Abstract
Human mesenchymal stem cells (MSCs) are multilineage somatic progenitor/stem cells that have been shown to possess immunomodulatory properties in recent years. Initially met with much skepticism, MSC immunomodulation has now been well reproduced across tissue sources and species to be clinically relevant. This has opened up the use of these versatile cells for application as 3rd party/allogeneic use in cell replacement/tissue regeneration, as well as for immune- and inflammation-mediated disease entities. Most surprisingly, use of MSCs for in immune-/inflammation-mediated diseases appears to yield more efficacy than for regenerative medicine, since engraftment of the exogenous cell does not appear necessary. In this review, we focus on this non-traditional clinical use of a tissue-specific stem cell, and highlight important findings and trends in this exciting area of stem cell therapy.
Similar content being viewed by others
Stem cell therapy for immune- and inflammation-mediated diseases
Stem cells are likely the most promising agent for the treatment of degenerative and ischemic diseases due to their self-renewal and multilineage differentiation capacity. The most exciting aspect of these unique cells is their potential therapeutic impact for regenerative medicine [1, 2]. The best studied type of stem cell is the hematopoietic stem cell (HSC), and transplantation of these tissue-specific stem cells have now become standard-of-care for numerous indications [3]. Over 50 years in the making, the success of HSC transplantation is illustrative of the paradigm for stem cell therapy: replacement and regeneration of pathological endogenous tissue with autologous or 3rd party/allogeneic stem cells. While research in stem cell biology is mainly focused on this goal, an unexpected new avenue of clinical application has emerged for the mesenchymal stem cell (MSC) as an immunotherapeutic agent. A type of somatic progenitor/stem cell, the MSC is capable of multilineage differentiation. However, in recent years, consistent reports on its immunomodulatory properties have opened up the use of these cells for indications other than regenerative medicine. The therapeutic application of MSCs in immune/inflammatory contexts may be more efficacious than traditional indications for regenerative medicine, since engraftment of infused/transplanted stem cells—which have proved surprisingly difficult to achieve [4]—appears not to be necessary for efficacy [5]. In this review, we specifically focus on this non-traditional application of a tissue-specific stem cell, and highlight important findings and trends in this exciting area of stem cell therapy.
Background: Functional capacity of Mesenchymal stem cells (MSCs)
MSCs were first isolated from the adult bone marrow (BM), and distinguished from marrow hematopoietic cells by their adherent nature in in vitro cell cultures and fibroblastic morphology [6, 7]. The function of BMMSCs was initially thought to be limited to supporting hematopoiesis; indeed, one of the first clinical use of these progenitor/stem cells was to enhance HSC engraftment [8]. Since these early reports, MSCs have been demonstrated to exist in a wide range of adult and fetal organs/tissues [9], and popular sources for isolation other than the BM include adipose tissue, umbilical cord blood, umbilical cord and placenta. In 2006, the International Society for Cellular Therapy (ISCT) established the following unified and minimal criteria to define MSCs [10].
-
Plastic-adherent cells
-
Expression of the surface markers CD73, CD90 and CD105, but not the hematopoietic markers CD45, CD34, CD14, CD11b, CD19, CD79a or HLA-DR
-
Trilineage mesenchymal differentiation capacity into osteoblasts, adipocytes and chondrocytes
In the early 2000’s, reports of immunomodulatory properties in BMMSCs began to emerge [11–13]. While initially met with much skepticism, the reproducibility of these findings using multiple species and disease models along with human case reports established that in vitro cultured MSCs clearly are immunosuppressive and immunomodulatory [14–16]. Moreover, these properties were not limited to MSCs from the BM, but also found with other sources of MSCs, especially fetal sources [17, 18]. Interestingly, despite the increasing number of reports on MSC immune-related functions, the question of why these somatic progenitor/stem cells harbor these properties remain much of a mystery. Regardless of this issue, MSC immunomodulatory functions have greatly expanded the clinical utility of this progenitor/stem cell over other stem cell types, since this allows 3rd party/allogeneic use. Moreover, use of MSCs for immune-/inflammation-mediated disease entities appear to yield more efficacy than for cell replacement/tissue regeneration, since engraftment of the exogenous cell is not necessary. These reasons, along with easily accessible sources for isolation, help explain the popularity of MSC therapy for immune-and inflammation-mediated diseases.
Clinical status of MSC therapy for immune-/inflammation-mediated diseases
Disease indications in clinical trials utilizing MSCs
The capacity of MSCs for multilineage differentiation as well as immunomodulation has meant that these somatic progenitor cells are highly versatile for a wide range of therapeutic applications. Moreover, a number of animal model and translational studies have reported on the capacity of MSCs to home to sites of injury and/or inflammation, thus adding to their attractiveness for clinical use [19]. Indeed, as of April 2016, there were over 500 MSC-related clinical trials registered on the NIH Clinical Trial Database (https://clinicaltrials.gov/). Surprisingly, while the immunomodulatory properties of MSCs have only more recently been identified, nearly half of all registered clinical trials—230 trials or 42 % of all registered trials—are being conducted for immune-/inflammation-mediated diseases (Fig. 1). The main clinical indications within these trials include autoimmune diseases (n = 51), organ transplantation and rejection (n = 67), and other inflammatory aspects of various diseases (n = 112). These trials generally are Phase 1 studies to evaluate safety (n = 49 or 21.3 %; 2 Phase 0 trial to establish dosage safety in a small number of subjects), Phase 2 studies to evaluate efficacy (n = 53 or 23.0 %), or combined Phase 1/2 studies (n = 103 or 44.8 %). A small number of trials are in Phase 3 (n = 10 or 4.3 %) or combined Phase 2/3 (n = 8 or 3.5 %). There is only one Phase 4 trial to monitor side effects after marketing, and there are 4 trials which did not specify a trial Phase (n = 4 or 1.7 %) (Table 1). Trials also differ in terms of the tissue source of MSCs used, with the most frequent reported source being adult BMMSCs (41.2 %). However, other tissue and fetal source MSCs are also popular choices, with 16.3 % of trials using adipose-derived MSCs, and 21.1 % of trials using fetal-source MSCs which includes MSCs isolated from umbilical cord, umbilical cord blood, and placenta (Table 1). While 32.5 % of all trials specify the use of autologous sources, over 50.9 % of trials appear to use allogeneic sources, i.e. trials which use fetal-source MSCs on adult patients. Unspecified donor sources account for approximately 16.7 % of trials. Clearly, the capacity to use allogeneic/3rd party source MSCs greatly contributes to the popularity of this stem cell source. In this review, we will focus our attention on disease indications which have a higher number of clinical trials being conducted.
