Abstract
Background
Fowl adenovirus is of major concern to the poultry industry worldwidely. In order to monitor the prevalent status of Fowl adenovirus in China, a total of 1920 clinical samples from apparently healthy birds in the 25 sites of poultry flocks, Slaughterhouse and living bird markets from 8 provinces in eastern China were collected and detected by PCR, sequencing, and phylogenetic analysis.
Results
The epidemiological survey showed that Fowl adenoviruses were detected in living bird markets, and circulating in a variety of fowl species, including chickens, ducks, goose and pigeons. Among the 1920 clinical samples, 166 samples (8.65%) were positive in the fowl adenovirus PCR detection. In this study, totally all the 12 serotypes (serotypes of 1, 2, 3, 4, 5, 6, 7, 8A, 8B, 9, 10 and 11) fowl adenoviruses were detected, the most prevalent serotype was serotype 1. Phylogenetic analysis indicated that 166 FAdVs of 12 serotypes were divided into 5 fowl adenovirus species (Fowl aviadenovirus A, B, C, D, E).
Conclusions
In the epidemiological survey, 8.65% of the clinical samples from apparently healthy birds were positive in the fowl adenovirus PCR detection. Totally all the 12 serotypes fowl adenoviruses were detected in a variety of fowl species, which provided abundant resources for the research of fowl adenoviruses in China. The newly prevalent FAdV serotypes provides valuable information for the development of an effective control strategy for FAdV infections in fowls.
Similar content being viewed by others
Background
Fowl adenoviruses (FAdV) belong to genus Aviadenovirus in the family Adenoviridae, which resulting in huge economic losses to the poultry industry worldwide [1, 2]. FAdV is a double stranded DNA viruses, with a genome of 43–45 kb in size and a diameter of 70–100 nm [3]. Serologically different FAdV types are classified into five species (Fowl aviadenovirus A-E) [2, 4]. FAdVs are further classified into 12 serotypes (FAdV-1 to 8a and 8b to 11) by cross-neutralization tests [2, 5]. FAdV-2, FAdV-7, FAdV-8a, FAdV-8b and FAdV-11 can cause inclusion body hepatitis (IBH) [1, 2, 4, 6], and FAdV-4 can result in hepatitis hydropericardium syndrome (HHS) [2, 6].
FAdV can cause severe immunosuppression in infected birds. Clinical cases of FAdV infection have been widely reported in avian populations worldwide, and multiple FAdV strains have been isolated from dead or sick animals [1, 2, 7,8,9,10]. In China, FAdV-4 was considered as the dominant serotype in recent years [7, 11,12,13], which has resulted in huge economic losses in poultry industry in China since 2015 [11]. Several outbreaks caused by FAdV-4 was reported in China [4, 7, 11,12,13,14]. FAdV-8a and FAdV-8b were also the circulating serotypes in China between 2007 to 2014 and 2015 to 2018 [11, 14]. But, FAdV-11 was the predominant serotype in some regions in China from 2007 to 2014, according to the previous report [14]. FAdV-1 [1], FAdV-2 [15], FAdV-7 [4] and FAdV-10 [15] were also identified in China.
In this study, a total of 1920 clinical samples were collected from apparently healthy birds in 25 poultry flocks, Slaughterhouse and living bird markets (LBMs) from 8 provinces in eastern China. This is a comprehensive survey from apparently healthy fowls. FAdVs were detected and serotyped to investigate the prevalence of FAdV and analyze the genetic epidemiology.
Results
Epidemiological analysis
Among the 1920 clinical samples, 166 samples (8.65%) were positive in the FAdV PCR detection. Spatial analysis showed that, except Hebei province, FAdVs were detected in all the other 7 provinces, including Hubei, Jiangxi, Shanghai, Guangxi, Jiangsu, Guangdong, Anhui (Fig. 1). The FAdV infection rate in Huhei province is the highest (23.75%). The details were showed in Table 1. All the FAdVs were detected from LBMs. All the samples collected from large-scale farms were negative in PCR detection. In the samples collected from 5 kinds of fowl, FAdV was detected in samples of chicken (13.27%), goose (2.50%), duck (0.48%) and pigeon (0.43%), while negative in the samples of partridge.
