Abstract
Background
Laparoscopic large para-oesophageal hiatal hernia (LPHH) repair using mesh reinforcement significantly reduces postoperative recurrence rates compared to conventional suture repair, especially within short follow-up times. However, the ideal strategy for repairing LPHH remains disputable because no clear guidelines are given regarding indications, mesh type, shape or position. The aim of this study was to survey our short-term results of LPHH management with a biosynthetic monofilament polypropylene mesh coated with titanium dioxide to enhance biocompatibility (TiO2Mesh™).
Methods
A retrospective study was performed at Ramon y Cajal University Hospital, Spain from December 2014 to October 2018. Data were collected on 27 consecutive patients with extensive hiatal hernia defects greater than 5 cm for which a laparoscopic repair was performed by primary suture and additional reinforcement with a TiO2Mesh™. Study outcomes were investigated, including clinical and radiological recurrences, dysphagia and mesh-related drawbacks.
Results
Twenty-seven patients were included in our analysis; 10 patients were male, and 17 were female. The mean age was 73 years (range, 63–79 years). All operations were performed laparoscopically. The median postoperative hospital stay was 3 days. After a mean follow-up of 18 months (range, 8-29 months), only 3 patients developed clinical recurrence of reflux symptoms (11%), and 2 had radiological recurrences (7%). No mesh-related complications occurred.
Conclusions
TiO2Mesh™ was found to be safe for laparoscopic repair of LPHH with a fairly low recurrence rate in this short-term study. Long-term studies conducted over a period of years with large sample sizes will be essential for confirming whether this mesh is suitable as a standard method of care with few drawbacks.
Similar content being viewed by others
Background
Large para-oesophageal hiatal hernia (LPHH) repair remains a controversial and challenging intervention for most practising surgeons [1, 2]. Clinical and/or radiological recurrence after conventional repair using sutures occurs in up to 33% of patients, even with good clinical outcome [3].
The standard method of repairing LPHH is still debatable, because although short-term follow-up revealed that mesh-reinforced repair critically reduces the recurrence rate compared to conventional repair with sutures [4,5,6,7,8], no clear guidelines are given regarding indications, mesh type, shape and position [9, 10].
The drawbacks of using mesh in LPHH repair include a prolonged procedure time for the application and safe fixation of the mesh as well as complications related directly to the mesh, such as oesophageal erosion and postoperative dysphagia [11,12,13]. A group of surgeons have considered avoiding the use of synthetic mesh [e.g., polytetrafluoroethylene (PTFE), polypropylene, …] and shifting to new type of biologic and synthetic bio-absorbable meshes (e.g., intestinal submucosa, cadaveric human skin, bovine pericardium, collagen cross-linked mesh) to prevent or minimize complications [14,15,16,17,18,19,20,21,22]. However, the short- and long-term results of these studies were not as satisfactory as previously imagined [23].
Currently, an ideal mesh for hiatal repair does not exist. Despite the availability of different brands, almost all brands use one of the same three basic components: polyester, polypropylene, and PTFE. Differences between brands were based on their combination with each other or the addition of extra substances such as omega 3 fatty acids, titanium, hyaluronate, and poliglecaprone 25. Numerous standards exist, and measures of a proper synthetic mesh should be assured, such as having optimal biocompatibility and being malleable with adequate strength in order to avoid recurrence, degeneration or retraction. To fulfil these standards, ultra-lightweight, large-pore meshes have been developed recently, while considering the need for stability [24]. Previous experimental studies have shown that addition of an extra layer of atomic titanium to the polypropylene filaments (TiO2Mesh™) has led to further enhancement of the biocompatibility and a significant reduction in shrinkage rates in comparison to the same mesh without a covering of titanium [25].
To date, no clinical randomized studies have been conducted on TiO2Mesh™ for the repair of hiatal hernias, and therefore, no scientific declarations can be provided about this type of mesh. The other available analyses in the literature relate exclusively to the lightweight titanium-coated polypropylene mesh TiMesh that was used in 18 patients who underwent laparoscopic repair of large para-oesophageal hiatus hernia. TiMesh, weighing 35 g/m2 (poor size > 1.24 mm), was created by a German company, Medizintechnik GmbH, in Nuremberg [26]. The aim of the current study is to present the short-term outcomes for patients who have undergone laparoscopic hiatal hernia repair with a synthetic monofilament polypropylene mesh with adherent titanium dioxide surface coating (TiO2Mesh™) that was synthesized by a German company named BioCer Entwicklungs-GmbH [large pored mesh structure (2,8 mm) and 47 g/m2], which differs from the lightweight TiMesh by the presence of a biocompatible coating, blue orientation strips and the hydrophilic implant surface that supports the intraoperative handling and placement of TiO2Mesh™.
