Abstract
Background
This study aimed to investigate the preventive effects of tofogliflozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, on atherosclerosis progression in type 2 diabetes (T2DM) patients without apparent cardiovascular disease (CVD) by monitoring carotid intima-media thickness (IMT).
Methods
This prospective, randomized, open-label, blinded-endpoint, multicenter, parallel-group, comparative study included 340 subjects with T2DM and no history of apparent CVD recruited at 24 clinical units. Subjects were randomly allocated to either the tofogliflozin treatment group (n = 169) or conventional treatment group using drugs other than SGLT2 inhibitors (n = 171). Primary outcomes were changes in mean and maximum common carotid IMT measured by echography during a 104-week treatment period.
Results
In a mixed-effects model for repeated measures, the mean IMT of the common carotid artery (mean-IMT-CCA), along with the right and left maximum IMT of the CCA (max-IMT-CCA), significantly declined in both the tofogliflozin (− 0.132 mm, SE 0.007; − 0.163 mm, SE 0.013; − 0.170 mm, SE 0.020, respectively) and the control group (− 0.140 mm, SE 0.006; − 0.190 mm, SE 0.012; − 0.190 mm, SE 0.020, respectively). Furthermore, the tofogliflozin and the conventional treatment group did not significantly differ in the progression of the mean-IMT-CCA (mean change (95% CI) 0.008 (− 0.009, 0.025) mm, P = 0.34), along with the right (mean change (95% CI) 0.027 (− 0.005, 0.059) mm, P = 0.10) and the left max-IMT-CCA (mean change (95% CI) 0.020 (− 0.030, 0.070), P = 0.43). Similar findings were obtained even after adjusting for traditional CV risk factors and/or administration of drugs at baseline. Relative to the control treatment effects, tofogliflozin significantly reduced the HbA1c, blood glucose level, body weight/body mass index, abdominal circumference, and systolic blood pressure, and significantly increased the HDL-C. The total and serious adverse events incidences did not significantly vary between the treatment groups.
Conclusions/interpretation
No IMT changes were observed between the tofogliflozin and the conventional treatment groups. However, tofogliflozin is a safe and effective treatment option for managing primary CVD risk factors in this population.
Clinical Trial Registration UMIN000017607 (https://www.umin.ac.jp/icdr/index.html).
Similar content being viewed by others
Background
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are antidiabetic agents that lower blood glucose levels by promoting urinary glucose excretion. Their risk of causing hypoglycemia, which is linked to increased cardiovascular (CV) events [1,2,3], is low since their mode of action is independent of insulin secretion. SGLT2 inhibitors are known to diminish various CV risk factors by reducing visceral adipose tissue, body weight, and blood pressure, improving the blood lipid profile, and generating a reno-protective effect independent of the glycemic effects [4, 5]. Because SGLT2 inhibitors have a pleiotropic antiatherogenic effect, they are expected to attenuate the progression of atherosclerosis, and therefore, to protect against CV events.
Clinical trials in patients with type 2 diabetes mellitus (T2DM) showed that SGLT2 inhibitors, such as empagliflozin and canagliflozin, significantly reduced the primary outcome, a composite of death from CV causes, nonfatal myocardial infarction, and nonfatal stroke, compared to that of placebo [6, 7]. However, although worsening heart failure was decreased, these treatments failed to reduce atherothrombotic events, such as myocardial infarction and stroke [5,6,7,8,9]. Thus, clinical evidence of the anti-atherosclerotic effect of SGLT2 inhibitors remains to be established. Furthermore, to our knowledge, only very few clinical trials have investigated whether SGLT2 inhibitors protect against atherosclerosis in subjects with T2DM but no apparent cardiovascular disease (CVD) [10].
Tofogliflozin, an SGLT2 inhibitor that has been clinically used in Japan since 2014, is associated with favorable metabolic effects, including improved glycemic control and serum lipid profile, along with decreased body weight, visceral adipose tissue, and blood pressure [11, 12]. Tofogliflozin has the highest selectivity of all clinically developed inhibitors with 2900-fold greater selectivity for SGLT2 than SGLT1 [13], which may contribute to the relatively low incidence of adverse events including hypoglycemia, compared to that of other SGLT2 inhibitors [14]. Moreover, among all SGLT2 inhibitors, tofogliflozin has the shortest half-life with a urinary excretion rate of more than 80% within 12 h after administration. Morning tofogliflozin administration reportedly reduces the risk of nocturnal hypoglycemia because its effects almost disappear by nighttime [15].
In patients with T2DM, a progressive thickening of the carotid artery intima-media is considered a CVD surrogate marker [16] used for evaluating the effects of various interventions on the progression of atherosclerosis [17,18,19,20,21,22,23,24]. Our randomized controlled trial investigated the preventive effects of tofogliflozin on the progression of intima-media thickness (IMT) in patients with apparent CVD-free T2DM.
Methods
Study design
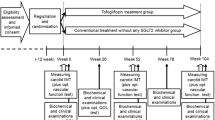
The Study of Using Tofogliflozin for Possible better Intervention against Atherosclerosis for type 2 diabetes patients (UTOPIA) trial was a multicenter prospective, randomized (1:1), open-label, blinded-endpoint (PROBE) study, as described previously [25]. This study is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), a nonprofit organization in Japan, and meets the requirements of the International Committee of Medical Journal Editors (UMIN000017607).
Study population
Japanese subjects with T2DM who periodically attended the outpatient diabetes clinics of 24 institutions in Japan (Additional file 1: Material S1) were asked to participate in this study, as described in detail previously [25]. The inclusion criteria were as follows: (1) Japanese with T2DM and inadequate glycemic control (HbA1c ≥ 6% but < 9%), along with the inability to achieve the blood glucose level stated in the Diabetes Treatment Guideline of 2014–2015 despite being on drugs—except SGLT2 inhibitors—with diet and physical therapy, on diet and physical therapy without being on drugs for at least 12 weeks, or on SGLT2 inhibitors in the past but without them for at least 12 weeks before signing the consent form, (2) without changes (including new prescriptions) in the antidiabetic, antithrombotic, antihypertensive medication, or a therapeutic agent for dyslipidemia management for at least 12 weeks before signing the consent form, (3) age 30–74 at the time of giving consent, and (4) able to provide informed consent. Furthermore, the following exclusion criteria were applied: (1) type 1 or secondary diabetes, (2) in the perioperative period or with a serious infection or injury, (3) a history of myocardial infarction, angina, stroke, or cerebral infarction, (4) severe renal dysfunction (estimated glomerular filtration rate (eGFR) of < 30 mL/min/1.73 m2) or end-stage renal failure (eGFR < 15 ml/min/1.73 m2, i.e., dialysis or renal transplantation is required), (5) serious liver functional impairment (aspartate aminotransferase ≥ 100 U/L), (6) moderate to severe heart failure (class 3 or worse based on the New York Heart Association Functional Classification), (7) urinary tract or genital infection, (8) pregnant, possibly pregnant, nursing, or planning to conceive a child, (9) history of hypersensitivity to the study drug, (10) present or past history of a malignant tumor (exceptions: patients not on medication for malignant tumor and no recurrence of the disease so far without recurrence risks during this study were allowed to participate), (11) prohibited to use tofogliflozin, (12) other ineligibility determined by an investigator.