Molecular serotype identification of FAdVs
Total 12 FAdV serotypes were detected in the 1920 clinical samples. The hexon gene fragment sequences of the FAdVs were submitted to the GenBank database, National Center for Biotechnology Information and were assigned the accession numbers of ON502449-ON502604, and ON462358-ON462367. In the detected 166 FAdVs, the most prevalent serotypes were serotype 1 (30/166, 18.07%), serotype 4 (28/166, 16.87%), serotype 3 (27/166, 16.27%), serotype 2 (26/166, 15.66%) (Table 1). These 4 serotypes were accounting for 66.87% of all the detected FAdVs. All the 166 FAdV strains inferred the 12 serotypes were likely to be circulating in chickens. Besides in chicken samples, serotype 4 FAdVs were detected in duck, goose and pigeon samples, while serotype 8B FAdV was detected in duck samples. The FAdV serotype distribution in the 8 provinces and 5 kinds of fowls was showed in Tables 1 and 2, respectively.
In 166 cases, 18.07% (30/166) of the isolates were related to FAdV-A (including serotype 1), and 3.61% (6/166) of the isolates were identified as FAdV-B (including serotype 5), and 21.69% (36/166) of the isolates were identified as FAdV-C (including serotypes 4 and 10), and 38.55% (64/166) of the isolates were identified as FAdV-D (including serotypes 2, 3, 9, and 11), and 18.07% (30/166) of the isolates were identified as FAdV-E (including serotypes 6, 7, 8A and 8B). The prevalent rate of circulating species was showed in Table 3.
Phylogenetic analysis of FAdVs
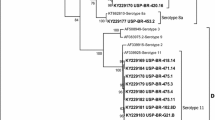
The results of the phylogenetic analysis of FAdVs detected in this study and reference strains are shown in Fig. 2. In the analysis, the FAdV strains clustered into five major groups. Cluster 1 is corresponding to Fowl aviadenovirus D (FAdV-D), including serotypes 2, 3, and 11 FAdVs. Cluster 2 is corresponding to Fowl aviadenovirus E (FAdV-E), including serotypes 6, 7, 8A and 8B FAdVs. Cluster 3 is corresponding to Fowl aviadenovirus B (FAdV-B), including serotype 5 FAdVs. Cluster 4 is corresponding to Fowl aviadenovirus A (FAdV-A), including serotype 1 FAdV. Cluster 5 is corresponding to Fowl aviadenovirus C (FAdV-C), including serotypes 4 and 10 FAdVs. The phylogenetic analysis result in this study was consistent with the previous studies [4, 14, 16].
Phylogenetic analysis of FAdVs detected in this study and reference strains. Phylogenetic relationships were calculated using the model with the Maximum likelihood (ML). Gaps were handled by pairwise deletion and bootstrap values were calculated from 1, 000 replicates. FAdV strains in this study were marked with “▲”
Discussion
FAdVs are commonly present in fowl farms worldwide [17]. As reported, more and more FAdVs have been isolated from dead or sick animals in recent years [2, 6, 18,19,20]. FAdV infections are associated with a range of avian infectious diseases, such as IBH and HHS.
In China, since 2015, sporadic outbreaks of HHS occurred with suddenly high mortality rates in layers in most areas in China [7]. It was previously reported that the FAdV infection was caused by a variety of different FAdV species in China, at least two or three species of FAdVs (species C, D or E) were detected [7, 11]. In our survey, the present epidemiology surveillance showed more abundant diversity and wider distribution than the previous reported studies in China [7, 12, 14], the surveillance showed that all the five species (species A, B, C, D and E) FAdVs were detected in the FAdV survey in 7 provinces (Jiangxi, Shanghai, Guangxi, Jiangsu, Guangdong, Anhui, Hebei) of China. Significantly, all the detected samples in this survey were collected from apparently healthy birds, no diseased or dead fowls were sampled. This is a comprehensive survey from apparently healthy fowls. It has great significance for the prevention and control of circulation of fowl adenovirus in China.