Clinical application of human mesenchymal stem cells (MSCs) for immune- and inflammation-mediated diseases. Graph is a summary of the number of clinical trials using MSC therapy in immune-/inflammation-mediated diseases, as registered on the website https://clinicaltrials.gov/ (accessed April 2016). MS, multiple sclerosis; T1DM, type 1 diabetes mellitus; GVHD, graft versus host disease; OA, osteoarthritis; IBD, inflammatory bowel disease
Mechanisms of human BM and other tissue source MSC immunomodulation
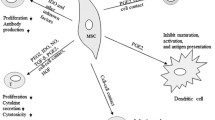
Since the first studies demonstrating immunomodulation by MSCs, there have been significant advances in understanding mechanisms involved in these properties, including interactions with specific leukocyte populations [16, 20]. MSC modulation of CD4 T lymphocyte populations has been best studied, with most reports demonstrating that secreted factors such as transforming growth factor β1 (TGF-β1) and prostaglandin E2 (PGE2) being involved in inhibiting T cell proliferation [21]. In addition, MSCs can modulate T lymphocyte fate, polarizing naïve CD4 towards a regulatory T cell (Treg) phenotype and shifting the cytokine profile from a T helper cell type 1 (Th1)—in which high levels of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) are secreted—to a Th2 milieu [22]. MSCs can suppress the cytotoxic activity of CD8 cytotoxic T cells [23, 24] as well as natural killer cells (NK) [25], and can also interfere with B cell maturation and antibody production [26, 27]. In addition to interacting with adaptive and innate lymphocyte populations, MSCs have also been shown to modulate the differentiation, expansion, and/or function of myeloid cells towards more immunosuppressive and immunomodulatory phenotypes. These interactions include myeloid populations ranging from monocytes [28, 29], dendritic cells (DCs) [30, 31], macrophages [32, 33], and myeloid-derived suppressor cells (MDSCs) [34]. Most recently, there is also data showing modulation of granulocytes by BM and placental MSCs [35, 36]. In studies using animal disease models, efficacy was especially prominent in experimental autoimmune encephalomyelitis (EAE) and moderate for collagen-induced arthritis (CIA), which are models for multiple sclerosis (MS) and rheumatoid arthritis (RA), respectively [20, 37]; an early human case report demonstrated efficacy of allogeneic BMMSCs towards graft-versus-host disease (GVHD) [14].
In correlation to animal studies and human case reports, the most common immune-/inflammation-mediated indications in MSC clinical trials were for GVHD (n = 46), osteoarthritis (OA; n = 38), inflammatory airway diseases (n = 29), MS (n = 23), and solid organ transplant rejection (n =21). The majority of trials are still ongoing, with less than 7 % of trials with published results; these published reports have been for clinical trials on MS [38], GVHD [39–41], OA [42–46], inflammatory bowel disease (IBD) [47, 48] and various pulmonary inflammatory diseases [49–51]. In this review, therefore, we will discuss the possible mechanisms and clinical efficacy of MSC treatment for these particular indications (Fig. 2).
State of MSC clinical research in specific immune-/inflammation-mediated diseases
Graft-versus-host disease (GVHD)
The most successful therapeutic application using stem cells has been with HSCs [52]. These tissue-specific stem cells can be isolated from adult BM, cord blood, or mobilized to peripheral blood, and represent a life-saving treatment for patients with hematopoietic malignancies and genetic diseases, including hereditary anemia and immunodeficiencies. Either autologous or matched allogeneic/3rd-party HSC transplantation may be performed depending on the clinical scenario. With allogeneic/3rd-party HSC transplantation, immunosuppression is necessary. But despite immunosuppressant therapy, immune rejection in the form of GVHD is still a major cause of morbidity and mortality, occurring in 30 ~ 40 % of allogeneic HSC transplantations [53]. The presence of allo-reactive donor lymphocytes is the crucial reason for GVHD, which are responsible for the inflammatory injury to multiple organs, most commonly the skin, gastrointestinal tract, and liver [54, 55]. The clinical application of MSCs for GVHD developed more rapidly than for any other type of immune-/inflammation-mediated diseases, likely due in large part to a case report in which a pediatric patient with severe GVHD was infused with haploindentical BMMSCs with dramatic therapeutic effect [14]. The scientific basis for this case largely rested on a few human in vitro report showing allogeneic BMMSCs suppressing lymphocyte proliferation and effector functions [11–13], along with clinical safety data from MSC-HSC co-transplantation engraftment trials [8]. In this case report, the patient was a 9 year-old boy with acute lymphoblastic leukemia post-allogeneic HSC transplantation. Despite being on multiple immunosuppressants including two types of corticosteroids, infliximab + daclizumab, as well as cyclosporin, the patient developed severe acute GVHD which lead to the inability to eat by day 24 post-transplantation. Haploidentical BMMSCs from his mother—a readily available donor—was infused at 2 × 106 cells/kg weight, and dramatic decreases in GVHD symptomatology could be seen within a week of MSC infusion. The patient eventually required a 2nd infusion of MSCs at a lower dose of 1 × 106 cells/kg, which along with low levels of immunosuppression (predinosolone + cyclosporine) resolved the GVHD and allowed for the patient to be alive and well many years post-HSC transplantation. Based on this one successful case report, numerous clinical trials for GVHD using autologous, haploidentical, and/or unmatched MSCs have subsequently been conducted. Among completed trials with published reports are two large-scale multicenter Phase 2 studies for treatment of steroid-resistant, severe acute GVHD, both of which showed striking efficacy [56, 57]. Smaller trials on other related complications have also been published: refractory cytopenias [58] and attenuated dry eye in patients with chronic GVHD [59, 60]. Currently, there are 46 registered trials of MSCs for GVHD and related complications. Most of these trials are Phase 2 (n = 20) or combined Phase 1/2 trials (n = 15), whereas a small number are Phase 1 (n = 3), Phase 3 (n = 3), combined Phase 2/3 (n =3), or undefined trials (n = 2). BM is the major source of MSCs in GVHD trials (n = 22), with a few trials utilizing MSCs from other sources including adipose tissues (n = 3), umbilical cord (n = 1) and umbilical cord blood (n = 3). 17 trials did not specify the source of MSC used. A few currently registered trials have published results, and all demonstrate safety of MSC use in GVHD patients as well as some efficacy [39–41].