The subjects were screened consecutively, and patients who met the above eligibility criteria were asked to participate in our study. All patients who agreed to participate were included in the study. The protocol was approved by the Osaka University Clinical Research Review Committee and the institutional review board of each participating institution in compliance with the Declaration of Helsinki and current legal regulations in Japan. Written informed consent was obtained from all participants after a full explanation of the study.
Randomization and study intervention
Patient registration was performed at the administration office of the UTOPIA trial via the internet; once enrolled, the subjects were randomly and equally assigned to a tofogliflozin treatment group or a conventional treatment group using drugs other than SGLT2 inhibitors. The randomization was performed using a dynamic balancing minimization method based on insulin use/non-use, age, and sex. Assignments were made by the electronic data capturing system using computer-generated random numbers and minimization software for group allocation. The computer programs for analyses were developed and run by biostatisticians, following the prespecified statistical analysis plan. Neither patients nor investigators were masked to treatment group assignment.
Treatment was continued to achieve the target value specified in the Japanese treatment guide for diabetes [26] (generally an HbA1c < 7.0%) in all patients. In the conventional treatment group, either the current therapy dosage was increased or a concomitant oral glucose-lowering drug (excluding any other SGLT2 inhibitor) was added 12 weeks following randomization. In the tofogliflozin group, 20 mg tofogliflozin once daily was started in addition to ongoing therapy. However, the addition of an alternative antidiabetic agent (excluding another SGLT2 inhibitor) was permitted 12 weeks after randomization. In the case of hypoglycemia, the dosage of the concomitant oral glucose-lowering drug was titrated. The use of antihyperlipidemic and antihypertensive drugs was allowed during the study.
Observation items and schedule
The study period was 104 weeks following patient registration (registration period: January to November 2016). All randomized participants were followed until the scheduled study end regardless of adherence to or discontinuation of study medication for any reason. Clinical outcomes, adherence, and adverse events were confirmed, and clinical and biochemical data were collected at 0, 26, 52, 78, and 104 weeks after randomization.
Study outcomes
Primary study outcomes were the changes in mean IMT of the common carotid artery (mean-IMT-CCA) and maximum IMT of the CCA (max-IMT-CCA) during the 104-week treatment period measured by carotid arterial echography. The most primary outcome of this study was preliminarily defined as the change in the mean derived from the left- and right-side mean-IMT-CCA values. Investigations were conducted at the beginning of the study and at 52 and 104 weeks.
Measurement of carotid IMT
Ultrasonography scans of the carotid artery based on the guideline of the Japan Society of Ultrasonics in Medicine [27] were performed by expert sonographers specifically trained to perform the prescribed study examination. To avoid inter-sonographer variability, each participant was examined by the same sonographer with the same equipment (high-resolution B-mode ultrasound scanner equipped with a high-frequency [> 7.5 MHz] linear transducer with a limit of detection of < 0.1 mm) throughout all the visits. Scanning of the extracranial CCA, the carotid sinus, and the internal carotid artery in the neck was performed bilaterally in at least three different longitudinal projections as well as transverse projections, and the site of greatest thickness, including plaque lesions, was sought along the arterial walls. The IMT was measured as the distance between two parallel echogenic lines corresponding to the vascular lumen and the adventitial layer.
To avoid inter-reader variability, all scans were electronically stored and sent to the central office (IMT Evaluation Committee, Osaka, Japan) for reading by a single experienced reader unaware of the subjects’ clinical characteristics in a random order using automated digital edge-detection software (Intimascope; MediaCross, Tokyo, Japan) [28]. The software system averaged about 200 points of IMT values in the segment 2 cm proximal to the dilation of the carotid sinus (mean-IMT-CCA). In addition, the maximum thicknesses of the intima and media layers, including the plaque lesions, in the common carotid arteries (max-IMT-CCA) were captured separately. The same systematic procedures for analyzing carotid IMT were used in our previous studies [21, 22]. Reproducibility analysis of replicate measurements in the randomly selected 20 subjects yielded absolute mean differences of 0.02 ± 0.01 and 0.01 ± 0.01 for mean-IMT-CCA and max-IMT-CCA, respectively. The intra-observer coefficients of variation for the measurements of mean-IMT-CCA and max-IMT-CCA were 1.1% and 0.7%, respectively.
Biochemical tests
Blood samples were collected after overnight fasting. Serum lipids (total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, triglycerides), HbA1c (National Glycohemoglobin Standardization Program), glucose, insulin, and creatinine were measured with standard techniques. Measurements of highly sensitive C-reactive protein were outsourced to a private laboratory (SRL Laboratory, Tokyo). Urinary albumin excretion (UAE) was measured by the improved bromocresol purple method using a spot urine sample. The estimated glomerular filtration rate (eGFR) was calculated using the following formula: eGFR (ml/min per 1.73 m2) = 194 × age − 0.287 × serum creatinine − 0.1094 (× 0.739 for females) [29].
Safety evaluation
All adverse events (AEs) were recorded during the study, as described in the Additional file 1.
Sample size
The progression of carotid IMT in diabetic patients is considered to be 0.034 ± 0.054 mm/year (mean ± standard deviation [SD]), and a 1% improvement in the HbA1c value is associated with a 0.02 mm/year improvement in IMT [30]. Therefore, during a 2-year observation period, the registration of at least 310 patients was required to obtain 90% power to detect a difference of 0.04 mm in IMT between the two treatment groups assuming an SD of 0.108 mm for individual differences, which was presumed to be common in both groups, and a 0.05 level of significance. The dropout and/or study discontinuation rate during the 2-year observation period was assumed to be 10%. According to this calculation, the target number of enrolled patients was set at 340 (170 per group) for the 2-year registration period.
