Abstract
Background
Laparoscopic choledochojejunostomy (LCJ) is an essential basic skill for biliary surgeons. Therefore, we established a convenient and effective LCJ 3D printing model to evaluate whether the model could simulate the actual operation situation and determine its effectiveness and validity in surgical training.
Methods
A 3D printing dry laboratory model was established to simulate LCJ. The face and content validity of the model were evaluated by six experienced biliary surgeons based on 5-point Likert scale questionnaires. A total of 15 surgeons with different levels of experience performed LCJ on the model and evaluated the structural validity of the model using the objective structured assessment of technical skills (OSATS). Simultaneously, the operation time of each surgery was also recorded. A study was also performed to further evaluate the learning curve of residents.
Results
The operating space score of the model was 4.83 ± 0.41 points. The impression score of bile duct and intestinal canal was 4.33 ± 0.52 and 4.17 ± 0.41 points, respectively. The tactile sensation score of bile duct suture and intestinal canal suture was 4.00 ± 0.63 and 3.83 ± 0.41points, respectively. The OSATS score for model operation in the attending group was 29.20 ± 0.45 points, which was significantly higher than that in the fellow group (26.80 ± 1.10, P = 0.007) and the resident group (19.80 ± 1.30, P < 0.001). In addition, there was a statistical difference in operation time among surgeons of different experience levels (P < 0.05). Residents could significantly improve the surgical score and shorten the time of LCJ through repeated training.
Conclusions
The 3D printing LCJ model can simulate the real operation scenes and distinguish surgeons with different levels of experience. The model is expected to be one of the training methods for biliary tract surgery in the future.
Similar content being viewed by others
Background
Choledochojejunostomy (CJ) is the most commonly used surgical method in treating biliary surgical diseases, as well as the common surgical regimen in treating malignant biliary obstruction. LCJ is an important skill, which is characterized by complexity, difficulty, challenge, and long learning curve. Lack of skilled operation experience may lead to postoperative cholangitis, bile duct stenosis, bile leakage, peritonitis, and even death [1,2,3]. During the current Covid-19 pandemic, the number of patients and operations has decreased substantially, which is reflected in the training and teaching of surgical residents [4, 5]. To alleviate this problem, surgical societies, including the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and the American College of Surgeons (ACS) are all in support of surgical simulation [6,7,8]. A recent system overview subdivides simulation based training tools into four categories: virtual reality, wet laboratory (animal organs and corpse models from animals or humans), dry laboratory (synthetic models), and E-Learning [9]. It has been proved that clinical training before surgical intervention can provide safe and effective training for surgical residents, so that trainees can rapidly acquire and maintain surgical skills. At the same time, the acquired operative skills can be directly transferred to the actual surgical environment [10,11,12].
Novice surgeons often face many challenges in their surgical training. The virtual training platforms can provide an immersive experience, which are conducive to the familiarity with surgical procedures. However, these platforms are expensive, lack tactile feedback, and are not widely available [13]. Although the training of animal organs or animals and human cadavers has realistic advantages, and surgeons have widely accepted its education and training value, there are still some limitations, including high cost, availability, non-repeatability, risk of infectious diseases, and potential ethical issues [14, 15]. Many residents support the use of dry laboratory model at home, which is simple, convenient, and inexpensive, and enables trainees to acquire basic laparoscopic skills, including laparoscopic suture, knotting, and coordination training, but does not simulate advanced operation [16].
With the availability of advanced materials and printing techniques, 3D printing technology has been widely used in the medical field, especially in anatomy education and surgical training, among which 3D printing technology has shown promising results and new applications [17]. Several randomized controlled trials in multiple surgical fields have demonstrated that 3D printing model can simulate relevant operations, thus acquiring early skills and carrying out advanced skill training [18,19,20,21,22]. Despite the wide application of 3D printing in various surgical fields, there remain a lack of relevant application in the surgical training of LCJ.