Among all the circulating FAdV species, FAdV-D showed the highest percentage of 38.55% (including serotypes 2, 3, 9, and 11) in this study, which is different with the previous study [4, 11]. In this study, FAdV-D strains mainly circulating in Southern provinces of China, which including Anhui, Guangdong, Guangxi, Hubei, Jiangsu, Jiangxi, and Shanghai. Meanwhile, the prevalence rate of FAdV-A, FAdV-C and FAdV-E is maintained between 18.07% and 21.69% in southern China. Although the prevalent rate of species FAdV-A, FAdV-C and FAdV-E was lower than the dominant species FAdV-D, the strict biosecurity measures may be necessary to the prevention and control of FAdVs.
In the previous study, FAdV-1 [1], FAdV-2 [15], FAdV-4 [11], FAdV-7 [4], FAdV-8a [11], FAdV-8b [11], FAdV-10 [15], and FAdV-11 [14] have been reported in China. In this study, 166 samples (166/1920, 8.65%) were detected FAdV positive in the 1920 clinical samples, and all the FAdVs were detected from LBMs, the results suggest that biosafety measures should be strengthened in the LBMs. Totally all the FAdV serotypes of 1, 2, 3, 4, 5, 6, 7, 8A, 8B, 9, 10 and 11 were detected. Slightly different from previous reports [4, 11], the most prevalent serotype in this study was serotype 1 (30/166, 18.07%), not serotype 4 [11], FAdV-11 [14] or FAdV-8a [11], FAdV-8b [11]. Of course, serotypes 2, 3 and 4 remained at a relatively high prevalence rate of 15.66%, 16.27% and 16.87%, respectively. This difference may be due to the fact that FADVs are screened from apparently healthy birds, and the published articles are from diseased birds. The prevalence rate of other serotypes of FAdVs was below 7% in China in this study. In this study, the circulating serotypes showed abundant diversity and distributed in 8 provinces in China, this increased the difficulty for the prevention and control of the FAdVs.
The results showed that all the FAdV-D strains and the most prevalent serotype 1 strains were detected from chickens in LPMs, no positive samples were detected from slaughterhouse or large-scale farms. This indicated that chicken may be an important risk, which indicated that chickens in the LPMs of southern China may play an important role in the transmission and circulation of FAdVs. According to the relevant reports around the world, duck adenovirus have been reported since 2014 [21,22,23,24,25,26], and infection of FAdV-4 in geese and pigeon adenovirus 1 have been reported in the previous study [27,28,29]. Meanwhile, FAdVs were also detected from ducks, geese and pigeons in this study. The role of waterfowl and birds (e.g. pigeon) in the spread of FAdVs should be paid more attention in the further study. All the cloacal/throat double swabs in this study were collected from clinical healthy birds according to a random sampling method. The results showed that 8.65% collected samples were positive in the FAdV detection. As reported, it is believed that the mechanism of FAdV infection is very complex in chicken flocks [7]. The pathogenicity, such as FAdV-D and FAdV-E, was not evaluated and, thus, further investigation are warranted.
Conclusion
Taken together, in the FAdV epidemiological survey, 8.65% of the clinical samples from apparently healthy birds were positive in the fowl adenovirus PCR detection. Totally all the 12 serotypes fowl adenoviruses were detected in a variety of fowl species, which provided abundant resources for the research of fowl adenoviruses in China. The present study provides new information about the epidemiology and characteristics of fowl adenoviruses associated with chickens, ducks and pigeons in China, which will provide a basis for further understanding of the disease, and would aid in the prevention and control of FAdVs. Surveillance for fowl adenovirus must be continued worldwide.
Materials and methods
Sample collection and Nucleic acid extraction
In 2019, totally 25 sites of large-scale farms, slaughterhouse and LBMs of the 8 provinces (Hubei, Jiangxi, Shanghai, Guangxi, Jiangsu, Guangdong, Anhui, Hebei) in China were selected at random. Cloacal/throat double swabs were collected from more than 60 clinical healthy birds at each farm according to a random sampling method. Totally, 1920 swab samples were collected, the sample details were listed in Table 4. The samples were stored at 4 °C by a preservation buffer of 1.2 mL phosphate buffered saline (PBS, pH 7.2) containing 10% glycerol. Based on the QIAxtractor platform, viral DNA was extracted using the cador Pathogen 96 QIAcube HT kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and stored at − 80 °C.