Statistical analysis
All allocated participants, except those without any IMT measurements during the observation period, were analyzed regardless of adherence using an intent-to-treat approach. Analyses of the efficacy were performed on the full dataset using the intent-to-treat approach principle and secondarily using the per-protocol set. Primary analysis was performed using the mixed-effects model for repeated measures with treatment group, time (week), interactions between treatment group and time (week), age, sex, use of insulin at baseline, and baseline IMT as fixed effects; an unstructured covariate was used to model the covariance of within-subject variability. The sensitivity analysis assessed differences in delta change in IMT from baseline between two groups using analysis of covariance (ANCOVA) models that included treatment group, age, sex, baseline IMT, systolic blood pressure, and administration of statin. For the occurrence of cardiovascular events as one of the secondary outcomes, the time to onset was analyzed using a log-rank test and the Cox proportional hazard model.
Baseline and follow-up group comparisons were performed with Student’s t test or Wilcoxon rank-sum test for continuous variables and Fisher’s exact test or Chi square test for categorical variables. Changes from baseline to treatment visits were assessed with a one-sample t-test and Wilcoxon signed-rank test within the group. The frequency and proportion of patients reporting AEs were derived from each treatment group and compared between the two treatment groups using Fisher’s exact test. All statistical tests were two-sided with a 5% significance level. All analyses were performed using the SAS software version 9.4 (SAS Institute, Cary, NC).
Role of the funding source
The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study population
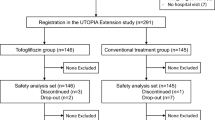
Between January 12, 2016, and November 25, 2016, 340 participants were randomly allocated to either the tofogliflozin group (n = 169) or the conventional treatment group (n = 171). After excluding 1 patient from further analysis because of no data for the primary endpoint, 169 and 170 patients of the tofogliflozin group and the conventional treatment group were included in the full analysis set, respectively. Among the study subjects, 140 of the tofogliflozin group and 146 of the conventional treatment group completed the allocated treatment regimen with the final patient visit on February 26, 2019 (Fig. 1). There were no significant differences in baseline characteristics between the two groups (Table 1).
Carotid intima-media thickness
For 104 weeks, both tofogliflozin and conventional treatment significantly reduced the mean-IMT-CCA and the right and left max-IMT-CCA values relative to the respective baseline values (Table 2). In a mixed-effects model for repeated measures, there were no significant differences in the progression in the mean-IMT-CCA and the right and left max-IMT-CCA (i.e., primary endpoints of the study) between the tofogliflozin and the conventional treatment group (Table 2). Similar findings were obtained even after adjusting the mixed-effects models for traditional CV risk factors and/or administration of drugs, including hypoglycemic agents, antihypertensive agents, statin, and antiplatelets at baseline (Table 3). Moreover, ANCOVA models that included treatment group, age, sex, use of insulin, baseline IMT, systolic blood pressure, and administration of statins produced findings that resembled those generated by the mixed-effects models (Additional file 1: Table S1).
Glucose metabolism and other parameters
Within 104 weeks, tofogliflozin treatment, but not conventional treatment, significantly reduced HbA1c and fasting blood glucose levels relative to baseline. The improvements (value at study end—value at baseline) in HbA1c (− 0.3 ± 0.8% vs. 0.1 ± 0.7%, P < 0.001) and fasting blood glucose (− 0.7 ± 1.9 mmol/l vs. 0.1 ± 1.8 mmol/l, P < 0.001) were significantly better in the tofogliflozin group than in the conventional group. The serum C-peptide level was also significantly decreased in the tofogliflozin group but not in the conventional group (Table 4).
Tofogliflozin treatment, but not conventional treatment, significantly reduced the body mass index (BMI), waist circumference, and systolic and diastolic blood pressure relative to baseline. Improvements during observation period in BMI (− 1.0 ± 1.4 kg/m2 vs. − 0.2 ± 1.8 kg/m2, P < 0.001), waist circumference (− 1.2 ± 6.0 cm vs. 1.5 ± 4.3 cm, P < 0.001), and systolic blood pressure (− 5.3 ± 16.3 mmHg vs. 0.5 ± 18.0 mmHg, P = 0.004) were significantly larger in the tofogliflozin group than in the conventional group (Table 4). Tofogliflozin treatment, but not conventional treatment, significantly increased HDL-C levels relative to baseline within 26, 52, and 78 weeks, and the HDL-C levels at these examination points were significantly improved by tofogliflozin treatment than by conventional treatment. Although the total cholesterol levels from each examination week did not significantly vary, the reductions relative to baseline values tended to be larger in the conventional group (Table 4).
The eGFRs significantly decreased in both groups during the study. UAE significantly increased in the conventional group but tended to decrease in the tofogliflozin group, and the UAE level change was significantly greater in the conventional group than in the tofogliflozin group (Table 5).
Serum adiponectin levels significantly increased during the observation period in both groups, but its increase was more significant in the tofogliflozin group than in the conventional group. However, the hsCRP and NT-proBNP levels did not significantly change during the study (Table 5).
Remarkably, over the course of the study, the DPP-4 inhibitor use was significantly higher, and after 52 weeks, the metformin use was significantly higher in the conventional group than in the tofogliflozin group (Additional file 1: Table S2). Furthermore, antihypertensive drugs, especially angiotensin II receptor blockers (ARBs), were significantly more used, and the use of lipid-lowering agents tended to be higher in the conventional group than in the tofogliflozin group during the study (Additional file 1: Table S3).
Adverse events
During the study, 168 patients, 76 in the tofogliflozin and 92 in the conventional group, developed adverse events (AEs), and 57 patients, 26 in the tofogliflozin and 31 in the conventional group, developed serious AEs. The total AE and serious AE incidences did not significantly vary between the treatment groups. Although a total 6 patients developed CV events, 3 patients in each group (hazard ratio, 0.96; 95% CI 0.21–5.03, P = 0.98), there were no CV-related deaths in either group during the follow-up period. A total of 34 hypoglycemic events, 17 in each group, were recorded (Table 6), but none of the affected patients experienced severe hypoglycemia. No significant variations in cancers, genital infections, urinary tract infections, or bone fractures were observed between the two groups, and no incidents of death, diabetic ketoacidosis, or leg amputation occurred.
Discussion
SGLT2 inhibitors, such as empagliflozin and canagliflozin, attenuated arteriosclerosis in animal models of the disease [31,32,33]. However, to date, there are no randomized controlled trials (RCT) monitoring the effect of SGLT2 inhibitors on the progression of arteriosclerotic lesions. Therefore, we conducted a PROBE study to investigate the preventive effects of a potent and selective SGLT2 inhibitor, tofogliflozin, on atherosclerosis progression in subjects with T2DM but no history of apparent CVD.
Effects of tofogliflozin on atherosclerosis
To the best of our knowledge, the UTOPIA study is the first RCT evaluating the effects of SGLT2 inhibitor on carotid IMT. We found statistically significant IMT reduction in the tofogliflozin treatment group, and while the control group also showed a statistically significant IMT reduction, there were no significant differences in the progression in IMT between the two treatment groups.