Methods
Patients
The study was performed retrospectively at Ramon y Cajal University Hospital, Spain, from December 2014 to October 2018. Data were collected on 27 consecutive patients with extensive hiatal hernia defects greater than 5 cm for which a laparoscopic repair was carried out by primary suture and extra reinforcement with a TiO2Mesh™.
Preoperative workup
Each patient experienced a standard preoperative workup, including a physical examination, blood work, chest X-ray, barium swallow, oral endoscopy and computed tomography (CT) scan. In our institution, 24-h pH-metry and oesophageal manometry are not routinely indicated in LPHH.
Surgical technique
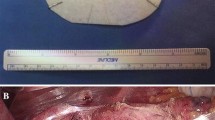
Initially, the herniated sac with its contained stomach was reduced, the hernial sac was removed, and the phrenogastric ligament was dissected. Short gastric vessels were routinely transected. The gastrohepatic omentum was then separated, and the lower oesophagus was dissected from its surroundings. The crura were then repaired utilizing 3-4 interrupted nonabsorbable sutures (Ethibond 0) (Johnson & Johnson, Somerville, NJ, USA) between both diaphragmatic crura. Because of the large size of the hiatal defect (5 cm), a 7 cm × 10 cm manufactured monofilament polypropylene mesh with an adherent titanium dioxide surface covering (TiO2Mesh™) was placed and settled on the pillars of the oesophageal hiatus utilizing interrupted stitches on the edges of the mesh with non-absorbable sutures (Ethibond 2-0). A “floppy” Nissen or Toupet fundoplication was then customized in every patient utilizing nonabsorbable sutures (Ethibond 2-0) (Fig. 1).
TiO2Mesh™ tissue reinforcement [25]
The TiO2Mesh™ tissue reinforcement is made of a synthetic monofilament polypropylene mesh thread and has a vast pored structure with blue orientation stripes. The mesh inserts are encompassed by a high-purity and adherent titanium dioxide surface covering to improve biocompatibility. Moreover, the lightweight character, together with both the large individual pores structure and the reduced material surface, promotes enhanced fibroblastic ingrowth and decreased shrinkage. Furthermore, its biocompatible covering is associated with a hydrophilic surface, and the blue orientation stripes are intended to encourage the intraoperative determination and fixation of TiO2Mesh™. This mesh is available in various standard sizes and shapes, and tailor-made mesh inserts for particular types of present-day hernia surgery are accessible. In our procedure, the mesh was custom-fitted specifically for large hiatal hernia (LHH) repair with a U-Shape of 7-cm × 10 cm.
Postoperative course and long-term clinical assessment
Postoperatively, patients were put on a liquid diet (day 0 surgery) and discharged to go home on a soft diet for 10–15 days before returning to a typical diet. Follow-up was carried out at approximately 1 and 2 weeks and at the first, third, sixth and twelfth months; after that point, follow-up was conducted annually. A barium swallow and CT scan check were performed at the six- and twelve-month follow-ups. Oral endoscopy, 24-h pH-metry and oesophageal manometry were performed only if new symptoms occurred.
The investigation during the follow-up period was aimed at detecting radiological recurrence of hiatal hernia, mesh-related problems, gastroesophageal reflux (GERD) manifestations and dysphagia.
The best estimated value for radiological recurrence of hiatal hernia was the vertical extension of the stomach over the diaphragm by no less than 2 cm [23].
GERD symptom recurrence was defined by the assessment of acid reflux in postoperative 24-h pH-metry (DeMeester Scoring system > 14.7) and by the ingestion of proton pump inhibitor (PPI) medication to treat “de novo” symptoms of reflux.
Statistical analysis
Statistical analysis was performed with SPSS 23.0 for Windows (IBM SPSS Inc., Chicago, IL, USA). Quantitative variables that were normally distributed were characterized by the mean and standard deviation. For non-Gaussian variable, the median and range were utilized. Categorical variables were characterized by the number and level of cases.