We described portable and soft model created by 3D printing for dry laboratory training in LCJ. Through the establishment of 3D printing, the model can be used in the dry laboratory of LCJ. This model included a liver with an embedded bile duct and a section of intestinal canal, which can be used for visualization, instrumentation, and laparoscopic anastomosis. Experts in the field of biliary surgery can evaluate the face and content validity of the model, and whether the model can simulate the real surgical situations, distinguish different levels of surgeons, share our experience, reduce learning costs and help surgeons improve their surgical skills.
Methods and materials
Participants
This study invited six surgical experts from biliary surgery center of Zhejiang Provincial People’s Hospital and Wenzhou Central Hospital to evaluate the face and content validity of the 3D printing model. All six experts performed more than 10 cases of LCJ in the previous year. At the same time, 15 surgeons from the biliary surgery center were also invited to participate in the structural evaluation of the model.
3D-printed dry lab LCJ model production
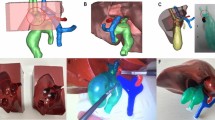
Anonymized Digital Imaging and Communication in Medicine files were obtained using Mimic 23.0 system from 3D computed tomography scans of disease-free human body to extract/reconstruct the anatomical models of liver, bile duct, and small intestine. The extracted STL file was repaired with Magic24 to obtain the sealing structure. Then, OBJ files were exported from Magic 24 and imported to Zbush for further modification. Mold designations were completed by NX 1899 and either positive mold or negative mold was designed depending on the shape of the organs. The STL files of the designed mold were imported to Magic 24 for further designation of support structure and positioning. Next, an FDM 3D printer was used to print the mold based on sliced data, and the surface treatment and support structure removal are carried out after printing. The mold cavity was treated with Vaseline to ensure smooth removal of models from the mold after curing. The model was produced via casting. The silica gel was poured into the mold from the vacuum box and cured at 25 °C for 1 h. Finally, the mold was removed to obtain the model after solidification. That printing files are available upon request.
The silica gel material used in the bile duct was yellow, with an elastic modulus of 0.16 MPa, and a tensile strength of 0.48 MPa (Fig. 1A). The silica gel material used in the small intestine was red, with an elastic of 0.17 MPa, and a tensile strength of 0.74 MPa (Fig. 1B). The stiffness of model was measured through ultrasound. Ultrasonic Elastic value of bile duct was 1.95 m/s (Fig. 1C), and that of small intestine was 1.47 m/s (Fig. 1D).
The box includes (Fig. 2): (1) Liver and its bile duct;(2) Small intestine;(3) Clip for fixing the intestinal canal;(4) The self-printed laparoscopic operation platform that can adjust the height of the platform up and down to simulate pneumoperitoneum, and the silica gel thickness of the abdominal wall skin can be used to place the laparoscopic cannula;(5) Lens fixer, a flexible metal snake bone lens that supports the 30-degree camera, allows surgeons to operate independently. In addition, monitors, sutures, lenses, and laparoscopic instruments were also used in the training.
Patient and public participation
Neither patients nor the public were directly involved in the design of the current study.
Model face validity and content validity
Referring to the relevant literature, the face validity and content validity of the model was comprehensively designed [23,24,25]. The six biliary surgery experts used the 5-point Likert scale (5: Strongly agree; 4: Agree; 3: Neutral; 2: Disagree; 1: Strongly disagree, Additional file 1) to evaluate the model, including the impression, fidelity, texture, appearance, operation space and tactile sensation of the 3D model, as well as its effectiveness for clinical treatment and training.
Model structure validity
A total of 15 attendings, fellows, and residents were recruited, with five members in each group, and basic information collection tables were issued. All surgeons provided informed written consent. Through pre-recorded video, the explanation and model training operation were performed. The resident group also completed eight-time LCJ training program on the model. The entire operation procedure was captured on video. Our study used a modified edition of OSATS [26, 27] (out of 30 points, Additional file 1). Two experts used OSATS to independently evaluate the recorded video. Respect for tissue, time and motion, instrument handling, flow of operation and forward planning, knowledge of specific procedure and overall performance of attendings, fellows and residents were mainly evaluated. The operation time was recorded at the same time, ignoring the identity of surgeons.