IMT is a quantitative indicator of arteriosclerosis-related changes linked to CV risk factors that lead to the development of CVDs [34] and have been validated against pathological specimens and confirmed as strong predictors of CV events [35, 36]. Changes in the IMT values are used as an alternative index of CVD that can be measured repeatedly with low-cost, low-invasive techniques [16, 36,37,38]. IMT measurements have been applied as a surrogate outcome for evaluating the effects of various drugs, including statins and antidiabetic drugs, on arteriosclerosis in numerous clinical trials [17,18,19,20,21,22,23,24].
In this study, repeated IMT measurements were performed in a blinded manner and the analyses were performed at a core laboratory to avoid bias and measurement errors between institutions: all scans were electronically stored and sent to the core laboratory for reading by a single experienced reader unaware of the patients’ clinical characteristics in a random order using automated digital edge-detection software. The same procedure for analyzing carotid IMT was used in our previous studies [21, 22]. Thus, the reliability and reproducibility of IMT measurements were certified in the current study.
Two earlier, non-randomized studies evaluated the effects of SGLT2 inhibitors on IMT in T2DM patients [39, 40]. A 3-month prospective cohort study with 35 Italian T2DM patients by Irace et al. [39] showed that empagliflozin and incretin-based therapy reduced IMT. However, it did not evaluate potential differences in IMT reduction between groups, and was limited by small sample size and short treatment duration. The second trial was a single-arm intervention study in 134 Japanese T2DM patients who reported no statistically significant changes in IMT following 52 weeks of ipragliflozin treatment [40]. However, the report could not assess the effect of the SGLT2 inhibitors on IMT, since it was a single-arm study.
As the study drugs and participants’ backgrounds differ, the results of the above two studies cannot be directly compared with those of our UTOPIA study. However, our study is superior in terms of certain aspects including study design, sample size, and observation duration. Like Irace et al. [39], our study reports that IMT decreased after SGLT2 inhibitor treatment compared with pre-treatment. Moreover, all three studies report that SGLT2 inhibitor treatment has not been proven to significantly prevent IMT progression compared to with the conventional treatment.
The effects of SGLT2 inhibitors on endothelial function, which is an arteriosclerosis-related change that occurs on a shorter timescale than IMT hyperplasia [41], were evaluated in earlier reports. In the double-blind RCT EMBLEM, the effect of empagliflozin on the reactive hyperemia peripheral arterial tonometry index, an indicator of endothelial function, was estimated; however, the change was not significant [42]. Similarly, in another study, dapagliflozin did not significantly affect the flow-mediated dilation, another indicator of endothelial function [10]. These reports do not contradict our findings.
CVD onset events, such as myocardial infarction and stroke, occur later than IMT hyperplasia. Several cardiovascular outcome trials (CVOTs) with SGLT2 inhibitors have been published [6, 7, 43]. Remarkably, three studies reported substantial inhibition of heart failure, but there were no statistically significant inhibitory effects on events more closely related to atherosclerosis, such as fatal or non-fatal myocardial infarction [6, 7, 43]. Meta-analyses of CVOTs with new classes of antidiabetic drugs, including SGLT2 inhibitors, have produced similar findings [44], suggesting that possible mechanisms may involve hemodynamic effects induced by glycosuria and natriuresis rather than a direct antiatherothrombotic effect [5, 6, 8, 9].
In addition, it is possible that the effects of SGLT2 inhibitor treatment differ depending on vessels size. Both IMT and FMD are indices used to evaluate angiopathies in large vessels. Conversely, complex onset mechanisms underlie cardiovascular events, involving disorders of both large and small vessels. Coronary artery disease and stroke develop through complex onset mechanisms, in which disorders of both large and small vessels are involved but the presence of large-vessel lesions have greater significance. Previous studies have proven that SGLT2 inhibitors reduce mortality and hospitalization due to heart failure [6, 7, 43, 44]. Tofogliflozin has been reported to improve cardiac diastolic function [45]. The same study has also shown that the diastolic function improvement does not correlate with the improvement in FMD-based vascular endothelial function in large vessels and has inferred that tofogliflozin may improve diastolic function by improving local hemodynamics in the coronary artery. These findings suggest that the beneficial effects of SGLT2 inhibitors on small vessels may be greater rather than those on large vessels. This particular point warrants further investigation.
Effects of tofogliflozin on glucose metabolism and other parameters
Within a 2-year observation period, relative to the control group parameters, tofogliflozin significantly reduced HbA1c, blood glucose, BMI, and abdominal circumference, which were accompanied by a significant HDL-C increase. These observations indicated that tofogliflozin is an effective drug for the management of CV risk factors in patients with T2DM.
In the tofogliflozin group, after 26 weeks, the HbA1c level was significantly decreased by an average of 0.4% from an initial level of 7.4 ± 0.7%, and even after 104 weeks, a significant average reduction of 0.3% was maintained. Since blood glucose was controlled in most subjects at the study start, the average reduction of 0.3–0.4% over 2 years appears to be clinically significant. Remarkably, the improvement in blood glucose control was accompanied by a significant reduction of body weight and abdominal circumference, but without an incident increase in hypoglycemic events. Since the beneficial effects of tofogliflozin on glycemic control has been reported to be more significant in subjects with larger visceral fat area at baseline, tofogliflozin would be more suitable for relatively obese subjects [46].
Tofogliflozin treatment was also associated with significant reduction of blood pressures and body weight, which were accompanied by a significant increase of red blood cell count, hemoglobin, and hematocrit. These findings were consistent with previous reports indicating the favorable hemodynamic effects of SGLT2 inhibitors [5,6,7,8,9]. SGLT2 inhibitor-induced plasma volume reduction and hemoconcentration leading to improvement of oxygen transport could contribute to the efficient myocardial energetics. Indeed, SGLT2 inhibitors including tofogliflozin significantly improve left ventricular diastolic function in patients with T2DM [45, 47]. It remains unclear whether there are differences in cardiac effects between agents belonging to this class of drugs, while a clinical comparison of tofogliflozin and empagliflozin based on a 48-week retrospective analysis showed that empagliflozin had a significantly stronger effect on the hematocrit than tofogliflozin [48].
Safety
Although this study could not specifically evaluate the effects of tofogliflozin on CVD, the incidence of CV events was low in both treatment groups, and there was no significant difference between these groups. This incidence rate was similar to that reported in a recent large-scale RCT, J-DOIT3, that included Japanese T2DM patients [49].
