, Jin X, Fang T, Pan S, Sun X, Xu L. Bufalin reverses resistance to sorafenib by inhibiting Akt activation in hepatocellular carcinoma: the role of endoplasmic reticulum stress. PLoS ONE. 2015;10(9):e0138485.
Liu M, Feng LX, Sun P, Liu W, Mi T, Lei M, Wu W, Jiang B, Yang M, Hu L, et al. Knockdown of apolipoprotein E enhanced sensitivity of Hep3B cells to cardiac steroids via regulating Na+/K+-ATPase signalosome. Mol Cancer Ther. 2016;15(12):2955–65.
Xia J, Inagaki Y, Gao J, Qi F, Song P, Han G, Sawakami T, Gao B, Luo C, Kokudo N, et al. Combination of cinobufacini and doxorubicin increases apoptosis of hepatocellular carcinoma cells through the Fas- and mitochondria-mediated pathways. Am J Chin Med. 2017;45(7):1537–56.
Amano Y, Cho Y, Matsunawa M, Komiyama K, Makishima M. Increased nuclear expression and transactivation of vitamin D receptor by the cardiotonic steroid bufalin in human myeloid leukemia cells. J Steroid Biochem Mol Biol. 2009;114(3–5):144–51.
Zhu Z, Li E, Liu Y, Gao Y, Sun H, Wang Y, Wang Z, Liu X, Wang Q, Liu Y. Bufalin induces the apoptosis of acute promyelocytic leukemia cells via the downregulation of survivin expression. Acta Haematol. 2012;128(3):144–50.
Kang XH, Xu ZY, Gong YB, Wang LF, Wang ZQ, Xu L, Cao F, Liao MJ. Bufalin reverses HGF-induced resistance to EGFR-TKIs in EGFR mutant lung cancer cells via blockage of Met/PI3k/Akt pathway and induction of apoptosis. Evid Based Complement Alternat Med. 2013;2013:243859.
Kang XH, Zhang JH, Zhang QQ, Cui YH, Wang Y, Kou WZ, Miao ZH, Lu P, Wang LF, Xu ZY, et al. Degradation of Mcl-1 through GSK-3beta activation regulates apoptosis induced by bufalin in non-small cell lung cancer H1975 cells. Cell Physiol Biochem. 2017;41(5):2067–76.
Zhang C, Fu L. Effects of bufalin combined with doxorubicin on the proliferation and apoptosis of human lung cancer cell line A549 in vitro. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(7):762–8.
Huang H, Cao Y, Wei W, Liu W, Lu SY, Chen YB, Wang Y, Yan H, Wu YL. Targeting poly (ADP-ribose) polymerase partially contributes to bufalin-induced cell death in multiple myeloma cells. PLoS ONE. 2013;8(6):e66130.
Chang Y, Zhao Y, Zhan H, Wei X, Liu T, Zheng B. Bufalin inhibits the differentiation and proliferation of human osteosarcoma cell line hMG63-derived cancer stem cells. Tumour Biol. 2014;35(2):1075–82.
Chang Y, Zhao Y, Gu W, Cao Y, Wang S, Pang J, Shi Y. Bufalin inhibits the differentiation and proliferation of cancer stem cells derived from primary osteosarcoma cells through Mir-148a. Cell Physiol Biochem. 2015;36(3):1186–96.
Lee CH, Shih YL, Lee MH, Au MK, Chen YL, Lu HF, Chung JG. Bufalin induces apoptosis of human osteosarcoma U-2 OS cells through endoplasmic reticulum stress, caspase- and mitochondria-dependent signaling pathways. Molecules. 2017;22(3):437.
Chen H, Zhang L, Zhang L, Du J, Wang H, Wang B. MicroRNA-183 correlates cancer prognosis, regulates cancer proliferation and bufalin sensitivity in epithelial ovarian caner. Am J Transl Res. 2016;8(4):1748–55.
Yeh JY, Huang WJ, Kan SF, Wang PS. Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate. 2003;54(2):112–24.
Zhai XF, Fang FF, Liu Q, Meng YB, Guo YY, Chen Z. MiR-181a contributes to bufalin-induced apoptosis in PC-3 prostate cancer cells. BMC Complement Altern Med. 2013;13:325.
Fridman E, Lichtstein D, Rosen H. Formation of new high density glycogen-microtubule structures is induced by cardiac steroids. J Biol Chem. 2012;287(9):6518–29.
Authors’ contributions
Conceived and designed the study: C-SC, JW, JC, LC, ZC, ZM. Collected the literature, wrote the manuscript: C-SC, JW, JC, K-TK, TJ, HG, LC. Collected and drew figures: C-SC, JW, JC. Revised the manuscript: C-SC, JW, JC, TJ, HG, ZC, ZM. All authors read and approved the final manuscript.
Acknowledgements
The authors deeply appreciate Dr. Xiaoyan Zhu, Dr. Yawen Geng, and Dr. Ye Li for their advices and constructive comments for this review paper in preparation.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The work was supported by National Natural Science Foundation of China (NSFC) [Grant No. 81573753].
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cheng, Cs., Wang, J., Chen, J. et al. New therapeutic aspects of steroidal cardiac glycosides: the anticancer properties of Huachansu and its main active constituent Bufalin. Cancer Cell Int 19, 92 (2019). https://doi.org/10.1186/s12935-019-0806-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-019-0806-1