General information for biliary surgeons
A total of 15 surgeons were invited to participate in the current study. All the surgeons involved in the study were right-handed. There were significant differences in the working year of surgeons in the three groups (14.20 ± 1.64vs 6.40 ± 1.14vs 2.80 ± 0.45, respectively; p < 0.001). There were significant differences in the number of cases of LCJ performed by the three groups of surgeons as the chief surgeon (P = 0.003) and the number of cases of LCJ performed as the first assistant (P = 0.007). There were no significant differences in the use of surgical simulation tools among the three groups (Table 1).
Surgical procedure
Laparoscopic end-to-side anastomosis of bile duct and small intestine with full-thickness continuous valgus suture was used in this study (Fig. 3). 4–0 absorbable suture was used to suture from the left corner of the intestinal opening from outside to inside, and from the left corner of the broken end of the biliary tract from inside to outside. One needle was knotted and the knot was tied outside the anastomosis. The suture needle was sutured into the biliary tract from the left corner of the biliary tract wall, and the second needle was sutured from intestinal tract to the biliary tract. The needle was sutured into the intestinal wall from inside to outside and the needle was sutured into the biliary tract wall from outside to inside. The posterior wall was continuously sutured to the right end. The needle distance and edge distance were maintained at about 2 ~ 3 mm; generally, 5–6 stitches were sutured. When the suture reached the right end of the posterior wall, the suture needle penetrated the suture line of the anterior wall of the anastomosis from the right corner of the intestinal wall. The anterior wall was sutured from right to left, the intestinal wall was sutured from outside to inside and the biliary wall was sutured from inside to outside. Continuous suture was performed until the left end of the anastomosis was knotted with the thread tail to complete the anastomosis.
LCJ instructions for 3D printing model A The first needle was inserted into the left lateral wall of the intestine. B The first needle was inserted into the left lateral wall of the bile duct. C The second needle was inserted into the posterior wall of the intestine from inside to outside. D The second needle was inserted into the posterior wall of the bile duct from outside to inside. E The posterior wall of the intestine had been anastomosed to the posterior biliary ductal wall. F The needle was pulled out at the right lateral wall of the intestine. G The needle was inserted into the anterior wall of the intestine from outside to inside. H Continuously suture the anterior wall of the intestine and the bile duct to complete the anastomosis
Statistics analysis
SPSS version 21.0 (IBM Corp., Armonk, NY, USA) was used for the subsequent data analyses and processing. A comparison of the differences between three groups was performed using the analysis of variance (ANOVA), and multiple comparisons of operative time and operative scores were made using Tukey’s method. Fisher’s exact test was used for intergroup comparisons of count data. The learning curve of the model operation was assessed using the cumulative sum method (CUSUM) and the results were calculated with Excel 2016. Hypothesis tests were all performed using two-tailed probabilities, and the significance level was set at α = 0.05.
Results
Face validity score of the model
A total of 6 biliary surgery experts were invited to conduct the face validity evaluation for this study (Fig. 4). The operation space score of the model was 4.83 ± 0.41. The impression score of bile duct, intestinal canal and liver was 4.33 ± 0.52, 4.17 ± 0.41 and 3.83 ± 0.41, respectively. The realism score of bile duct was 4.17 ± 0.75, which was higher than the other two parts. The texture score of bile duct was 4.17 ± 0.41, which was higher than the other two parts. The appearance score of bile duct was4.00 ± 0.63, which was higher than the other two parts. The tactile sensations score of bile duct and intestinal canal was 4.00 ± 0.63 and 3.83 ± 0.41, respectively.
Content validity score of the model
Except that the similarity score between the model and the real operation was less than “agree” (4 points), the evaluation given by all experts was greater than 4 points (Table 2). The rationality of model training and suggestion to use the model in LCJ training have been strongly supported by all experts. The model was considered to be easy to operate, which can reduce the risk of patients, improve the skills of participants, shorten the learning curve, and enhance the operative confidence and training interest.