In addition, all adverse events, hypoglycemia, genital infections, urinary tract infections, and bone fractures did not significantly vary between the two treatment groups. The fairly low incidence of adverse events related to genital infections and the absence of significant difference from the control group were consistent with the results of previous studies investigating tofogliflozin as a study drug in Japanese patients with T2DM [11, 12], although genital infections have been commonly known as an adverse event related to SGLT2 inhibitor treatment and thus should be administered with caution. Furthermore, there were no concerns documented in our study records about the safety of tofogliflozin.
These findings suggest that tofogliflozin is a safe and effective drug for managing T2DM in patients without apparent CVD.
Limitations
Our study also has some limitations. First, although many clinical trials of antilipidemic and antidiabetic agents have used the carotid IMT as a surrogate clinical endpoint for cardiovascular events [17,18,19,20,21,22,23,24], there is no sufficient evidence whether the progression of carotid IMT reflects an increased risk of subsequent cardiovascular events. Some previous studies indicated that the progression of carotid IMT and carotid stenosis could be used as a surrogate endpoint of cardiovascular events [16, 36,37,38, 50, 51]. However, recent meta-analyses indicated that there was no significant association between the carotid IMT progression and the development of combined endpoints [52,53,54,55].
Second, this study was not a double-blind, placebo-controlled trial but rather a prospective, randomized study with open-label medications and blinded endpoint. Although the endpoint determination was blinded and conducted by expert committees, the medications were open label, which may induce unexpected bias. Furthermore, it is possible that the administration of additional antidiabetic, antilipidemic, and antihypertensive agents, which was more frequent in the control group at baseline and during the treatment periods (Additional file 1: Tables S2 and S3), may have affected the outcomes. These drugs presumably have direct anti-arteriosclerotic properties in addition to their effects on the reduction of blood glucose, blood pressure, and lipid levels. Specifically, biguanides [19, 20], DPP-4 inhibitors [21, 22], ARBs [23, 24], and statins [17, 18] exert strong inhibitory effects on the progression of IMT, and these effects are assumed to be independent of blood glucose, blood pressure, and lipid levels improvements. Thus, it is possible that the inhibition of IMT progression following tofogliflozin treatment might have been masked by the analogous effects of other drugs used in managing diabetes, as described above.
Third, we should consider that the relatively small number of subjects recruited for the study could be an important bias. Although the sample size had been considered as sufficient for detecting a difference in the IMT progression between the two treatment groups, it might have lacked the power to detect a smaller effect, which might be the reason why this study failed to observe a significant difference between the two treatment groups. The IMT progression rate observed for the conventional treatment group in our study was lower than the value expected based on a report by Yokoyama et al. [30] One possible explanation for this is that the mean HbA1c level (= 7.3%) in our control group was relatively better than that (= 7.9%) in the study by Yokoyama et al. since it comprised the results of meta-analysis of multiple studies conducted in early 2000s, which since then, the standard treatments of diabetes have improved. In addition, drugs anticipated to have vascular protective effects are currently being used more proactively. This may have resulted in a significant decrease in IMT progression rates in diabetic patients compared to rates during early 2000s. In addition, unlike Yokoyama et al., our participants were patients with no history of CVD; which it may be also related to a smaller IMT progression rate.
Fourth, there may have been measurement errors in IMT due to inter-sonographer differences, which were not evaluated in this study. However, IMT was measured by the same expert sonographer in each institution throughout all the visits based on the study protocol. In addition, we did not find significant heterogeneity in changes in IMT among institutions (data not shown).
Fifth, subjects in this study were Japanese patients with T2DM, a cohort with relatively low CV risks. Therefore, it would be premature to generalize our findings to other racial or ethnic groups. Furthermore, the average BMI of the study subjects was relatively high (27.0 ± 5.8 kg/m2 in the tofogliflozin and 27.0 ± 4.6 kg/m2 in the conventional group), since average BMI of Japanese patients with T2DM is approximately 25 kg/m2. Thus, there might have been some bias in the patient selection process during enrollment.
Finally, multiple statistical analyses were performed on these subjects, which would generate false-positive results derived from multiple testing. Thus, secondary endpoint results should be interpreted with caution.
Conclusion
We found no differences in IMT change between the tofogliflozin and conventional treatment groups. However, tofogliflozin is a safe and effective treatment option for managing primary CVD risk factors, including high blood glucose levels in T2DM patients without apparent CVD.
Availability of data and materials
The datasets generated and/or analyzed during our study will be available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Adverse event
- ANCOVA:
-
Analysis of covariance
- ARB:
-
Angiotensin II receptor blocker
- BMI:
-
Body mass index
- CV:
-
Cardiovascular
- CVD:
-
Cardiovascular disease
- CVOT:
-
Cardiovascular outcome trial
- DPP-4:
-
Dipeptidyl peptidase-4
- eGFR:
-
Estimated glomerular filtration rate
- IMT:
-
Intima-media thickness
- max-IMT-CCA:
-
Maximum IMT of common carotid artery
- mean-IMT-CCA:
-
Mean IMT of common carotid artery
- PROBE:
-
Prospective, randomized, open label, blinded-endpoint
- RCT:
-
Randomized controlled trials
- SGLT2:
-
Sodium-glucose cotransporter 2
- UAE:
-
Urinary albumin excretion
- UTOPIA:
-
Using Tofogliflozin for Possible better Intervention against Atherosclerosis for type 2 diabetes patients
References
Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34(Suppl 2):S132–7.
Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38:316–22.
Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL, Chan WL, Chen CH, Chou P, Chuang SY. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes. Diabetes Care. 2013;36:894–900.
Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:211721–8.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews D, CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–9.
Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016;39:717–25.
Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, Hirose T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16:84.
Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, Tanizawa Y, Araki E, Ueda M, Suganami H, Watanabe D. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65.
Tanizawa Y, Kaku K, Araki E, Tobe K, Terauchi Y, Utsunomiya K, Iwamoto Y, Watada H, Ohtsuka W, Watanabe D, Suganami H, Tofogliflozin 004 and 005 Study group. Long-term safety and efficacy of tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open-label, randomized controlled trials. Expert Opin Pharmacother. 2014;15:749–66.
Suzuki M, Honda K, Fukazawa M, Ozawa K, Hagita H, Kawai T, Takeda M, Yata T, Kawai M, Fukuzawa T, Kobayashi T, Sato T, Kawabe Y, Ikeda S. Tofogliflozin, a potent and highly specific sodium/glucose cotransporter 2 inhibitor, improves glycemic control in diabetic rats and mice. J Pharmacol Exp Ther. 2012;341:692–701.