Screening of FAdV in clinical samples
In order to screen and analyze FAdV, conventional PCR using degenerate primers specific for the L1 loop of the FAdV hexon genes was applied according to the published report [30]. The primers were designed by alignment comparison of the conserved sequences of FAdV1, FAdV8 and FAdV9, and were able to amplify the 12 serotypes of FAdVs [30]. A 897-base fragment of hexon gene was amplified using primers: hexon A (forward, in the position 144–161 of FAdV1 with the GenBank accession number of U46933) 5′- CAARTTCAGRCAGACGGT-3′ and hexon B (reverse, in the position 1229–1211 of FAdV1 with the GenBank accession number of U46933) 5′- TAGTGATGMCGSGACATCAT -3′. The DNA was tested by PCR with the Premix Taq™ (Ex Taq™ Version 2.0) (Takara, Dalian, China; RR003Q), and the reaction system included 12.5 μL of Premix Taq, 1 μL each of the forward and reverse primers (10 pM), 3 μL of DNA, and 7.5 μL of RNase-free H2O. The thermocycling conditions were as follows: 95 °C for 5 min, followed by 40 cycles of 98 °C for 30 s, 60 °C for 30 s and 72 °C for 60 s, followed by a final extension for 10 min at 72 °C. The gene fragment PCR results were analyzed using a QIAxcel DNA Screening Kit (2400) (Qiagen) using the QIAxcel Advanced System.
As reported in the previous studies [4, 14, 16], it is shown that the amplification of the Hexon gene by molecular biology method can also be used to identify the serotype of FAdVs. We identified the serotype of FAdVs by using molecular biology methods in this study.
Sequencing
PCR products were purified using a QIAquick PCR purification kit (Qiagen, Hilden, Germany), cloned into the pMD18-T vector (Takara, Dalian, China), and then sequenced using synthetic oligonucleotides (Sangon Biotech, Shanghai, China). The sequences were submitted to the GenBank database, National Center for Biotechnology Information.
Phylogenetic analysis
All the hexon sequences derived from the detected FAdV were aligned with 22 selected hexon genes of the 12 serotypes FAdV reference strains in the ICTV classification system and some other selected field strains, following the published reports [30]. Hexon gene sequences of the representative strains were downloaded from NCBI, GenBank accession numbers of these sequences were listed in Table 5. The phylogenetic tree based upon the results of multiple sequence alignment was constructed using the Molecular Evolutionary Genetics Analysis (MEGA) software version 7.0 [31, 32] applying the model with the maximum likelihood (ML) method, The robustness of the phylogenetic constructions was evaluated by bootstrapping with 1,000 replicates [31]. Serotypes were identified based on the phylogenetic analysis and pairwise identities as described in previous studies [30].
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the the GenBank database of National Center for Biotechnology Information, persistent web link is https://www.ncbi.nlm.nih.gov/, the GenBank accession numbers of FAdVs are ON502449-ON502604 and ON462358-ON462367.
Abbreviations
- FAdV:
-
Fowl adenovirus
- LBMs:
-
Living bird markets
References
Zhang M, Hu Y, Zhang C, Guo M, Cao Y, Zhang X, et al. Nearly complete genome sequence of a serotype 1 fowl adenovirus strain isolated in Jiangsu, China. Microbiol Resour Announc. 2019;8(30):e00310–19.
Schachner A, Matos M, Grafl B, Hess M. Fowl adenovirus-induced diseases and strategies for their control - a review on the current global situation. Avian Pathol. 2018;47(2):111–26.
Benko M, Aoki K, Arnberg N, Davison AJ, Echavarria M, Hess M, et al. ICTV virus taxonomy profile: adenoviridae 2022. J Gen Virol. 2022;103(3):001721.
Niu Y, Sun Q, Zhang G, Sun W, Liu X, Xiao Y, et al. Epidemiological investigation of outbreaks of fowl adenovirus infections in commercial chickens in China. Transbound Emerg Dis. 2018;65(1):e121–6.