Construct validity of the model
There were significant differences in OSATS scores among researchers in the three groups (P < 0.01). The score of the attending group was significantly higher than those of fellow group (29.20 ± 0.45vs26.80 ± 1.10, P = 0.007) and resident group (29.20 ± 0.45vs19.80 ± 1.30, P < 0.001), as shown in Table 3 and Fig. 5A. There were significant differences in the operation time among researchers in the three groups (P < 0.05). The operation time of the attending group was significantly shorter than those of fellow group (13.32 ± 1.49 vs19.92 ± 2.02, P = 0.016) and resident group (13.32 ± 1.49 vs 39.84 ± 4.88, P < 0.001), as shown in Table 3 and Fig. 5B.
Learning curve assessment
Four residents were willing were receive a total of eight-time LCJ trainings. The other one resident was unable to participate due to personal reason. The operation times of the four residents were showed in Fig. 6A. The operation scores were shown in Fig. 6B. The average operation scores and operation times of the five fellows were marked with a line. As the number of training increases, the operation times and scores presented gradual progress. It was found that the number of turning points in the learning curve of the residents were 4th case, 4th case, 5th case, and 5th case in the training (Fig. 6C).
Discussion
Traditional surgical teaching and training methods are facing various pressures. As an alternative educational tool, surgical simulation training figures prominently in the training curriculum [26]. A series of simulation training tools have been developed and verified as effective ones in the medical field, including laparoscopic box trainer and virtual reality simulator [28]. Several randomized controlled trials and systematic evaluations have shown that the technical skills acquired in these simulators can indeed be transformed into skills in the real environment of the operating room [29,30,31,32,33]. Unfortunately, there is still a lack of sufficiently realistic models to simulate the real anatomical structures. The dry laboratory model is made of hard material, which is not conducive to suture. The mechanical simulation of soft tissue has not been optimized, which significantly hinders its application in surgical training (such as suture and cutting). Virtual reality is expensive. It cannot completely simulate the same surgical environment, nor can it include real anatomy and tactile feedback.
3D printing technology has developed rapidly, and become one of the application fields of medicine, biomaterials, tissue engineering and surgery [34, 35].3D printing surgical training model can not only be conducive to preoperative anatomical details of organs, so as to enhance the familiarity with the surgical procedure, improve surgical skills and shorten the learning curve, but also be affordable and have low surgical cost [36, 37].
A recent systemic evaluation showed that 3D models could provide surgeons with equal teaching and training effects as cadaver simulation. With the development of 3D printing and biomaterial technology, they may replace the role of cadaver simulation tools [38]. The six experts in the study believed that the established model can achieve favorable surface validity. They all agreed that the model reconstructed the realistic LCJ and supported its application in LCJ training. In addition, the 3D model can effectively improve surgical skills, self-confidence, learning interest, shorten learning curve and reduce the risks of patients. This indicates that the model can simulate real surgical scenes and play a potential role in surgical training.
According to our previous experience in using 3D printing models for surgical training, we chose LCJ in this study, because CJ is a commonly used choledochojejunostomy in the field of hepatobiliary and pancreatic surgery. Due to the technical difficulty of LCJ, few studies have been conducted to evaluate LCJ. Due to the high error cost, LCJ is not often exposed to surgical resident or fellow. Additionally, variations of the bile ducts pose difficulties that are uneasy to encounter. In this model, we simulated the narrow space by providing the helium part of the liver, as well as typical LCJ situations, such as the anastomosis of left and right hepatic ducts, or the anastomosis to the common hepatic duct. Moreover, the diameter of the bile duct can range from 2 mm to 2 cm. Therefore, the training can be stepwised from easy to difficult. Trainees only needs to take out the model to check the anastomotic stoma. The tube simulating the bile duct is connected to the water pump, which can objectively evaluate and compare the leakage or stenosis of the anastomotic stoma.