Nagata T, Fukazawa M, Honda K, Yata T, Kawai M, Yamane M, Murao N, Yamaguchi K, Kato M, Mitsui T, Suzuki Y, Ikeda S, Kawabe Y. Selective SGLT2 inhibition by tofogliflozin reduces renal glucose reabsorption under hyperglycemic but not under hypo- or euglycemic conditions in rats. Am J Physiol Endocrinol Metab. 2013;304:E414–23.
Takeishi S, Tsuboi H, Takekoshi S. Comparison of tofogliflozin 20 mg and ipragliflozin 50 mg used together with insulin glargine 300 U/mL using continuous glucose monitoring (CGM): a randomized crossover study. Endocr J. 2017;64:995–1005.
Katakami N, Mita T, Gosho M, Takahara M, Irie Y, Yasuda T, Matsuoka TA, Osonoi T, Watada H, Shimomura I. Clinical utility of carotid ultrasonography in the prediction of cardiovascular events in patients with diabetes—a combined analysis of data obtained in five longitudinal studies. J Atheroscler Thromb. 2018;25:1053–66.
Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: arterial biology for the investigation of the treatment effects of reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–60.
MacMahon S, Sharpe N, Gamble G, Hart H, Scott J, Simes J, White H. Effects of lowering average of below-average cholesterol levels on the progression of carotid atherosclerosis: results of the LIPID Atherosclerosis Substudy. LIPID Trial Research Group. Circulation. 1998;97(18):1784–90.
Katakami N, Yamasaki Y, Hayaishi-Okano R, Ohtoshi K, Kaneto H, Matsuhisa M, Kosugi K, Hori M. Metformin or gliclazide, rather than glibenclamide, attenuate progression of carotid intima-media thickness in subjects with type 2 diabetes. Diabetologia. 2004;47:1906–13.
Matsumoto K, Sera Y, Abe Y, Tominaga T, Yeki Y, Miyake S. Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabet Res Clin Pract. 2004;64:225–8.
Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, Shiraiwa T, Kosugi K, Umayahara Y, Yamamoto T, Yokoyama H, Kuribayashi N, Jinnouchi H, Gosho M, Shimomura I, Watada H, Collaborators on the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A) Trial. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the sitagliptin preventive study of intima-media thickness evaluation (SPIKE): a randomized controlled trial. Diabetes Care. 2016;39:455–64.
Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, Shiraiwa T, Kosugi K, Umayahara Y, Yamamoto T, Yokoyama H, Kuribayashi N, Jinnouchi H, Gosho M, Shimomura I, Watada H, Collaborators on the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A) Trial. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care. 2016;39:139–48.
Mörtsell D, Malmqvist K, Held C, Kahan T. Irbesartan reduces common carotid artery intima-media thickness in hypertensive patients when compared with atenolol: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) study. J Intern Med. 2007;261:472–9.
Ono H, Minatoguchi S, Watanabe K, Yamada Y, Mizukusa T, Kawasaki H, Takahashi H, Uno T, Tsukamoto T, Hiei K, Fujiwara H. Candesartan decreases carotid intima-media thickness by enhancing nitric oxide and decreasing oxidative stress in patients with hypertension. Hypertens Res. 2008;31:271–9.
Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, Umayahara Y, Kaneto H, Osonoi T, Yamamoto T, Kuribayashi N, Maeda K, Yokoyama H, Kosugi K, Ohtoshi K, Hayashi I, Sumitani S, Tsugawa M, Ohashi M, Taki H, Nakamura T, Kawashima S, Sato Y, Watada H, Shimomura I, UTOPIA Study Investigators. Rationale, design, and baseline characteristics of the UTOPIA trial for preventing diabetic atherosclerosis using an SGLT2 inhibitor: a prospective, randomized, open-label, parallel-group comparative study. Diabetes Ther. 2017;8:999–1013.
The Japan Diabetes Society: Treatment Guide for Diabetes 2014–2015, ed by the Editorial Committee Members of “Treatment Guide for Diabetes” (Araki E, Inagaki N, Inoguchi T, Utsunomiya K, Tanizawa Y, Nakamura J, Noda M, Watada H, Imamura S), Bunkodo Co., Ltd., Tokyo, Japan, 2014.
Terminology and Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine. Subcommittee for preparing guidelines for ultrasound diagnosis of carotid artery: standard method for ultrasound evaluation of carotid artery lesions. Jpn J Med Ultrasonics. 2009;36:501–18.
Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, Nomura M, Inoguchi T, Nawata H. Evaluation of a new carotid intima-media thickness measurement by B-Mode ultrasonography using an innovative measurement software, Intimascope. Am J Hypertens. 2006;19:1206–12.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Yokoyama H, Katakami N, Yamasaki Y. Recent advances of intervention to inhibit progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Stroke. 2006;37:2420–7.
Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim-Mitsuyama S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148.
Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE -/- mice fed a western diet. Diabetologia. 2017;60:364–76.
Nasiri-Ansari Ν, Dimitriadis GK, Agrogiannis G, Perrea D, Kostakis ID, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc Diabetol. 2018;17:106.
Wang X, Dalmeijer GW, den Ruijter HM, Anderson TJ, Britton AR, Dekker J, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Holewijn S, Ikeda A, Kauhanen J, Kitagawa K, Kitamura A, Kurl S, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, Polak JF, Price JF, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CDA, Tuomainen TP, Peters SAE, Bots ML. Clustering of cardiovascular risk factors and carotid intima-media thickness: the USE-IMT study. PLoS ONE. 2017;21:e0173393. https://doi.org/10.1371/journal.pone.0173393.
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67.
Goldberger ZD, Valle JA, Dandekar VK, Chan PS, Ko DT, Nallamothu BK. Are changes in carotid intima-media thickness related to risk of nonfatal myocardial infarction? A critical review and meta-regression analysis. Am Heart J. 2010;160:701–14.
Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Nakamura F, Miyamoto Y. Impact of intima-media thickness progression in the common carotid arteries on the risk of incident cardiovascular disease in the Suita Study. J Am Heart Assoc. 2018;7:e007720.
Sabeti S, Schlager O, Exner M, Mlekusch W, Amighi J, Dick P, Maurer G, Huber K, Koppensteiner R, Wagner O, Minar E, Schillinger M. Progression of carotid stenosis detected by duplex ultrasonography predicts adverse outcomes in cardiovascular high-risk patients. Stroke. 2007;38:2887–94.
Irace C, Casciaro F, Scavelli FB, Oliverio R, Cutruzzolà A, Cortese C, Gnasso A. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc Diabetol. 2018;17:52.