Results
The study included 17 females (63%) and 10 males (37%), and the mean age was 73 years (range, 63–79 years). Assessment of patient risk was performed using the American Society of Anesthesiology (ASA) Scoring System [ASA I: 3 cases (11%), ASA II: 15 cases (56%), ASA III: 9 cases (33%)].
Nineteen cases were primary LPHH (70%), five cases were associated with gastric volvulus (18%), and 8 cases were large recurrent hiatal hernias (30%). The median surgical time was 120 min (range, 100–146 min) and overall hospital stay was 3 days (range, 2-3 days). A Nissen fundoplication was performed in 23 cases (85%), a Toupet in three cases (11%), and an angulation of the His angle in the last case (4%). One patient underwent conversion to open surgery (4%) due to bleeding of the lesser curvature after removal of the hernial sac. In one patient, laparoscopic incisional epigastric hernia repair was simultaneously performed. Intraoperative complications occurred in 5 patients (18%). Three patients developed a pneumothorax during dissection of the sac. However, they were managed intraoperatively without the placement of a chest tube. One patient had trocar site bleeding. Regarding postoperative complications (7%), there was 1 case of hyponatraemia, abdominal pain, anaemia and wall haematoma in an elderly patient with concomitant incisional hernia repair who was treated with a blood transfusion and pain killers, prolonging the hospital stay to 21 days. The patient who converted to open surgery developed a contained skin evisceration and was managed conservatively (primary reinforcement of skin with a running suture). In the remaining patients who underwent isolated LPHH repair, postoperative care was uneventful.
A complete follow-up assessment was obtained for all patients after a mean follow-up period of 18 months (range, 8-29 months). Regarding the most important items for assessment of the short-term outcome of TiO2Mesh™, 3 patients developed clinical recurrence of reflux symptoms (11%), assessed by the presence of acid reflux in 24-h pH-metry, and PPI treatment was added. There were two cases of radiological recurrence (7%). No mesh-related complications were found.
Discussion
LHH repair remains a controversial and challenging intervention for most practising surgeons [1, 2]. Although repair with primary suture results in good clinical outcomes for the hiatal hernia defect, clinical and radiological recurrence have been reported in up to 33% of patients when treating these LPHH [3].
Evidence indicates that the utilization of mesh-strengthened hiatal repair has brought about a noteworthy decrease in recurrence rates in correlation with primary suturing of the hiatus, mainly in short-term follow-up [4,5,6,7,8]. Nevertheless, the standard of care for repairing LPHH remains controversial [1, 2, 9], particularly since no rules have been provided with respect to mesh position, type, method of fixation and indication [10].
To evaluate whether mesh type (biological or synthetic mesh) influences outcomes, Huddy et al. [27] published the results of a meta-analysis of four randomized controlled trials [22, 28,29,30] and five comparative studies [22, 23, 28, 29, 31] involving 676 patients undergoing LHH repair with a median follow-up ranging from 12-58 months using different techniques (primary suture vs. synthetic mesh vs. biological mesh). The authors showed that the recurrence rate was lower in patients receiving mesh implants (14.5% vs. 24.5%), with no statistically significant difference in complications between mesh and suture repair. However, when comparing synthetic mesh vs. suture repair, the recurrence rate was doubled in patients who did not receive mesh implants (12.6% vs. 24.6%), without any differences in complications (6.7% vs. 5.6%) or revisional surgery (5.9% vs. 6.7%). Interestingly, the rates of recurrence (17.1% vs. 23.4%), complications (12.5% vs. 15.6%) and reoperations (0% vs. 6.8%) were lower in the biological mesh group than in the suture repair group. However, and in spite of these good outcomes of biological meshes, not only costs are higher than the synthetic ones, but also, when compare overall recurrence rates, these were reduced in the synthetic mesh compared to biological mesh group (12.6% vs. 17.1%).
Simultaneously, reports from many other studies have encouraged the use of mesh-strengthened hiatal occlusion because of a considerable reduction in recurrence rates relative to those of suture repair alone [32,33,34,35,36,37] Despite these excellent outcomes, a few authors have reported increasing disappointment in the long-term outcomes of LPHH repair [23, 38].