Soft silica gel material was used in this study to print the parenchyma of bile duct and small intestine. The materials were continuously modified and adjusted during this period to meet the criteria of surgical training. The model not only simulated the anatomical structure, texture, appearance and tactile sensation of real organs, but also performed the mechanical test. According to literature, the maximum stress of small intestine in human body is 0.9 MPa [39]. The maximum stress of bile duct and small intestine under uniaxial tension is 0.48 MPa and 0.74 MPa respectively, which is close to the maximum stress of human small intestine. All the participants thought bile duct and small intestinal are easy to suture because they are easy to be stretched.
Ultrasonic elastography is a kind of ultrasonic imaging method used to evaluate the hardness of tissue. Through the measurement of strain generated in tissue, the mechanical properties of tissue can be non-invasively evaluated. The detection of 3D printing components through ultrasonic elastography, the mechanical properties of tissues can be evaluated to promote the improvement of materials, so as to make the texture of surgical training materials much closer to the normal human body and improve the training experience. In this study, soft silica gel was used to simulate CJ model and its hardness. The hardness of small intestine was slightly higher than that of normal small intestine. Literature reported that the hardness of normal small intestine was 1.42 ± 0.6 m/s [40]. Currently, there is no relevant literature report on the ultrasonic elasticity of human bile duct. Ultrasonic elasticity of our bile duct model showed its hardness of 1.95 m/s, which was higher than that of small intestine. It was consistent with the fact that the hardness of bile duct is higher than that of small intestine in actual operation. Currently, our team is studying to use hydrogel as a 3D printing material to print CJ model. Its hardness is similar to that of normal CJ. However, the hydrogel is not easy to preserve and is expensive. We believe that with the improvement of 3D printing technology and materials, the issue of tissue preservation and price will be solved. A method of FDM was selected for the model used in this study. This method can quickly and massively manufacture the model by melting the printing mold, and the printing cost of FDM is relatively low.
Evaluation of surgical skills and abilities is an essential aspect of medical education. Through the model operation, the study of operation score and time can be carried out to effectively reflect the operation level of surgeons [41, 42]. A recent systemic review of 24 multidisciplinary studies showed that, through effective evaluation tools, the technical skills of surgeons could be evaluated, which can positively influence the prognosis and outcome of patients [43]. The reliability and effectiveness of OSATS evaluation tool are similar to objective structured clinical examination, which can be widely used in various medical specialties and has been proved to be an effective tool for evaluating the technical ability of surgeons [44, 45]. The surgical score criteria of this study are based on this kind of improved scoring design. Three groups of surgeons were selected in our study to score the construct validity of this model. This model can effectively distinguish the differences in surgical scores and operation time among three groups of surgeons, which further illustrates the training effect of this model, and can distinguish different levels of surgeons, and roughly assess whether biliary surgeons are ready for LCJ surgery.
In the repeated training part, four residents presented gradual progress through repeated. After 8 training sessions, all residents gradually shorten the operation time and improve score, there was still a gap between their levels and the average fellow surgeon level. The turning point in the CUSUM curve indicates a point of trend transition. It was found that the operation time was relatively stable after training for 4–5 cases, and entered a plateau. After more training, we believe that the learning curve of residents should shorten, and their operation time will reach the fellow surgeon levels, even the levels of attending surgeon.
Our study still has several limitations. Firstly, we selected 15 surgeons to perform LCJ on the 3D model, and more experienced surgeons are needed to evaluate the effectiveness of the model. Secondly, it is uncertain whether the skills acquired by the current LCJ model will be directly transferred to the surgical environment, and future validation studies are needed. Thirdly, the current model does not contain blood vessels and simulation of intraoperative bleeding. Our group is currently working on this model for tissue bleeding, which will more perfectly mimic the real situation. Fourthly, soft silica gel material was used to simulate intestinal canal and bile ducts in this study, but its hardness was slightly higher than that of normal tissue. In addition, the intestinal tract and bile duct used in this study were bilayer structures, lacking the multilayered structure of normal tissue. In the future we need to try different materials to achieve better material simulation, such as hydrogel organs and tissues, and compare the effectiveness of their expert evaluation and training. Although the model was anatomically realistic, the training task was limited to laparoscopic sutures. We are working on hydrogels and believe that these technical organizations will be solved in the future.