Nomiyama T, Shimono D, Horikawa T, Fujimura Y, Ohsako T, Terawaki Y, Fukuda T, Motonaga R, Tanabe M, Yanase T, Collaborators of Fukuoka Study of Ipragliflozin (FUSION) trial. Efficacy and safety of sodium-glucose cotransporter 2 inhibitor ipragliflozin on glycemic control and cardiovascular parameters in Japanese patients with type 2 diabetes mellitus; Fukuoka Study of Ipragliflozin (FUSION). Endocr J. 2018;65:859–67.
Fathi R, Marwick TH. Noninvasive tests of vascular function and structure: why and how to perform them. Am Heart J. 2001;141:694–703.
Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, Takamura T, Taguchi I, Hisauchi I, Toyoda S, Matsuzawa Y, Tomiyama H, Yamaoka-Tojo M, Yoshida H, Sato Y, Ikehara Y, Ueda S, Higashi Y, Node K, EMBLEM Investigators. Effect of empagliflozin on endothelial function in patients with type 2 diabetes and cardiovascular disease: results from the multicenter, randomized, placebo-controlled, double-blind EMBLEM trial. Diabetes Care. 2019;42:e159–61.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Fei Y, Tsoi MF, Chung BMY. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol. 2019;18:112.
Tochiya M, Makino H, Tamanaha T, Matsuo M, Hishida A, Koezuka R, Ohata Y, Tomita T, Son C, Miyamoto Y, Yasuda S, Hosoda K. Effect of tofogliflozin on cardiac and vascular endothelial function in patients with type 2 diabetes and heart diseases: a pilot study. J Diabetes Investig. 2020;11:400–4.
Kamei S, Iwamoto M, Kameyama M, Shimoda M, Kinoshita T, Obata A, Kimura T, Hirukawa H, Tatsumi F, Kohara K, Nakanishi S, Mune T, Kaku K, Kaneto H. Effect of tofogliflozin on body composition and glycemic control in Japanese subjects with type 2 diabetes mellitus. J Diabetes Res. 2018. https://doi.org/10.1155/2018/6470137.
Higashikawaa T, Ito T, Mizuno T, Ishigami K, Kohori M, Mae K, Usuda D, Takagi S, Sangen R, Saito A, Iguchi M, Kasamaki Y, Fukuda A, Kanda T, Okuro M. Effects of tofogliflozin on cardiac function in elderly patients with diabetes mellitus. J Clin Med Res. 2020;12:165–71.
Kobayashi K, Toyoda M, Hatori N. Clinical comparison of tofogliflozin and empagliflozin based on an analysis of 24-h accumulated urine in Japanese patients with type 2 diabetes mellitus. Obes Med. 2019. https://doi.org/10.1016/j.obmed.2019.100088.
Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, Haraguchi M, Morita A, Ohashi K, Hara K, Morise A, Izumi K, Ishizuka N, Ohashi Y, Noda M, Kadowaki T, J-DOIT3 Study Group. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951–64.
Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–9.
Espeland MA, O’Leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med. 2005;6:3.
Costanzo P, Perrone-Filardi P, Vassallo E, Paolillo S, Cesarano P, Brevetti G, Chiariello M. Does carotid intima-media thickness regression predict reduction of cardiovascular events? A meta-analysis of 41 randomized trials. J Am Coll Cardiol. 2010;56:2006–20.
Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG, Group P-IS. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053–62.
Lorenz MW, Price JF, Robertson C, Bots ML, Polak JF, Poppert H, Kavousi M, Dörr M, Stensland E, Ducimetiere P, Ronkainen K, Kiechl S, Sitzer M, Rundek T, Lind L, Liu J, Bergström G, Grigore L, Bokemark L, Friera A, Yanez D, Bickel H, Ikram MA, Völzke H, Johnsen SH, Empana JP, Tuomainen TP, Willeit P, Steinmetz H, Desvarieux M, Xie W, Schmidt C, Norata GD, Suarez C, Sander D, Hofman A, Schminke U, Mathiesen E, Plichart M, Kauhanen J, Willeit J, Sacco RL, McLachlan S, Zhao D, Fagerberg B, Catapano AL, Gabriel R, Franco OH, Bülbül A, Scheckenbach F, Pflug A, Gao L, Thompson SG. Carotid intima-media thickness progression and risk of vascular events in people with diabetes: results from the PROG-IMT collaboration. Diabetes Care. 2015;38(10):1921–9.
Lorenz MW, Gao L, Ziegelbauer K, Norata GD, Empana JP, Schmidtmann I, Lin HJ, McLachlan S, Bokemark L, Ronkainen K, Amato M, Schminke U, Srinivasan SR, Lind L, Okazaki S, Stehouwer CDA, Willeit P, Polak JF, Steinmetz H, Sander D, Poppert H, Desvarieux M, Ikram MA, Johnsen SH, Staub D, Sirtori CR, Iglseder B, Beloqui O, Engström G, Friera A, Rozza F, Xie W, Parraga G, Grigore L, Plichart M, Blankenberg S, Su TC, Schmidt C, Tuomainen TP, Veglia F, Völzke H, Nijpels G, Willeit J, Sacco RL, Franco OH, Uthoff H, Hedblad B, Suarez C, Izzo R, Zhao D, Wannarong T, Catapano A, Ducimetiere P, Espinola-Klein C, Chien KL, Price JF, Bergström G, Kauhanen J, Tremoli E, Dörr M, Berenson G, Kitagawa K, Dekker JM, Kiechl S, Sitzer M, Bickel H, Rundek T, Hofman A, Mathiesen EB, Castelnuovo S, Landecho MF, Rosvall M, Gabriel R, de Luca N, Liu J, Baldassarre D, Kavousi M, de Groot E, Bots ML, Yanez DN, Thompson SG, PROG-IMT study group. Predictive value for cardiovascular events of common carotid intima media thickness and its rate of change in individuals at high cardiovascular risk—results from the PROG-IMT collaboration. PLoS ONE. 2018;13:e0191172.
Acknowledgements
The authors thank all the staff and patient participants of this study. The authors gratefully acknowledge the assistance of D. Takayama and H. Yamada (Soiken Holdings Inc., Tokyo, Japan) and thank Editage (http://www.editage.com) for English language editing.
Funding
Financial support for this study, including the fees for the assistances and for processing of the article by the journal, was provided by Kowa Co., Ltd., Tokyo, Japan.
Author information
Authors and Affiliations
Consortia
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Consent for publication
Not applicable.
Competing interests
Naoto Katakami is a staff member of the endowed chair established by funds from Kowa Co., Ltd., and has received research funds from MSD, and lecture fees from Astellas Pharma Inc., AstraZeneca K.K., Boehringer Ingelheim, Daiichi Sankyo Inc., Eli Lilly, Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Co., Novartis Pharmaceuticals, Novo Nordisk Pharma, Ono Pharmaceutical Co., Taisho Toyama Pharmaceutical Co., Takeda Pharmaceutical Co., and Sanofi-Aventis, and Shionogi & Co.