The dangers of mesh-related complications, for example, migration of the mesh, oesophageal erosion, stenosis and postoperative dysphagia, have been the basis of contention against routine use of mesh reinforcement for several authors [11,12,13]. We have recently reported our long-term results and complications identified with Crurasoft® mesh in 93 patients who underwent open or laparoscopic fundoplication for gastroesophageal reflux disease (GERD) or LPHH [V-moulded mesh with permeable PTFE on one side and extended polytetrafluorethylene (e-PTFE) on the other side (Bard® Davol INC)] repair for para-oesophageal hernias. In our investigation, the recorded mortality rate was 4% within the first 30 postoperative days. The reoperation rate was 5%, and the mesh was removed in 3 cases (3%). With a follow-up of 76 months, only 8 patients (9%) developed a repeated hiatal hernia [39]. Of the five reoperation cases, one patient had oesophageal perforation that was probably secondary to the manipulation of the oesophagus rather than erosion of the mesh due to a prolonged surgical time. Two patients developed oesophageal and gastric perforation due to mesh erosion, and the last two patients were reoperated due to dysphagia related to fibrosis around the mesh. Mortality due to direct mesh-related complications was recorded in only 1 case due to oesophageal perforation if we excluded mortality that was secondary to pulmonary embolism, pneumonia, and multiple organ failure.
With a specific end goal to prevent or decrease this hazard, a few surgeons have stopped using synthetic mesh for another type of biologic and manufactured bio-absorbable meshes [14,15,16,17,18,19,20,21,22]. However, the short- and long-term consequences of these studies were not as satisfactory as expected [23, 40,41,42]
In 2014, we published [20] our transient short-term results for laparoscopic repair of extensive para-oesophageal hiatal hernias with a manufactured mesh (Gore Bio A®). With a median follow-up of 20.3 months, only one out of 10 patients developed a recurrent hiatal hernia (10%), and no mesh-related complexities were observed. Unfortunately, we do not have long-term results; however, we concluded that the use of this mesh is safe and feasible, but the lack of long-term follow-up together with the small number of patients limits its use.
Currently, an ideal mesh for hiatal repair does not exist. Regardless of the considerable choice in available brands, almost all synthetic meshes for hernia surgery continue to use one of three essential materials: polyester, polypropylene and ePTFE. These materials are utilized alone or as part of a blend with extra elements. The ideal manufactured mesh ought to guarantee a high level of biocompatibility, be easy to handle and provide adequate fastness to avoid recurrence, shrinkage or degradation. To meet these criteria, more lightweight, large pored meshes have been produced recently, while assessing the dependability needed [24]. In experimental investigations, an extra coating of nuclear titanium on the polypropylene fibres (TiO2Mesh™) has been shown to further increase the biocompatibility and reduce shrinkage rates compared with those of an indistinguishable polypropylene mesh without a titanium coating. Most of the studies mentioned the use of titanium in tacks for mesh fixation, although there is no available literature comparing titanium versus non-titanium mesh use in hernia repair. Therefore, our experience determined the biocompatibility of the titanium-coated polypropylene mesh [25].
The TiO2Mesh™ tissue fortification is a manufactured monofilament polypropylene mesh thread and has a large pored structure with blue orientation stripes. The mesh inserts are encompassed by a high-purity and adherent titanium dioxide surface coating to improve biocompatibility. Together with its lightweight character, the large pored structure and the reduced material surface promote enhanced fibroblastic ingrowth and decreased shrinkage. Moreover, its biocompatible coating, together with a hydrophilic surface and blue orientation stripes, is designed to facilitate intraoperative handling and placement. Indeed, due to the high tensile strength of 55 N/cm, this mesh can be utilized for all regular open and laparoscopic systems of inguinal or incisional hernia repair, e.g., Lichtenstein strategy, transabdominal preperitoneal (TAPP) herniaplastic repair, total extraperitoneal (TEP) herniaplastic repair or all techniques for repairing ventral hernia [43,44,45].
To date, no trials or clinical investigations have been completed on this TiO2Mesh™ for the repair of hiatal hernias, and thus, no logical claims can be issued regarding this mesh. Therefore, the available literature relates only to the titanized polypropylene mesh TiMesh [26, 46,47,48]. Hazebroek et al. [26] described 18 patients who underwent LPHH repair with TiMesh, demonstrating postoperative complications in two patients (11%). Quality of life instrument (QOLRAD 5.79, p < 0.001) did not significantly change 2 years after hiatal hernia repair, and no differences were found between pre- and postoperative dysphagia scores. No indications of stricture development or prosthetic disintegration were observed by endoscopic evaluation at the follow-up time. One patient had a small (2 cm) sliding hiatal hernia detected by barium swallow, which was asymptomatic. Our results were comparable to the TiMesh in terms of recurrence rate and postoperative complication in the repair of large-sized hiatus hernias.