In conclusion, through this study, we established a new 3D printing model, which can simulate the real LCJ surgical situations, and has similar mechanical properties and hardness to distinguish surgeons with different experience levels. In addition, LCJ model can also help trainees acquire complex surgical skills, shorten the learning curve, reduce risks of patients. Moreover, the training is not limited by time and environment.
Conclusions
The results of this study on 3D printing LCJ model, the evaluation of face, content and construct validity, have an important influence on surgical training. Our goal is to extend this pattern to biliary surgery and other surgical specialties. Although the model is anatomically real, it is limited to laparoscopic suture, and does not involve cutting and anastomosis parts, such as ultrasonic scalpels and staplers. In future hydrogel studies, we believe that these manipulations will be addressed. In addition, whether the skills acquired from the models will be directly transferred to the surgical setting still needs to be verified in the future.
Availability of data and materials
Additional data and materials can be made available upon request to the corresponding author.
References
Chuang SH, Lin CS. Single-incision laparoscopic surgery for biliary tract disease. World J Gastroenterol. 2016;22(2):736–47.
Birgin E, Téoule P, Galata C, Rahbari NN, Reissfelder C. Cholangitis following biliary-enteric anastomosis: a systematic review and meta-analysis. Pancreatology. 2020;20(4):736–45.
Li T, Tuerxun K, Keyoumu Y, Apaer S, Zeng Q, Aierken A, et al. Laparoscopic versus open roux-en-Y Choledochojejunostomy: a single-institute experience with literature review. Surg Laparosc Endosc Percutan Tech. 2020;31(3):321–5.
Poelmann FB, Koëter T, Steinkamp PJ, Vriens MR, Verhoeven B, Kruijff S. The immediate impact of the coronavirus disease 2019 (COVID-19) pandemic on burn-out, work-engagement, and surgical training in the Netherlands. Surgery. 2021;170(3):719–26.
Nassar AH, Zern NK, McIntyre LK, Lynge D, Smith CA, Petersen RP, et al. Emergency restructuring of a general surgery residency program during the coronavirus disease 2019 pandemic: the University of Washington experience. JAMA Surg. 2020;155(7):624–7.
Rogers AT, Dirks R, Burt HA, Haggerty S, Kohn GP, Slater BJ, et al. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines development: standard operating procedure. Surg Endosc. 2021;35(6):2417–27.
Peters JH, Fried GM, Swanstrom LL, Soper NJ, Sillin LF, Schirmer B, et al. Development and validation of a comprehensive program of education and assessment of the basic fundamentals of laparoscopic surgery. Surgery. 2004;135(1):21–7.
Satava RM, Stefanidis D, Levy JS, Smith R, Martin JR, Monfared S, et al. Proving the effectiveness of the fundamentals of robotic surgery (FRS) skills curriculum: a single-blinded, multispecialty, Multi-institutional Randomized Control Trial. Ann Surg. 2020;272(2):384–92.
Lee R, Raison N, Lau WY, Aydin A, Dasgupta P, Ahmed K, et al. A systematic review of simulation-based training tools for technical and non-technical skills in ophthalmology. Eye (Lond). 2020;34(10):1737–59.
Kantar RS, Alfonso AR, Ramly EP, Cohen O, Rifkin WJ, Maliha SG, et al. Knowledge and skills acquisition by plastic surgery residents through digital simulation training: a prospective, randomized, Blinded Trial. Plast Reconstr Surg. 2020;145(1):184e–92e.
Carlsen CG, Lindorff-Larsen K, Funch-Jensen P, Lund L, Konge L, Charles P. Module based training improves and sustains surgical skills: a randomised controlled trial. Hernia. 2015;19(5):755–63.