Tomoya Mita has received lecture fees from Astellas Pharma Inc., Daiichi Sankyo Inc., Eli Lilly, Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma, Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited and Sanofi-Aventis, scholarship donations from MSD K.K., Astellas Pharma Inc., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Sanofi-Aventis K.K., Daiichi Sankyo Company, Limited, Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Terumo Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Pfizer Japan Inc., Benefit One Health Care Inc., Mochida Pharmaceutical Co., Ltd., and Nitto Boseki Co., Ltd. as well as funds of endowed chair from MSD K.K., Takeda Pharmaceutical Company Limited.
Toshihiko Shiraiwa has received lecture fees from Sanofi-Aventis K.K. and Takeda Pharmaceutical Company Limited and research funding from Novo Nordisk Pharma Ltd., Sanofi-Aventis K.K., Takeda Pharmaceutical Company Limited, AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd., and Mitsubishi Tanabe Pharma Corporation.
Tetsuyuki Yasuda has received lecture fees from Nippon Boehringer Ingelheim Co., Ltd. and Sanofi-Aventis K.K.
Yosuke Okada has received lecture fees from Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bayer Holding Ltd., Novartis Pharmaceuticals Corp., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., and Kissei Pharmaceutical Co., Ltd. as well as research funding from Kowa Pharmaceutical Co. Ltd. and Mitsubishi Tanabe Pharma Corporation.
Hideaki Kaneto has received lecture fees from Nippon Boehringer Ingelheim Co., Ltd., Sanofi-Aventis K.K., Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, AstraZeneca K.K., Astellas Pharma Inc., Novartis Pharmaceuticals Corp., and Sumitomo Dainippon Pharma Co.; scholarship donations from Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi-Aventis K.K., Eli Lilly Japan K.K., Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Takeda Pharmaceutical Company Limited, AstraZeneca K.K., Mochida Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Kissei Pharmaceutical Co., Ltd., and Kyowa Hakko Kirin Co. Ltd; and research funding from Taisho Pharmaceutical Co., Sumitomo Dainippon Pharma Co.
Takeshi Osonoi has received lecture fees from Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., Novo Nordisk Pharma Ltd., and Sanwa Kagaku Kenkyusho Co., Ltd.; manuscript fees from Sanwa Kagaku Kenkyusho Co., Ltd.; and research funding from Takeda Pharmaceutical Company Limited, Novo Nordisk Pharma Ltd., Astellas Pharma Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Daiichi Sankyo Company, Limited, Bayer Holding Ltd., Kowa Pharmaceutical Co. Ltd., and AbbVie Inc.
Nobuichi Kuribayashi has received lecture fees from Takeda Pharmaceutical Company Limited, Sanofi-Aventis K.K., Novo Nordisk Pharma Ltd., MSD K.K., and Mitsubishi Tanabe Pharma Corporation.
Satoru Sumitani has received lecture fees from Sumitomo Dainippon Pharma Co., Ltd.
Yasunori Sato has received lecture fees from Mochida Pharmaceutical Co., Ltd.
Hirotaka Watada has received lecture fees from Sumitomo Dainippon Pharma CO., Ltd., Bayer Yakuhin, Ltd. Sanofi-Aventis K.K., MSD K.K., Astellas Pharma Inc., Takeda Pharmaceutical Company Ltd, Mitsubishi Tanabe Pharma Co., AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Kowa Co., Ltd., Novartis Pharmaceuticals Corp., Daiichi Sankyo Company, Ltd, Kyowa Hakko Kirin Co. Ltd., Ono Pharmaceutical Co., Ltd., and Kissei Pharmaceutical Co., Ltd. and research support from Novartis Pharmaceuticals Corp., Otsuka Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., MSD K.K., Astellas Pharma Inc., Bayer Yakuhin, Ltd. Teijin Pharma Ltd., Ono Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co. Ltd., Kowa Pharmaceutical Co. Ltd., Sanofi-Aventis K.K., Sanwa Kagaku Kenkyusho Co., Ltd., Daiichi Sankyo Company, Ltd, Sumitomo Dainippon Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Ltd, Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Pfizer Japan Inc., Shionogi & Co., Ltd. Yakult, and Kissei Pharmaceutical Co., Ltd.
Iichiro Shimomura has received lecture fees from Astellas Pharma Inc., AstraZeneca K.K., MSD K.K., Ono Pharmaceutical Co., Kyowa Kirin Co., Ltd., Kowa Company, Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co., Daiichi Sankyo Co., Takeda Pharma K.K., Mitsubishi Tanabe Pharma Co., Teijin Pharma, Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Novartis Pharma K.K., Novo Nordisk Pharma, Mochida Pharmaceutical Co., Taisho Pharmaceutical Co. Ltd., Nippon Chemiphar Co., Ltd., Covidien Japan Inc., Amgen Astellas Biopharma K.K., KOBAYASHI Pharmaceutical Co., Ltd., Dainippon Sumitomo Pharma Co., Rohto Pharmaceutical Co., Ltd.; and research funds from Astellas Pharma Inc., MSD K.K, Ono Pharmaceutical Co., Kaken Pharmaceutical Co., Kyowa Kirin Co., Ltd., Sanofi K.K., Shionogi & Co., Daiichi Sankyo Co., Dainippon Sumitomo Pharma Co., Takeda Pharma K.K., Mitsubishi Tanabe Pharma Co., Teijin Pharma, Novartis Pharma K.K., Novo Nordisk Pharma, Eli Lilly Japan K.K, Kowa Company, Ltd.; and consulting fees from AstraZeneca K.K., MSD K.K., Taisho Pharmaceutical Co. Ltd., Novo Nordisk Pharma, Lotte Co., Ltd.
Hidenori Yoshii, Yutaka Umayahara, Tsunehiko Yamamoto, Kazuhisa Maeda, Hiroki Yokoyama, Keisuke Kosugi, Kentaro Ohtoshi, Isao Hayashi, Mamiko Tsugawa, Kayoko Ryomoto, Hideki Taki, Tadashi Nakamura, and Satoshi Kawashima declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Materials S1:
Safety evaluation. List of UTOPIA trial site investigators. Table S1. Effects of tofogliflozin on intima-media thickness were analyzed using covariance models. Table S2. Changes in concomitantly used anti-diabetic agents. Table S3. Changes in concomitantly used cardiovascular medications.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Katakami, N., Mita, T., Yoshii, H. et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol 19, 110 (2020). https://doi.org/10.1186/s12933-020-01079-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-020-01079-4