To maintain the properties of polypropylene [49] in terms of reducing the rate of recurrence and avoiding the mesh-related complications of these kinds of meshes, we decided to change our preferred mesh from Crurasoft to TiO2Mesh™. Our preliminary results appear to be highly satisfactory. Intraoperatively, we consider the TiO2Mesh™ to be excellent regarding handling, placement and consistency in comparison to Crurasoft and Gore BioA mesh. In the present study, the postoperative course related to LPHH repair was uneventful, with a median of 3 days of hospital stay without any vomiting or postoperative dysphagia. Moreover, with a follow-up of 18 months, the radiological recurrence rate was 7%. Unfortunately, in 3 patients (11%), “de novo” GERD symptoms were detected in 24-h pH-metry. All patients were on PPIs, but one of them had to be re-operated due to uncontrollable GERD symptoms without PPIs 14 months after primer surgery. In this case, wrap migration through the hiatus was ruled out during the intervention. Mesh was correctly integrated in the hiatus, and symptoms were related more to a disruption of the Nissen fundoplication. For this reason, a new Nissen fundoplication was performed, and the patient is currently asymptomatic 4 months after this second surgery. The remaining patients were controlled with PPIs.
Because this retrospective study had a small number of cases, a short duration of follow-up, no direct comparison mesh evaluated other than by historical controls, there were some limitations, but our preliminary results with this type of mesh are encouraging. Moreover, this study is the first to be published describing results with TiO2Mesh™ regarding the repair of LPHH.
Conclusion
The use of TiO2Mesh™ for the laparoscopic repair of large para-oesophageal and recurrent hiatal hernias was safe and had an acceptably low recurrence rate of GERD in the short evaluation period. Long-term studies will be important for evaluating whether this new manufactured mesh is not only safe but also effective in preventing recurrence.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computerized Tomography
- ePTFE:
-
expanded Polytetrafluoroethylene
- GERD:
-
Gastroesophageal Reflux Disease
- LPHH:
-
Large Paraesophageal Hiatal Hernias
- PH:
-
Potential hydrogen
- PTFE:
-
Polytetrafluoroethylene
- PVDF:
-
Polyvinylidene Fluoride
- QOLRAD:
-
Quality-of-Life in Reflux and Dyspepsia
- TAPP:
-
Transabdominal Preperitoneal Hernioplasty
- TEP:
-
Total Extraperitoneal Hernioplasty
References
Targarona E, Bendahan G, Balague C, et al. Mesh in the hiatus. A controversial issue. Arch Surg. 2004;139:1286–96.
Oleynikov D, Jolley JM. Paraesophageal hernia. Surg Clin Norh Am. 2015;95(3):555–65.
Mattar SG, Bowers SP, Galloway KD, et al. Long-term outcome of laparoscopic repair of paraesophageal hernia. Surg Endosc. 2002;16(5):745–9.
Johnson JM, Carbonell AM, Carmody BJ, et al. Laparoscopic mesh hiatoplasty for paraesophageal hernias and fundoplications. Surg Endosc. 2006;20:362–6.
Antoniou SA, Antoniou GA, Koch OO, et al. Lower recurrence rates after mesh-reinforced versus simple hiatal hernia repair: a meta-analysis of randomized trials. Surg Laparosc Endosc Percutan Tech. 2012;22(6):498–502.
Granderath FA, Schweiger UA, Kamolz T, et al. Laparoscopic Nissen fundoplication with prosthetic hiatal closure reduces postoperative intrathoracic wrap herniation: preliminary results of a prospective randomized functional and clinical study. Arch Surg. 2005;140(1):40–8.
Hazebroek EJ, Smith GS. Objective follow-up after laparoscopic repair of large type III hiatal hernia: assessment of safety and durability. World J Surg. 2008;32(7):1563–4.
Frantzides CT, Carlson MA, Loizides S, et al. Hiatal hernia repair with mesh: a survey of SAGES members. Surg Endosc. 2010;24(5):1017–24.