Despite the promising results of several MSC trials for GVHD treatment, this trend was not consistently seen in all trials [61]. A recent meta-analysis revealed much heterogeneity in conducted trials both on the patient end—which include differences between pediatric vs. adult patients, type of HSC transplanted (BM, peripheral blood, or cord blood)—as well as with the MSCs utilized [62]. A striking difference in published trials conducted in Europe (with generally positive results) compared to North America (with more equivocal results) has been in the MSCs used in terms of culture conditions, passage number, and whether cryopreservation was involved [63, 64]. Adding to the problem may be the fact that detailed mechanisms on acute GVHD are still somewhat unclear, and even more so for chronic GVHD [65]. Thus, there is continued research using mouse and other animal models to further understand the pathophysiology of these diseases. A number of mouse GVHD models—including humanized mouse models—have been developed, and the infusion of mouse and human BMMSCs have generally demonstrated efficacy against the disease by suppressing donor leukocyte inflammatory responses [66–68]. MSC factors involved include PGE2 [69] and nitric oxide (NO) [70]; and effects can be enhanced with pretreatment of IFN-γ to the MSCs [68]. Animal model studies also demonstrate that sources of MSCs other than BMMSCs may also ameliorate GVHD, and may involve vascular endothelial growth factor (VEGF), PGE2, and TGF-β [71–74]. One advantage of MSC immunodulation compared with immunosuppressant drug therapy may be the capacity of MSCs to inhibit GVHD processes while preserving graft-versus-leukemia (GVL) effects, a process thought to eliminate primary or secondary cancer/tumor formation [69]. This may be due to the fact that MSCs—regardless of source—highly expand Tregs [18, 75], a CD4 population now thought to be critical for simultaneously inhibiting GVHD without compromising GVL responses [76]. Clearly, MSCs have strong potential as therapeutic agents for GVHD, but detailed tailoring of patient population and stringent MSC processing criteria are necessary to deliver consistent and reproducible results.
Multiple sclerosis (MS)
MS is an inflammatory and demyelinating disorder of the central nerve system (CNS), and current studies have found that both Th1 and interleukin-17A (IL-17A)-secreting CD4 (Th17) lymphocytes are involved in the pathogenesis of this autoimmune disease [77, 78]. MS has long been known to be a CD4 T-cell mediated autoimmune disease that targets myelin-based protein (MBP), a protein found specifically in myelin sheaths [79]. The resulting demyelination leads to neuronal damage and conduction impairment, which explains the ‘waxing and waning’ nature of the disease. Symptoms are progressive and debilitating, and include blurred vision, blindness, partial or total paralysis, memory and cognitive deficits [80]. Currently without cure, MS is the most common autoimmune disease of the CNS and as of 2013, an estimated 2.3 million people are affected with the disease, with women twice as likely as men to be affected [81].
One of the best animal models for MS is EAE in mice and using this model, treatment with MSCs has demonstrated strong therapeutic effects [37, 82]. Intravenous administration of either mouse or human MSCs can be detected in the lymphoid organs and demyelinating regions of EAE mice, and results in amelioration of inflammation as well as symptoms and disease course [82, 83]. MSC treatment suppresses auto-reactive Th1/Th17 proliferation and infiltration in both in vitro and in vivo studies [82, 84, 85]. Other reports show that MSC treatment increases accumulation of Th2 cytokines—IL-4 and IL-5—and generation of Treg in vivo, both of which help reduce EAE symptomatology [83, 86]. Molecular mechanisms by which MSCs polarize CD4 T cells in EAE models include via indoleamine-2,3-dioxygenase (IDO) and monocyte chemoattractant protein-1/CC chemokine ligand 2 (MCP-1/CCL2) [87]. Interestingly, an in vitro human study found that while MSCs can effectively inhibit proliferation and IL-2 production by T cells isolated from MS patients as well as normal controls, T cells of MS patients still produce higher levels of IL-2 compared to normal control T cells, demonstrating the inherent pathological immune responses in these patients [88]. Based on these and many other preclinical studies demonstrating MSC therapeutic efficacy in animal disease models, these stem/progenitor cells were considered as strong candidates for treatment of patients with MS.
To date, there are 23 registered clinical trials using MSCs for treatment of MS, with 4 in Phase 1, 4 in Phase 2, and 15 as combined Phase 1/2. Sources of MSCs used in these trials are from the BM (n = 11), umbilical cord (n = 4) and adipose tissue (n =2), with 6 studies using unspecified sources. In general, the number of MSCs transplanted is approximately 2 × 106 cells/kg given either intravenously or intrathecally. One clinical trial has published results on determination of safety and efficacy of intravenously administration of autologous BM-MSCs for MS patients [38]. This Phase 2A trial, which included 10 MS patients and 8 healthy controls, demonstrated that the treatment was safe. While efficacy was difficult to evaluate, a few outcome parameters—mainly of optic nerve-based measures—demonstrated statistically significant or near significant improvement. The importance of this trial was also to establish detailed trial design and clinical efficacy measures for MSC therapy in MS. Resolution of these critical issues will help to pave the way for use of MSCs, which is one of the most novel methods of treating MS, in this incurable CNS disease.
Joint diseases: Osteoarthritis (OA) & Rheumatoid Arthritis (RA)
MSCs are an important therapeutic option for joint disease, since cartilage does not regenerate and these progenitor/stem cells are the endogenous progenitor for this tissue. Two major joint disease entities have been targeted for MSC treatment: OA and RA. OA is the most common joint disorder which is due to gradual deterioration of joint cartilage from ‘wear-and-tear.’ This subsequently induces an immune response with further resultant damage to joints [89]. Since cartilage does not regenerate, OA is a progressive and irreversible condition, with the incidence increasing with age and body weight. While immune injury is not the causative reason for OA, by the time patients seek medical help due to pain and joint stiffness, inflammation is well underway. Moreover, inhibition of the vicious cycle of cartilage destruction and immune attack is necessary in order for joint repair to occur. As such, MSCs are particularly suited for use in OA, since cartilage regeneration and immunosuppression can be achieved simultaneously [90]. Indeed, both small and large animal studies demonstrate that MSCs decrease inflammation in OA and allow for cartilage repair [91–93]. Currently, there are 38 clinical trials registered, with 9 in Phase 1, 16 in joint-Phase 1/2, and 8 in Phase 2. Not surprisingly, more than 18 % of studies have published results on the safety and efficacy of MSCs for OA treatment [42–46, 94]. Overall, these studies demonstrate quite positive results regarding improvement in symptomatology—including pain—and joint repair as seen by cartilage regeneration.