Dawe SR, Windsor JA, Broeders JA, Cregan PC, Hewett PJ, Maddern GJ. A systematic review of surgical skills transfer after simulation-based training: laparoscopic cholecystectomy and endoscopy. Ann Surg. 2014;259(2):236–48.
Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664–9.
Schmitt B, Wacker C, Ikemoto L, Meyers FJ, Pomeroy C. A transparent oversight policy for human anatomical specimen management: the University of California, Davis experience. Acad Med. 2014;89(3):410–4.
Brook NR, Dell'Oglio P, Barod R, Collins J, Mottrie A. Comprehensive training in robotic surgery. Curr Opin Urol. 2019;29(1):1–9.
Li MM, George J. A systematic review of low-cost laparoscopic simulators. Surg Endosc. 2017;31(1):38–48.
Qiu K, Haghiashtiani G, McAlpine MC. 3D printed organ models for surgical applications. Annu Rev Anal Chem (Palo Alto, Calif). 2018;11(1):287–306.
Hojo D, Murono K, Nozawa H, Kawai K, Hata K, Tanaka T, et al. Utility of a three-dimensional printed pelvic model for lateral pelvic lymph node dissection education: a randomized controlled trial. J Am Coll Surg. 2019;229(6):552–559.e553.
Li A, Tang R, Rong Z, Zeng J, Xiang C, Yu L, et al. The use of three-dimensional printing model in the training of Choledochoscopy techniques. World J Surg. 2018;42(12):4033–8.
Torres IO, De Luccia N. A simulator for training in endovascular aneurysm repair: the use of three dimensional printers. Eur J Vasc Endovasc Surg. 2017;54(2):247–53.
Lim KH, Loo ZY, Goldie SJ, Adams JW, McMenamin PG. Use of 3D printed models in medical education: a randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat Sci Educ. 2016;9(3):213–21.
Li Z, Li Z, Xu R, Li M, Li J, Liu Y, et al. Three-dimensional printing models improve understanding of spinal fracture--a randomized controlled study in China. Sci Rep. 2015;5:11570.
Ahmed K, Jawad M, Abboudi M, Gavazzi A, Darzi A, Athanasiou T, et al. Effectiveness of procedural simulation in urology: a systematic review. J Urol. 2011;186(1):26–34.
Alwani MM, Svenstrup TJ, Bandali EH, Sharma D, Higgins TS, Wu AW, et al. Validity testing of a three-dimensionally printed endoscopic sinonasal surgery simulator. Laryngoscope. 2020;130(12):2748–53.
Lee S, Ahn JY, Han M, Lee GH, Na HK, Jung KW, et al. Efficacy of a three-dimensional-printed training simulator for endoscopic biopsy in the stomach. Gut Liver. 2018;12(2):149–57.
Malas T, Al-Atassi T, Brandys T, Naik V, Lapierre H, Lam BK. Impact of visualization on simulation training for vascular anastomosis. J Thorac Cardiovasc Surg. 2018;155(4):1686–1693.e1685.
Birkmeyer JD, Finks JF, O'Reilly A, Oerline M, Carlin AM, Nunn AR, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369(15):1434–42.
Moulton CA, Dubrowski A, Macrae H, Graham B, Grober E, Reznick R. Teaching surgical skills: what kind of practice makes perfect?: a randomized, controlled trial. Ann Surg. 2006;244(3):400–9.
Price J, Naik V, Boodhwani M, Brandys T, Hendry P, Lam BK. A randomized evaluation of simulation training on performance of vascular anastomosis on a high-fidelity in vivo model: the role of deliberate practice. J Thorac Cardiovasc Surg. 2011;142(3):496–503.
Taksøe-Vester C, Dyre L, Schroll J, Tabor A, Tolsgaard M. Simulation-based ultrasound training in obstetrics and gynecology: a systematic review and Meta-analysis. Ultraschall Med. 2021;42(6):e42–54.
Mazzone E, Puliatti S, Amato M, Bunting B, Rocco B, Montorsi F, et al. A systematic review and Meta-analysis on the impact of proficiency-based progression simulation training on performance outcomes. Ann Surg. 2021;274(2):281–9.