McFerran JB, Smyth JA. Avian adenoviruses. Rev Sci Tech. 2000;19(2):589–601.
Kajan GL, Affranio I, TothneBistyak A, Kecskemeti S, Benko M. An emerging new fowl adenovirus genotype. Heliyon. 2019;5(5):e01732.
Li H, Wang J, Qiu L, Han Z, Liu S. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect Genet Evol. 2016;45:230–41.
Grafl B, Aigner F, Liebhart D, Marek A, Prokofieva I, Bachmeier J, et al. Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathol. 2012;41(6):599–604.
Choi KS, Kye SJ, Kim JY, Jeon WJ, Lee EK, Park KY, et al. Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poult Sci. 2012;91(10):2502–6.
Niu YJ, Sun W, Zhang GH, Qu YJ, Wang PF, Sun HL, et al. Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J Gen Virol. 2016;97(10):2684–90.
Chen L, Yin L, Zhou Q, Peng P, Du Y, Liu L, et al. Epidemiological investigation of fowl adenovirus infections in poultry in China during 2015–2018. BMC Vet Res. 2019;15(1):271.
Zhang H, Jin W, Ding K, Cheng X, Sun Y, Wang J, et al. Genetic Characterization of Fowl Adenovirus Strains Isolated from Poultry in China. Avian Dis. 2017;61(3):341–6.
Zhang T, Jin Q, Ding P, Wang Y, Chai Y, Li Y, et al. Molecular epidemiology of hydropericardium syndrome outbreak-associated serotype 4 fowl adenovirus isolates in central China. Virol J. 2016;13(1):188.
Changjing L, Haiying L, Dongdong W, Jingjing W, Youming W, Shouchun W, et al. Characterization of fowl adenoviruses isolated between 2007 and 2014 in China. Vet Microbiol. 2016;197:62–7.
Cui J, Xu Y, Zhou Z, Xu Q, Wang J, Xiao Y, et al. Pathogenicity and Molecular Typing of Fowl Adenovirus-Associated With Hepatitis/Hydropericardium Syndrome in Central China (2015–2018). Front Vet Sci. 2020;7:190.
Brown Jordan A, Blake L, Bisnath J, Ramgattie C, Carrington CV, Oura CAL. Identification of four serotypes of fowl adenovirus in clinically affected commercial poultry co-infected with chicken infectious anaemia virus in Trinidad and Tobago. Transbound Emerg Dis. 2019;66(3):1341–8.
Asthana M, Chandra R, Kumar R. Hydropericardium syndrome: current state and future developments. Arch Virol. 2013;158(5):921–31.
Kajan GL, Kecskemeti S, Harrach B, Benko M. Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Vet Microbiol. 2013;167(3–4):357–63.
Niczyporuk JS, Kozdrun W, Czekaj H, Piekarska K, Stys-Fijol N. Characterisation of adenovirus strains represented species B and E isolated from broiler chicken flocks in eastern Poland. Heliyon. 2021;7(2):e06225.
Niczyporuk JS, Kozdrun W. Current epidemiological situation in the context of inclusion body hepatitis in poultry flocks in Poland. Virus Res. 2022;318:198825.
Shi S, Liu R, Wan C, Cheng L, Chen Z, Fu G, et al. Isolation and characterization of duck adenovirus 3 circulating in China. Arch Virol. 2019;164(3):847–51.
Huang Y, Kang H, Dong J, Li L, Zhang J, Sun J, et al. Isolation and partial genetic characterization of a new duck adenovirus in China. Vet Microbiol. 2020;247:108775.
Yin L, Zhou Q, Mai K, Yan Z, Shen H, Li Q, et al. Epidemiological investigation of duck adenovirus 3 in southern China, during 2018–2020. Avian Pathol. 2022;51(2):171–80.
Chu L, Ye S, Wang J, Peng D, Wang X, Qian Y, et al. An insertion and deletion mutant of adenovirus in Muscovy ducks. Arch Virol. 2022;167(9):1879–83.
Chen S, Lin F, Jiang B, Xiao S, Jiang D, Lin C, et al. Isolation and characterization of a novel strain of duck aviadenovirus B from Muscovy ducklings with acute hepatitis in China. Transbound Emerg Dis. 2022;69(5):2769–78.