While translational and clinical data are generally positive for MSC therapy in OA, surprisingly this is not the case for RA. To date, there are only 5 clinical trials utilizing MSCs for RA treatment registered, with 1 trial in Phase 1, 3 in Phase 1/2 and 1 in Phase 2/3; no trials have yet published results. Unlike OA, RA is an autoimmune disease with a well-established animal model being the CIA model, in which autoimmune joint disease can be reproduced in rodent models [95]. Even with animal models, there are discrepant results with regards to MSC therapeutic effects [20, 96]. Clearly, there are detailed mechanistic differences between RA and OA which still need to be resolved, and may likely explain the therapeutic divergence in MSC therapy for the two joint diseases.
Inflammatory bowel diseases (IBD)
The etiology and progression of human IBD which includes Crohn’s disease (CD) and ulcerative colitis (UC) are multifactorial, but a critical part of these diseases is the uncontrolled immune responses to intestinal microbes [97]. Both CD and UC are progressively fatal without curative treatment, making MSCs an attractive therapeutic option for these chronic inflammatory diseases.
There are several experimental animal models for IBD, and among the commonly used models are the chemically-induced acute colitis models, with dextran sodium sulfate (DSS) supplemented in drinking water or 2, 4, 6-trinitrobenzene-sulfonate acid (TNBS) administrated by enema [98]. These are also the models in which MSC therapeutic effects were tested on [99, 100]: MSCs can be given by intraperitoneal or intravenous routes, and this can prevent DSS-induced morphological and immunogenic injury of the intestines. Moreover, application of MSCs can specifically reduce Th1 and Th17 responses as well as serum levels of proinflammatory IL-1β, IL-6, IL-17, TNF-α, IFN-γ levels, while enhancing the numbers of Tregs and splenic MDSCs [101, 102]. In TNBS animal models, injection of MSCs resulted in decreased immune cells infiltration and TNF-α expression, but increases of TGF-β levels in sites of injury [103]. To improve the efficacy of MSC treatment for IBD, these progenitor/stem cells have also been coated with antibodies against mucosal addressin cell adhesion moledule-1 (MAdCAM-1) and vascular cell adhesion molecule-1 (VCAM-1), both of which were shown to increase cell delivery to inflamed intestinal regions [104]. Immunosuppression was also enhanced when MSCs was modified with IL-12p40 or IL-37ß [105, 106].
Currently, there are 19 registered clinical trials using MSCs for IBD, with 3 for UC & 16 for CD. With the exception of 4 trials which are in Phase 3, all other trials are in Phase 1 and/or 2. Interestingly, there are already quite a number of published reports regarding treatment of MSCs for fistulas in CD in particular [48]. BM or adipose-derived MSCs were used in these trials, with 2 trials using autologous sources, 11 trials using allogenic source and 2 trials using undefined source. Collectively, a review of 15 trials (some registered at Clinicaltrials.gov but some are not) overwhelmingly demonstrate that MSC therapy is not only safe but therapeutically relevant, with some patients showing durable effects [107]. A very recent trial using allogeneic placenta-derived MSC-like cells (which was not registered) also showed favorable responses [108]. Thus, MSC therapy for IBD—especially CD fistula formation—appears to be safe and a highly viable option.
Inflammatory airway & pulmonary diseases
Inflammation is now known to affect many disease processes of the pulmonary system, including obstructive diseases like chronic obstructive lung diseases (COPD) and asthma, as well as restrictive diseases including idiopathic pulmonary fibrosis (IPF) and acute respiratory distress syndrome (ARDS). Whether as cause or consequence, the acute and chronic lung injury found in these diseases invariably involves aberrant immune activity and fibrosis [109, 110]. MSC therapy, indeed most cell therapies, may be particularly suited for use in pulmonary diseases since it has been consistently shown that the overwhelming majority (usually 80 ~ 90 %) of MSCs delivered intravenously—likely the most clinically feasible method for introduction of cellular products—will rapidly reach the lungs [111]. Under conditions of pulmonary inflammation, this percentage increases even further (Fig. 3) [112, 113]. A recent study also suggest that the lung may represent a unique niche for MSCs [114]. Thus, there has been rapid development of using MSCs for a wide range of pulmonary diseases.
Mechanisms involved in MSC therapy for inflammatory pulmonary diseases based on preclinical animal studies. Immunomodulatory effects include enhancing bacterial clearance by direct killing and enhancement of macrophage phagocytosis; decreasing inflammatory response by modulation of macrophages towards an M2 phenotype and inhibition of neutrophil recruitment; as well as reducing damage to alveolar epithelium
Interestingly, while specific inflammatory/immune processes are distinct for pulmonary diseases even within the same classification, i.e. COPD vs. asthma, MSCs have been shown in preclinical studies to impart therapeutic effects despite these pathophysiological differences. In COPD, inflammation driven by alveolar macrophages, cytotoxic T cells, and neutrophils leads to progressive limitations in airflow, with small airway fibrosis and alveolar destruction [115, 116]. In asthma, however, mast cells, eosinophils and Th2 lymphocytes are involved in further aggravation of airway hyperresponsiveness and bronchoconstriction [117]. In rodent models of elastase-induced emphysema or cigarette-induced COPD, MSC infusion reduces lung destruction and aberrant inflammation [118, 119]. MSC-secreted epidermal growth factor (EGF) leads to induction of secretory leukocyte protease inhibitor (SLPI), an inhibitor which protects epithelial tissues from serine protease degradation [120, 121]. Infusion of MSCs in a rat model of cigarette smoke-induced lung injury also results in down-regulation of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and MCP-1/CCL2, and up-regulation of VEGF and TGF-β [122]. In addition, MSC treatment can inhibit cyclooxygenase-2 (COX-2) and COX-2-mediated PGE2 production in alveolar macrophages to decrease inflammation [123]. For asthma, in rodent disease models using inhalation of toluene diisocyanate, ovalbumin or cockroach extract, MSC treatment modulated the immune milieu through generation of Tregs and inhibition of Th2 responses [124–126]. Reversal of disease symptomatology along with decreases in Th2 cytokines including IL-4, IL-5, and IL-13, as well as immunoglobulin E (IgE) levels, matrix metalloproteinase deposition, and mucus production were seen [127–129].