Nippita S, Haviland MJ, Voit SF, Perez-Peralta J, Hacker MR, Paul ME. Randomized trial of high- and low-fidelity simulation to teach intrauterine contraception placement. Am J Obstet Gynecol. 2018;218(2):258.e251–11.
Palter VN, Orzech N, Reznick RK, Grantcharov TP. Validation of a structured training and assessment curriculum for technical skill acquisition in minimally invasive surgery: a randomized controlled trial. Ann Surg. 2013;257(2):224–30.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85.
Kuruoglu D, Yan M, Bustos SS, Morris JM, Alexander AE, Sharaf B. Point of care virtual surgical planning and 3D printing in facial gender confirmation surgery: a narrative review. Ann Transl Med. 2021;9(7):614.
Wang DD, Qian Z, Vukicevic M, Engelhardt S, Kheradvar A, Zhang C, et al. 3D printing, computational modeling, and artificial intelligence for structural heart disease. JACC Cardiovasc Imaging. 2021;14(1):41–60.
Smith B, Dasgupta P. 3D printing technology and its role in urological training. World J Urol. 2020;38(10):2385–91.
Ghazi A. A call for change. Can 3D printing replace cadavers for surgical training? Urol Clin North Am. 2022;49(1):39–56.
Egorov VI, Schastlivtsev IV, Prut EV, Baranov AO, Turusov RA. Mechanical properties of the human gastrointestinal tract. J Biomech. 2002;35(10):1417–25.
Goertz RS, Lueke C, Wildner D, Vitali F, Neurath MF, Strobel D. Acoustic radiation force impulse (ARFI) elastography of the bowel wall as a possible marker of inflammatory activity in patients with Crohn's disease. Clin Radiol. 2018;73(7):678.e671–5.
Pucci JU, Christophe BR, Sisti JA, Connolly ES Jr. Three-dimensional printing: technologies, applications, and limitations in neurosurgery. Biotechnol Adv. 2017;35(5):521–9.
Bartel T, Rivard A, Jimenez A, Mestres CA, Müller S. Medical three-dimensional printing opens up new opportunities in cardiology and cardiac surgery. Eur Heart J. 2018;39(15):1246–54.
Fecso AB, Szasz P, Kerezov G, Grantcharov TP. The effect of technical performance on patient outcomes in surgery: a systematic review. Ann Surg. 2017;265(3):492–501.
Bhatti NI. Assessment of surgical skills and competency. Otolaryngol Clin N Am. 2017;50(5):959–65.
Navrazhina K, Murad A, Tung R, Cressey BD, Decker A, Surprenant D, et al. A blinded, multirater and multi-institutional study evaluating the objective structured assessment of technical skills (OSATS) tool in dermatology education. J Am Acad Dermatol. 2021;85(5):1346–8.
Acknowledgements
We would like to thank for all the participant’s wonderful cooperation and.
the fund of Subproject of the Key R&D Program of the Ministry of Science and Technology (2018YFB1107104).
Funding
This work was supported by the fund of Subproject of the Key R&D Program of the Ministry of Science and Technology (2018YFB1107104). The funder had role in the design and conduct of the study and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
WZF and XJF conceived and designed the analysis. WZF and XJF supervised the study. ZJ and XXD Manufactured and inspected the model. WZF, XJF and YJ performed all the teaching and operation experiments. MJL and CH analyzed the data. XJF, MJL and CH wrote the manuscript. WZF and JY reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received ethical approval from the Ethics Committee of Wenzhou Central Hospital, and all participants provided written informed consent to participate in this study. Besides, all the experiment protocol involving humans was in accordance with Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Pre-LCJ questionnaire.
Additional file 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xia, J., Mao, J., Chen, H. et al. Development and evaluation of a portable and soft 3D-printed cast for laparoscopic choledochojejunostomy model in surgical training. BMC Med Educ 23, 77 (2023). https://doi.org/10.1186/s12909-023-04055-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12909-023-04055-0