Memon MA, Memon B, Yunus RM. Khan S (2015) suture cruroplasty versus prosthetic hiatal herniorrhaphy for large hiatal hernia. Ann Surg. 2016;263(2):258–66.
Guidelines for the Management of Hiatal Hernia—A SAGES Guideline. http://www.sages.org/publications/guidelines/guidelines-for-the-management-of-hiatal-hernia/. Accessed 2 Jan 2015.
Stadlhuber RJ, Sherif AE, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28 cases series. Surg Endosc. 2009;23:1219–26.
Zugel N, Lang RA, Kox M, et al. Severe complication of laparoscopic mesh hiatoplasty for paraesophageal hernia. Surg Endosc. 2009;23:2563–7.
Griffith PS, Valenti V, Qurashi K, et al. Rejection of goretex mesh used in prosthetic cruroplasty: a case series. Int J Surg. 2008;6(2):106–9.
Fumagalli U, Bona S, Caputo M, et al. Are Surgisis biomeshes effective in reducing recurrences after laparoscopic repair of large hiatal hernias? Surg Laparosc Endosc Percutan Tech. 2008;18(5):433–6.
Diaz DF, Roth JS. Laparoscopic paraesophageal hernia repair with acellular dermal matrix cruroplasty. JSLS. 2011;15(3):355–60.
Lee YK, James E, Bochkarev V, et al. Long-term outcome of cruroplasty reinforcement with human acellular dermal matrix in large paraesophageal hiatal hernia. J Gastrointest Surg. 2008;12(5):811–5.
Wisbach G, Peterson T, Thoman D. Early results of the use of acellular dermal allograft in type III paraesophageal hernia repair. JSLS. 2006;10(2):184–7.
Bell RCW, Fearon J, Freeman KD. Allograft dermal matrix hiatoplasty during laparoscopic primary fundoplication, paraesophageal hernia repair and reoperation for failed hiatal hernia repair. Surg Endosc. 2013;27:1997–2004.
Jacobs M, Gomez E, Plasencia G, et al. Use of Surgisis mesh in laparoscopic repair of hiatal hernias. Surg Laparosc Endosc Percutan Tech. 2007;17(5):365–8.
Priego Jiménez P, Salvador Sanchís JL, Ángel V, Escrig-Sos J. Short-term results for laparoscopic repair of large paraesophageal hiatal hernias with Gore bio a® mesh. Int J Surg. 2014;12(8):794–7.
Massullo JM, Singh TP, Dunnican WJ, et al. Preliminary study of hiatal hernia repair using polyglycolic acid: trimethylene carbonate mesh. JSLS. 2012;16:55–9.
Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg. 2006;244(4):481–90.
Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011;213(4):461–8.
Köckerling F, Schug-Pass C. What do we know about tetanized polypropylene meshes? An evidence-based review of the literature. Hernia. 2014;18(4):445–57.
Scheidbach H, Tannapfel A, Schmidt U, et al. Influence of titanium coating on the biocompatibility of a heavyweight polypropylene mesh. Eur Surg Res. 2004;36:313–7.
Hazebroek EJ, Yong DH, Berry H, et al. Evaluation of lightweight titanium-coated polypropylene mesh (Timesh) for laparoscopic repair of large hiatal hernias. Surg Endosc. 2008;22(11):2428–32.
Huddy JR, Markar SR, Ni MZ, et al. Laparoscopic repair of hiatus hernia: does mesh type influence outcome? A meta-analysis and European survey study. Surg Endosc. 2016;30(12):5209–21.
Chilintseva N, Brigand C, Meyer C, et al. Laparoscopic prosthetic hiatal reinforcement for large hiatal hernia repair. J Visc Surg. 2012;149(3):215–20.
Müller-Stich BP, Holzinger F, Kapp T, et al. Laparoscopic hiatal hernia repair. Surg Endosc. 2006;20(3):380–4.
Dallemagne B, Kohnen L, Perretta S, et al. Laparoscopic repair of paraesophageal hernia: long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg. 2011;253(2):291–6.
Leeder PC, Smith G, Dehn TCB. Laparoscopic management of large paraesophageal hiatal hernia. Surg Endosc. 2003;17(9):1372–5.
Antoniou SA, Koch OO, Antoniou GA, et al. Mesh-reinforced hiatal hernia repair: a review on the effect on postoperative dysphagia and recurrence. Langenbeck's Arch Surg. 2012;39(1):19–27.