Even for fibrotic pulmonary diseases, MSC treatment appears to be efficacious. In fact, one of the earliest studies documenting therapeutic efficacy of MSC infusion was in mouse models of bleomycin-induced lung fibrosis, which is an animal model for IPF [112]. Subsequently, the same group demonstrated that MSC-secreted IL-1 receptor antagonist (IL-1RA) mediated the anti-inflammatory and anti-fibrotic effects [130]. Using the same disease model, infusion of umbilical cord MSCs were also shown to have therapeutic effects [131, 132]. In addition to anti-inflammatory effects, MSC treatment may reduce fibrosis through enhancing the resident lung bronchioalveolar stem cell population for repair and regeneration of healthy lung parenchyma [133]. Such profound effects induced by MSC treatment may account for the rapid push to clinical studies in this field, since about half of the basic and animal studies in this field were published within the past 3 years.
Most interestingly, MSC treatment can have therapeutic results in pneumonia of infectious etiology, especially bacterial pneumonia which clearly elicits intense inflammatory and immune responses. This is somewhat surprising given the strong immunosuppressive effects of MSCs towards effector cell functions. A lethal consequence of infection-induced pneumonia is ARDS, which is a complication with high mortality and morbidity despite medical advancements [134]. Using mice with lung injury induced by lipopolysaccharide, a component of gram-negative bacterial cell wall, delivery of MSCs or MSC-conditioned medium improved tissue damage and survival, which involved MSC-derived factors such as insulin-like growth factor I (IGF-I) and TNF-stimulated gene 6 protein (TSG-6) for generation of anti-inflammatory M2 macrophages and suppression of inflammatory cell infiltration [135–137]. In Escherichia coli (E. coli)-induced pneumonia rodent models, MSCs improved bacterial clearance by secreting antimicrobial peptide LL-37, antibacterial protein lipocalin 2 (LCN-2) and keratinocyte growth factor (KGF) directly against bacteria or by enhancing macrophage phagocytosis [138–140]. Moreover, administration of BMMSC-conditioned medium-derived microvesicles can also alleviate pulmonary inflammation and injury [141]. MSC treatment for viral pneumonia and subsequent lung injury, on the other hand, may not be as potent, with some reports demonstrating therapeutic effects [142] but not other reports [143, 144]. The dichotomous results of MSC treatment on bacterial compared to viral pneumonia may be due to the fact that MSCs have been shown by multiple studies to modulate neutrophil—the key leukocyte involved in bacterial but not viral infections—life span and functions [35, 36, 145, 146].
To date, 29 clinical studies of using MSCs for pulmonary disorders have been registered. Targeted disease entities include asthma, COPD, ARDS, bronchial pulmonary dysplasia (BPD), and fibrosis (including but not exclusive for IPF), with trials being in Phase 1 (n = 14), Phase 2 (n = 4), or combined Phase 1/2 (n = 11). There are a few published reports of MSC trials for various lung diseases, with the largest published trial being a Phase 2 multicenter study with 62 patients evaluating allogenic BMMSCs for COPD [50]. While safe, the trial did not demonstrate much efficacy. Other published studies are for Phase 1 trials using various tissue-source allogeneic MSCs infused intravenously (except where noted): two trials on ARDS, one using adipose-derived MSCs [147] and one using BMMSCs [51]; one using placental-derived MSCs for IPF [148]; and one using umbilical cord blood MSCs (delivered intratracheally) for preterm BPD [49]. All three reports showed safety of MSC infusion, but efficacy was weak at best. The strong evidence shown in preclinical animal studies does not seem to be replicated in human trials so far, and this may be a consequence of the diversity of lung diseases targeted, as well as the fact that multiple tissue source of MSCs were used. In addition, whether differences in MSC tissue source affect homing capacity is also a critical issue. Thus, careful selection of patient populations and more research into whether tissue-specific MSCs harbor distinct therapeutic effects are warranted.
Conclusion
The immunomodulatory properties of MSCs have become increasingly relevant for clinical use. Based on hundreds of clinical trials, the safety of this therapy appears clear; less certain is the efficacy of such cell therapy. The overwhelming positive results seen in preclinical animal studies have largely not yet translated into clinical efficacy. Clearly, there is still much to learn and optimize with regards to the in vivo interactions of MSCs in human pathological states. As we improve our understanding on the mechanistic properties of MSC immunomodulation, we also need to clarify pathophysiological details and subsets within disease entities to better tailor MSC therapy. One important aspect is to delineate tissue-specific functional differences in MSCs from difference sources; the current ISCT standardization does not include immune-related functional tests or more detailed molecular validation. In addition, standardization of in vitro culture protocols with stringent criteria for testing of functional parameters is necessary as well. While there is clearly much still to do in this field, it must be remembered that even for HSC transplantation—a clinically established treatment modality—continued evolution in improving engraftment and decreasing complications is still ongoing. Nevertheless, based on current development and results, the tremendous potential of MSC therapy can be expected in the near future to achieve clinical relevance.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- BM:
-
Bone marrow
- BPD:
-
Bronchial pulmonary dysplasia
- CCL2:
-
CC chemokine ligand 2
- CD:
-
Crohn’s disease
- CIA:
-
Collagen-induced arthritis
- CNS:
-
Central nerve system
- COPD:
-
Chronic obstructive lung diseases
- COX2:
-
Cyclooxygenase-2
- DC:
-
Dendritic cell
- DSS:
-
Dextran sodium sulfate
- E. coli:
-
Escherichia coli
- EAE:
-
Experimental autoimmune encephalomyelitis
- EGF:
-
Epidermal growth factor
- GVHD:
-
Graft-versus-host disease
- GVL:
-
Graft-versus-leukemia
- HSC:
-
Hematopoietic stem cell
- IBD:
-
Inflammatory bowel disease
- IDO:
-
Indoleamine-2, 3-dioxygenase
- IFN-γ:
-
Interferon-γ
- IgE:
-
Immunoglobulin E
- IGF-I:
-
Insulin-like growth factor I
- IL:
-
Interleukin
- IL-1RA:
-
IL-1 receptor antagonist
- IPF:
-
Idiopathic pulmonary fibrosis
- ISCT:
-
International society for cellular therapy
- KGF:
-
Keratinocyte growth factor
- LCN-2:
-
Antibacterial protein lipocalin 2
- MAdCAM-1:
-
Mucosal addressin cell adhesion moledule-1
- MBP:
-
Myelin-based protein
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDSC:
-
Myeloid-derived suppressor cell
- MS:
-
Multiple sclerosis
- MSC:
-
Mesenchymal stem cell
- NK:
-
Natural killer cell
- NO:
-
Nitric oxide
- OA:
-
Osteoarthritis
- PGE2:
-
Prostaglandin E2
- RA:
-
Rheumatoid arthritis
- SLPI:
-
Secretory leukocyte protease inhibitor
- T1DM:
-
Type 1 diabetes mellitus
- TGF-β1:
-
Transforming growth factor β1
- Th:
-
T helper cell type
- TNBS:
-
2, 4, 6-Trinitrobenzene-sulfonate acid
- TNF-α:
-
Tumor necrosis factor-α
- Treg:
-
Regulatory T cell
- TSG-6:
-
TNF-stimulated gene 6 protein
- UC:
-
Ulcerative colitis
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
References
Hochedlinger K, Jaenisch R. Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N Engl J Med. 2003;349(3):275–86.
Korbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349(6):570–82.
Boo M, Van Walraven SM, Chapman J, Lindberg B, Schmidt AH, Shaw BE, Switzer GE, Yang E, Egeland T. Remuneration of hematopoietic stem cell donors: principles and perspective of the World Marrow Donor Association. Blood. 2011;117(1):21–5.
Rosenzweig A. Cardiac cell therapy--mixed results from mixed cells. N Engl J Med. 2006;355(12):1274–7.
Von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–8.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–59.
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–16.
Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. Int Soc Cell Ther position statement Cytotherapy. 2006;8(4):315–7.
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8.
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43.
Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20.
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41.
Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36.
Gotherstrom C, Ringden O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190(1):239–45.
Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24(11):2466–77.
Griffin MD, Elliman SJ, Cahill E, English K, Ceredig R, Ritter T. Concise review: adult mesenchymal stromal cell therapy for inflammatory diseases: how well are we joining the dots? Stem Cells. 2013;31(10):2033–41.
Tyndall A. Mesenchymal stem cell treatments in rheumatology: a glass half full? Nat Rev Rheumatol. 2014;10(2):117–24.
Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012;91(11):1003–10.
Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2(4):34.
Engela AU, Baan CC, Litjens NH, Franquesa M, Betjes MG, Weimar W, Hoogduijn MJ. Mesenchymal stem cells control alloreactive CD8(+) CD28(−) T cells. Clin Exp Immunol. 2013;174(3):449–58.
Li M, Sun X, Kuang X, Liao Y, Li H, Luo D. Mesenchymal stem cells suppress CD8+ T cell-mediated activation by suppressing natural killer group 2, member D protein receptor expression and secretion of prostaglandin E2, indoleamine 2, 3-dioxygenase and transforming growth factor-beta. Clin Exp Immunol. 2014;178(3):516–24.
Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49.
Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482–90.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72.
Chen PM, Liu KJ, Hsu PJ, Wei CF, Bai CH, Ho LJ, Sytwu HK, Yen BL. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J Leukoc Biol. 2014;96(2):295–303.
Cutler AJ, Limbani V, Girdlestone J, Navarrete CV. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol. 2010;185(11):6617–23.
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6.
Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolome ST, Sambuceti G, Traggiai E, Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108(42):17384–9.
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9.
Oh JY, Ko JH, Lee HJ, Yu JM, Choi H, Kim MK, Wee WR, Prockop DJ. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells. 2014;32(6):1553–63.
Yen BL, Yen ML, Hsu PJ, Liu KJ, Wang CJ, Bai CH, Sytwu HK. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports. 2013;1(2):139–51.
Chen CP, Chen YY, Huang JP, Wu YH. The effect of conditioned medium derived from human placental multipotent mesenchymal stromal cells on neutrophils: possible implications for placental infection. Mol Hum Reprod. 2014;20(11):1117–25.
Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29(6):1001–11.
Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10(7):649–56.
Connick P, Kolappan M, Patani R, Scott MA, Crawley C, He XL, Richardson K, Barber K, Webber DJ, Wheeler-Kingshott CA, Tozer DJ, Samson RS, Thomas DL, Du MQ, Luan SL, Michell AW, Altmann DR, Thompson AJ, Miller DH, Compston A, Chandran S. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12:62.
Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L, Vanbellinghen JF, Hafraoui K, Lejeune M, Gothot A, Fillet G, Beguin Y. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2010;16(6):838–47.
Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, Longoni D, Pavan F, Masciocchi F, Algarotti A, Mico C, Grassi A, Deola S, Cavattoni I, Gaipa G, Belotti D, Perseghin P, Parma M, Pogliani E, Golay J, Pedrini O, Capelli C, Cortelazzo S, D’Amico G, Biondi A, Rambaldi A, Biagi E. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. 2014;20(3):375–81.
Perez-Simon JA, Lopez-Villar O, Andreu EJ, Rifon J, Muntion S, Diez CM, Sanchez-Guijo FM, Martinez C, Valcarcel D, Canizo CD. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica. 2011;96(7):1072–6.
Emadedin M, Ghorbani LM, Fazeli R, Mohseni F, Moghadasali R, Mardpour S, Hosseini SE, Niknejadi M, Moeininia F, Aghahossein FA, Baghban ER, Vosough DA, Labibzadeh N, Mirazimi BA, Baharvand H, Aghdami N. Long-Term Follow-up of Intra-articular Injection of Autologous Mesenchymal Stem Cells in Patients with Knee, Ankle, or Hip Osteoarthritis. Arch Iran Med. 2015;18(6):336–44.
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–66.
Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentis J, Sanchez A, Garcia-Sancho J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: 2-year follow-up results. Transplantation. 2014;97(11):e66–8.
Vega A, Martin-Ferrero MA, Del Canto F, Alberca M, Garcia V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sanchez A, Garcia-Sancho J. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99(8):1681–90.
Vives J, Oliver-Vila I, Pla A. Quality compliance in the shift from cell transplantation to cell therapy in non-pharma environments. Cytotherapy. 2015;17(8):1009–14.
Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12(1):64–71.
Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga JJ, Verspaget HW, Fibbe WE, van der Meulen-de Jong AE, Hommes DW. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2015;149(4):918–927 e916.
Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, Park WS. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164(5):966–972 e966.
Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–8.
Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32.
Quesenberry P, Levitt L. Hematopoietic stem cells. N Engl J Med. 1979;301(14):755–61.
Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125(4):606–15.
Levine JE, Paczesny S, Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S116–24.
Szyska M, Na IK. Bone Marrow GvHD after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2016;7:118.
Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, Horn B, Yu L, Talano JA, Nemecek E, Mills CR, Chaudhury S. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20(2):229–35.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86.
Sanchez-Guijo FM, Lopez-Villar O, Lopez-Anglada L, Villaron EM, Muntion S, Diez-Campelo M, Perez-Simon JA, San Miguel JF, Caballero D, Del Canizo MC. Allogeneic mesenchymal stem cell therapy for refractory cytopenias after hematopoietic stem cell transplantation. Transfusion. 2012;52(5):1086–91.