Shi X, Zhang X, Sun H, Wei C, Liu Y, Luo J, et al. Isolation and pathogenic characterization of duck adenovirus 3 mutant circulating in China. Poult Sci. 2022;101(1):101564.
Wei Z, Liu H, Diao Y, Li X, Zhang S, Gao B, et al. Pathogenicity of fowl adenovirus (FAdV) serotype 4 strain SDJN in Taizhou geese. Avian Pathol. 2019;48(5):477–85.
Kajan GL, Davison AJ, Palya V, Harrach B, Benko M. Genome sequence of a waterfowl aviadenovirus, goose adenovirus 4. J Gen Virol. 2012;93(Pt 11):2457–65.
Marek A, Kajan GL, Kosiol C, Harrach B, Schlotterer C, Hess M. Complete genome sequences of pigeon adenovirus 1 and duck adenovirus 2 extend the number of species within the genus Aviadenovirus. Virology. 2014;462–463:107–14.
Meulemans G, Boschmans M, Berg TP, Decaesstecker M. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol. 2001;30(6):655–60.
Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013;30(5):1229–35.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33(7):1870–4.
Chiocca S, Kurzbauer R, Schaffner G, Baker A, Mautner V, Cotten M. The complete DNA sequence and genomic organization of the avian adenovirus CELO. J Virol. 1996;70(5):2939–49.
Meulemans G, Couvreur B, Decaesstecker M, Boschmans M, van den Berg TP. Phylogenetic analysis of fowl adenoviruses. Avian Pathol. 2004;33(2):164–70.
Chen H, Dou Y, Zheng X, Tang Y, Zhang M, Zhang Y, et al. Hydropericardium hepatitis syndrome emerged in Cherry Valley Ducks in China. Transbound Emerg Dis. 2017;64(4):1262–7.
Marek A, Kosiol C, Harrach B, Kajan GL, Schlotterer C, Hess M. The first whole genome sequence of a Fowl adenovirus B strain enables interspecies comparisons within the genus Aviadenovirus. Vet Microbiol. 2013;166(1–2):250–6.
Marek A, Kajan GL, Kosiol C, Benko M, Schachner A, Hess M. Genetic diversity of species Fowl aviadenovirus D and Fowl aviadenovirus E. J Gen Virol. 2016;97(9):2323–32.
Alvarado IR, Villegas P, El-Attrache J, Jensen E, Rosales G, Perozo F, et al. Genetic characterization, pathogenicity, and protection studies with an avian adenovirus isolate associated with inclusion body hepatitis. Avian Dis. 2007;51(1):27–32.
Slaine PD, Ackford JG, Kropinski AM, Kozak RA, Krell PJ, Nagy E. Molecular characterization of pathogenic and nonpathogenic fowl aviadenovirus serotype 11 isolates. Can J Microbiol. 2016;62(12):993–1002.
Thiermann AB. International standards: the World Organisation for Animal Health Terrestrial Animal Health Code. Rev Sci Tech. 2015;34(1):277–81.
Acknowledgements
Not applicable.
Funding
This work was supported by The Innovation Fund of CAHEC (DW20211008). The funder did not have any roles in the design and conduct of the experiment, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Qiong Chen, Ran Zhao, Jinping Li and Guangyu Hou assisted the sample collection; Fuyou Zhang, Chenglong Zhao, Suchun Wang and Qingye Zhuang conducted the experiments and analyzed the data. Qingye Zhuang, Lei Ju, Pin Guo and Xiaoying Chen analyzed the data; Qingye Zhuang and Kaicheng Wang drafted the original manuscript; Qingye Zhuang, Kaicheng Wang and Fuliang Sun reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the animal welfare guidelines of the World Organization for Animal Health [40], and approved by the Animal Welfare Committee of the China Animal Health and Epidemiology Center. The present study were carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest related to this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhuang, Q., Wang, S., Zhang, F. et al. Molecular epidemiology analysis of fowl adenovirus detected from apparently healthy birds in eastern China. BMC Vet Res 19, 5 (2023). https://doi.org/10.1186/s12917-022-03545-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03545-5