Kepenekci I. Laparoscopic fundoplication with prosthetic hiatal closure. World J Surg. 2007;31:2169–76.
Gryska PV, Vernon JK. Tension-free repair of hiatal hernia during laparoscopic fundoplication: a ten-year experience. Hernia. 2005;9(2):150–5.
Granderath FA, Schweiger UM, Pointner R. Laparoscopic antireflux surgery: tailoring the hiatal closure to size of hiatal surface area. Surg Endosc. 2007;21:542–8.
Granderath FA, Carlson MA, Champion JK, et al. Prosthetic closure of the esophageal hiatus in large hiatal hernia repair and laparoscopic antireflux surgery. Surg Endosc. 2006;20(3):367–79.
Soricelli E, Basso N, Genco A, et al. Long-term results of hiatal hernia mesh repair and antireflux laparoscopic surgery. Surg Endosc. 2009;23(11):2499–504.
Rathore MA, Andrabi SIH, Bhatti MI, et al. A meta-analysis of recurrence after laparoscopic repair of paraesophageal hernia. JSLS. 2007;11:456–60.
Priego P, Perez de Oteyza J, Galindo J, et al. Long-term results and complications related to Crurasoft® mesh repair for paraesophageal hiatal hernias. Hernia. 2017;21(2):291–8.
Watson DI, Thompson SK, Devitt PG, et al. Laparoscopic repair of very large hiatus hernia with sutures versus absorbable mesh versus nonabsorbable mesh: a randomized controlled trial. Ann Surg. 2015;261(2):282–9.
Ringley CD, Bochkarev V, Ahmed SI, et al. Laparoscopic hiatal hernia repair with human acellular dermal matrix patch: our initial experience. Am J Surg. 2006;192(6):767–72.
Schmidt E, Shaligram A, Reynoso JF, et al. Hiatal hernia repair with biologic mesh reinforcement reduces recurrence rate in small hiatal hernias. Dis Esophagus. 2014;27(1):13–7.
Schug-Pass C, Jacob DA, Lippert H, et al. Differences in biomechanical stability using various fibrin glue compositions for mesh fixation in endoscopic inguinal hernia repair. Surg Endosc. 2012;26(11):3282–6.
D'Amore L, Ceci F, Mattia S, et al. Adhesion prevention in ventral hernia repair: an experimental study comparing three lightweight porous meshes recommended for intraperitoneal use. Hernia. 2017;21(1):115–23.
Delibegovic S, Koluh A, Cickusic E, et al. Formation of adhesion after intraperitoneal application of TiMesh: experimental study on a rodent model. Acta Chir Belg. 2016;116(5):293–300.
Koetje JH, Irvine T, Thompson SK, Devitt PG, et al. Quality of life following repair of large hiatal hernia is improved but not influenced by use of mesh: results from a randomized controlled trial. World J Surg. 2015;39(6):1465–73.
Fenton-Lee D, Tsang C. A series of complications after paraesophageal hernia repair with the used of Timesh: a case report. Surg Laparosc Endosc Percutan Tech. 2010;20(3):e95–6.
Hazebroek EJ, Ng A, Yong DH, et al. Clinical evaluation of laparoscopic repair of large hiatal hernias with TiMesh. ANZ J Surg. 2008;78(10):914–7.
Granderath FA, Kamolz T, Schweiger UM, et al. Laparoscopic refundoplication with prosthetic hiatal closure for recurrent hiatal hernia after primary failed antireflux surgery. Arch Surg. 2003;138:902–7.
Acknowledgments
Special thanks, admiration, and respect to all our department members for their kind help, guidance, and valuable support.
Funding
No funding resources.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. Study concept and design: IK, PP, MC. Acquisition of data: MC, FG, AB. Analysis and interpretation of data: AB, IK, PB. Drafting of the manuscript: IK, PP, MC, MF. Critical revision of the manuscript for intellectual content: PP, JG, EL. Statistical Analysis: IK.MF.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been reviewed by our Research ethics committee in Ramon y Cajal University, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
We have obtained a written consent from all the patients included in our study with institutional consent forms.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Khaled, I., Priego, P., Faisal, M. et al. Assessment of short-term outcome with TiO2 mesh in laparoscopic repair of large paraesophageal hiatal hernias. BMC Surg 19, 156 (2019). https://doi.org/10.1186/s12893-019-0607-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-019-0607-4