Weng J, He C, Lai P, Luo C, Guo R, Wu S, Geng S, Xiangpeng A, Liu X, Du X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther. 2012;20(12):2347–54.
Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, Wu SJ, Luo CW, Guo R, Ling W, Deng CX, Liao PJ, Xiang AP. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45(12):1732–40.
Baker M. Stem cells: Fast and furious. Nature. 2009;458(7241):962–5.
Rizk M, Monaghan M, Shorr R, Kekre N, Bredeson CN, Allan DS. Heterogeneity in studies of mesenchymal stromal cells to treat or prevent GVHD: a scoping review of the evidence. Biol Blood Marrow Transplant. 2016;22(8):1416–23.
Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15(1):2–8.
Moll G, Alm JJ, Davies LC, Von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M, Lonnies H, Lambris JD, Teramura Y, Nilsson-Ekdahl K, Nilsson B, Le Blanc K. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32(9):2430–42.
Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117(12):3268–76.
Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26(10):2531–41.
Robles JD, Liu YP, Cao J, Xiang Z, Cai Y, Manio M, Tang EH, Chan GC. Immunosuppressive mechanisms of human bone marrow derived mesenchymal stromal cells in BALB/c host graft versus host disease murine models. Exp Hematol Oncol. 2015;4:13.
Tobin LM, Healy ME, English K, Mahon BP. Human mesenchymal stem cells suppress donor CD4(+) T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease. Clin Exp Immunol. 2013;172(2):333–48.
Auletta JJ, Eid SK, Wuttisarnwattana P, Silva I, Metheny L, Keller MD, Guardia-Wolff R, Liu C, Wang F, Bowen T, Lee Z, Solchaga LA, Ganguly S, Tyler M, Wilson DL, Cooke KR. Human mesenchymal stromal cells attenuate graft-versus-host disease and maintain graft-versus-leukemia activity following experimental allogeneic bone marrow transplantation. Stem Cells. 2015;33(2):601–14.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50.
Gregoire-Gauthier J, Selleri S, Fontaine F, Dieng MM, Patey N, Despars G, Beausejour CM, Haddad E. Therapeutic efficacy of cord blood-derived mesenchymal stromal cells for the prevention of acute graft-versus-host disease in a xenogenic mouse model. Stem Cells Dev. 2012;21(10):1616–26.
Jang MJ, Kim HS, Lee HG, Kim GJ, Jeon HG, Shin HS, Chang SK, Hur GH, Chong SY, Oh D, Chung HM. Placenta-derived mesenchymal stem cells have an immunomodulatory effect that can control acute graft-versus-host disease in mice. Acta Haematol. 2013;129(4):197–206.
Jang YK, Kim M, Lee YH, Oh W, Yang YS, Choi SJ. Optimization of the therapeutic efficacy of human umbilical cord blood-mesenchymal stromal cells in an NSG mouse xenograft model of graft-versus-host disease. Cytotherapy. 2014;16(3):298–308.
Luz-Crawford P, Torres MJ, Noel D, Fernandez A, Toupet K, Alcayaga-Miranda F, Tejedor G, Jorgensen C, Illanes SE, Figueroa FE, Djouad F, Khoury M. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells. 2016;34(2):456–69.
Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516–25.
Verneris MR. Natural killer cells and regulatory T cells: how to manipulate a graft for optimal GVL. Hematology Am Soc Hematol Educ Program. 2013;2013:335–41.
Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125(2):161–9.
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–5.
Carnegie PR. Amino acid sequence of the encephalitogenic basic protein from human myelin. Biochem J. 1971;123(1):57–67.
Feinstein A, Freeman J, Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol. 2015;14(2):194–207.
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800.
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–61.
Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57(11):1192–203.
Lanz TV, Opitz CA, Ho PP, Agrawal A, Lutz C, Weller M, Mellor AL, Steinman L, Wick W, Platten M. Mouse mesenchymal stem cells suppress antigen-specific TH cell immunity independent of indoleamine 2,3-dioxygenase 1 (IDO1). Stem Cells Dev. 2010;19(5):657–68.
Sajic M, Hunt DP, Lee W, Compston DA, Schweimer JV, Gregson NA, Chandran S, Smith KJ. Mesenchymal stem cells lack efficacy in the treatment of experimental autoimmune neuritis despite in vitro inhibition of T-cell proliferation. PLoS One. 2012;7(2), e30708.
Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F. Mesenchymal stem cells generate a CD4 + CD25 + Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65.
Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K, Basik M, Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182(10):5994–6002.
Ben-Ami E, Miller A, Berrih-Aknin S. T cells from autoimmune patients display reduced sensitivity to immunoregulation by mesenchymal stem cells: role of IL-2. Autoimmun Rev. 2014;13(2):187–96.
Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–7.
Noth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nature clinical practice. Rheumatology. 2008;4(7):371–80.
Horie M, Choi H, Lee RH, Reger RL, Ylostalo J, Muneta T, Sekiya I, Prockop DJ. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthritis Cartilage. 2012;20(10):1197–207.
Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, Roberts S, Baba H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1):R31.
Toghraie F, Razmkhah M, Gholipour MA, Faghih Z, Chenari N, Torabi NS, Nazhvani DS, Ghaderi A. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;15(8):495–9.
Soler R, Orozco L, Munar A, Huguet M, Lopez R, Vives J, Coll R, Codinach M, Garcia-Lopez J. Final results of a phase I-II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23(4):647–54.
Seki N, Sudo Y, Mizuhara H, Orito K, Imasaki A, Ono S, Hamaoka T, Senoh H, Fujiwara H. Type II collagen-induced murine arthritis: induction of arthritis depends on antigen-presenting cell function as well as susceptibility of host to an anticollagen immune response. J Immunol. 1992;148(10):3093–9.
Bouffi C, Djouad F, Mathieu M, Noel D, Jorgensen C. Multipotent mesenchymal stromal cells and rheumatoid arthritis: risk or benefit? Rheumatology (Oxford). 2009;48(10):1185–9.
Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–78.
Chinnadurai R, Ng S, Velu V, Galipeau J. Challenges in animal modelling of mesenchymal stromal cell therapy for inflammatory bowel disease. World J Gastroenterol. 2015;21(16):4779–87.
Abdel Salam AG, Ata HM, Salman TM, Rashed LA, Sabry D, Schaalan MF. Potential therapeutic utility of mesenchymal stem cells in inflammatory bowel disease in mice. Int Immunopharmacol. 2014;22(2):515–21.
He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57(12):3136–44.
Forte D, Ciciarello M, Valerii MC, De Fazio L, Cavazza E, Giordano R, Parazzi V, Lazzari L, Laureti S, Rizzello F, Cavo M, Curti A, Lemoli RM, Spisni E, Catani L. Human cord blood-derived platelet lysate enhances the therapeutic activity of adipose-derived mesenchymal stromal cells isolated from Crohn’s disease patients in a mouse model of colitis. Stem Cell Res Ther. 2015;6:170.
Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, Zanotti L, D’Alessio S, Scaldaferri F, Luca G, Arato I, Calafiore R, Sgambato A, Rutella S, Locati M, Danese S, Vetrano S. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology. 2015;149(1):163–176 e120.
Zuo D, Tang Q, Fan H, Shou Z, Liu X, Cao D, Zou Z. Modulation of nuclear factor-kappaB-mediated pro-inflammatory response is associated with exogenous administration of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis. Mol Med Rep. 2015;11(4):2741–8.
Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18(7):1365–72.
Kim DJ, Kim KS, Song MY, Seo SH, Kim SJ, Yang BG, Jang MH, Sung YC. Delivery of IL-12p40 ameliorates DSS-induced colitis by suppressing IL-17A expression and inflammation in the intestinal mucosa. Clin Immunol. 2012;144(3):190–9.
Wang WQ, Dong K, Zhou L, Jiao GH, Zhu CZ, Li WW, Yu G, Wu WT, Chen S, Sun ZN, Wang YM, Liu WT, Zhang J, Wang BM, Feng XM. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 2015;36(11):1377–87.
Garcia-Olmo D, Schwartz DA. Cumulative Evidence That Mesenchymal Stem Cells Promote Healing of Perianal Fistulas of Patients With Crohn’s Disease--Going From Bench to Bedside. Gastroenterology. 2015;149(4):853–7.
Melmed GY, Pandak WM, Casey K, Abraham B, Valentine J, Schwartz D, Awais D, Bassan I, Lichtiger S, Sands B, Hanauer S, Richards R, Oikonomou I, Parekh N, Targan S, Johnson K, Hariri R, Fischkoff S. Human Placenta-derived Cells (PDA-001) for the Treatment of Moderate-to-severe Crohn's Disease: A Phase 1b/2a Study. Inflamm Bowel Dis. 2015;21(8):1809–16.
Inamdar AC, Inamdar AA. Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp Lung Res. 2013;39(8):315–27.
Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2(12):1016–26.
Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci. 2004;117(Pt 23):5655–64.
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–11.
Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117.
Sinclair K, Yerkovich ST, Chambers DC. Mesenchymal stem cells and the lung. Respirology. 2013;18(3):397–411.
Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56(4):515–48.
Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014;5:435.
Postma DS, Rabe KF. The Asthma-COPD Overlap Syndrome. N Engl J Med. 2015;373(13):1241–9.
Kim YS, Kim JY, Huh JW, Lee SW, Choi SJ, Oh YM. The Therapeutic Effects of Optimal Dose of Mesenchymal Stem Cells in a Murine Model of an Elastase Induced-Emphysema. Tuberc Respir Dis (Seoul). 2015;78(3):239–45.
Tibboel J, Keijzer R, Reiss I, De Jongste JC, Post M. Intravenous and intratracheal mesenchymal stromal cell injection in a mouse model of pulmonary emphysema. COPD. 2014;11(3):310–8.
Broekman W, Amatngalim GD, De Mooij-Eijk Y, Oostendorp J, Roelofs H, Taube C, Stolk J, Hiemstra PS. TNF-alpha and IL-1beta-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir Res. 2016;17(1):3.
Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, Saijo Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19(1):196–203.
Guan XJ, Song L, Han FF, Cui ZL, Chen X, Guo XJ, Xu WG. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem. 2013;114(2):323–35.
Gu W, Song L, Li XM, Wang D, Guo XJ, Xu WG. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci Rep. 2015;5:8733.
Gao P, Zhou Y, Xian L, Li C, Xu T, Plunkett B, Huang SK, Wan M, Cao X. Functional effects of TGF-beta1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma. J Immunol. 2014;192(10):4560–70.
Lee SH, Jang AS, Kwon JH, Park SK, Won JH, Park CS. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy, Asthma Immunol Res. 2011;3(3):205–11.
Ou-Yang HF, Huang Y, Hu XB, Wu CG. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood). 2011;236(12):1461–7.
Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):L760–70.
Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, Tse HF, Xu G, Lian Q, Shi J, Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012;30(12):2692–9.
Xu T, Zhou Y, Qiu L, Do DC, Zhao Y, Cui Z, Wang H, Liu X, Saradna A, Cao X, Wan M, Gao P. Aryl Hydrocarbon Receptor Protects Lungs from Cockroach Allergen-Induced Inflammation by Modulating Mesenchymal Stem Cells. J Immunol. 2015;195(12):5539–50.
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104(26):11002–7.
Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18(8):869–86.
Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175(1):303–13.
Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Alex MS, Kourembanas S, Kim CF. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302(9):L829–37.
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93.
Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, Sullivan DE. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2(3):27.
Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179(3):1855–63.
Ionescu L, Byrne RN, Van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L967–77.
Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67(6):533–9.
Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–38.
Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187(7):751–60.
Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med. 2015;192(3):324–36.
Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, Nicholls JM, Fang X, Guan Y, Lee JW, Chan RW, Webster RG, Matthay MA, Peiris JS. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A. 2016;113(13):3621–6.
Darwish I, Banner D, Mubareka S, Kim H, Besla R, Kelvin DJ, Kain KC, Liles WC. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS One. 2013;8(8), e71761.
Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol. 2014;307(5):L395–406.
Hall SR, Tsoyi K, Ith B, Padera Jr RF, Lederer JA, Wang Z, Liu X, Perrella MA. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013;31(2):397–407.
Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26(1):151–62.
Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39.
Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, Yerkovich ST, Khalil D, Atkinson KM, Hopkins PM. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19(7):1013–8.
Acknowledgement
Not applicable.
Funding
This work was supported in part by funding from the NHRI (CS-105-PP-06) and the Taiwan Ministry of Science & Technology (MOST-104-2321-B-400-021 and MOST-104-2314-B-400-002).
Availability of data and materials
Not applicable (review article).
Authors’ contributions
LZ W, CHT, & BLY conceived the concept, researched and analyzed the literature, and wrote the manuscript; MLY, KJL, HKS, & KKW analyzed the literature and edited the manuscript. All read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable (review article).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, LT., Ting, CH., Yen, ML. et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci 23, 76 (2016). https://doi.org/10.1186/s12929-016-0289-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12929-016-0289-5