Abstract
Background
Axial spondyloarthritis including ankylosing spondylitis (AS) is characterized by chronic inflammation and new bone formation in the axial skeleton. On the other hand, bone loss, osteoporosis and an increased risk of vertebral fractures is known to frequently occur in AS. In the MEASURE 1 study, the clinically efficacious interleukin-17A inhibitor secukinumab was shown to have limited radiographic progression through 4 years in patients with active AS. Here we present a post hoc analysis to evaluate the effect of secukinumab on bone mineral density (BMD) and bone turnover biomarkers over 2 years in this study.
Methods
BMD was measured by dual-energy X-ray absorptiometry at the lumbar spine, total hip, and femoral neck. Spinal radiographs performed at baseline and Week 104 were assessed by modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) and analyzed in relation to BMD change, considering baseline syndesmophytes. Bone turnover biomarkers were assessed at baseline and at Weeks 52 or 104.
Results
Among 104 patients included in this analysis, 66% were male, with a mean (SD) age of 40.4 (12.3) years. In postmenopausal women and men ≥50 years of age (T-score), the proportion of patients having normal BMD at baseline and Week 104 were 54.5%/54.5% (lumbar spine), 31.6%/55.6% (total hip), and 42.1%/44.4% (femoral neck). Similarly, at baseline, the proportion of patients with osteopenia/osteoporosis was 31.8%/13.6% (lumbar spine), 57.9%/10.5% (total hip), 42.1%/15.8% (femoral neck), and 36.4%/9.1% (lumbar spine), 44.4%/0% (total hip) and 55.6%/0% (femoral neck) at Week 104, respectively. In premenopausal women and men < 50 years of age (Z-score), the proportion of patients having BMD below the expected range for age at baseline and Week 104 were 25.0%/21.2% (lumbar spine), 11.3%/17.8% (total hip), and 9.9%/8.9% (femoral neck). In relation to mSASSS change scores ≥2 over 2 years, the increase in lumbar spine BMD was not related to radiographic progression and syndesmophyte formation. No significant changes were observed in the bone turnover markers over time.
Conclusion
The high proportion of AS patients with diminished BMD was confirmed in this study. An increase of BMD in the lumbar spine after 2 years of secukinumab treatment in patients with AS was found that was probably unrelated to radiographic progression. No relevant effects of secukinumab on bone turnover biomarkers were documented.
Trial registration
MEASURE 1 (post hoc analysis) Clinicaltrials.gov, NCT01358175; Registered, 23 May 2011.
Similar content being viewed by others
Background
Ankylosing spondylitis (AS) or radiographic axial spondyloarthritis (axSpA), is a chronic inflammatory disease that primarily affects the axial skeleton, including the sacroiliac joints and the vertebral column [1]. It is often characterized by inflammatory back pain and spinal stiffness leading to impaired physical function [2]. Extra-musculoskeletal manifestations such as psoriasis [3] and comorbidities such as osteoporosis can have substantial impact on quality of life in patients with AS [4, 5]. Structural bone damage and spinal radiographic progression in AS typically manifests by syndesmophyte formation and ankylosis [6, 7].
As a consequence of ongoing systemic inflammation, osteopenia or decreased bone mineral density (BMD) are common findings in patients with AS [8, 9]. Low BMD at the spine and hip is associated with an increased risk of fracture in patients with AS [10, 11]. The prevalence of osteoporosis in patients with AS is usually > 10% but varies according to age, disease duration, gender, the burden of inflammation and new bone formation, diet, vitamin D levels and immobility [12, 13]. Bone formation and loss of bone mineral content may occur in parallel in AS. The exact pathomechanisms leading to bone growth and loss, and the association between them is still unclear [5, 14, 15]. Due to significantly increased fracture risk an early diagnosis and treatment of osteoporosis is very important in patients with AS [12, 13, 16]. In addition to BMD assessment using dual-energy X-ray absorptiometry (DXA), biochemical markers of bone metabolism, known as bone turnover biomarkers, including markers of bone formation (growth), bone resorption (loss) and bone turnover regulators, can be useful diagnostic tools for the assessment of fracture risk in patients with AS [16, 17].
Biologics, such as interleukin-17 (IL-17) inhibitors and tumor necrosis factor inhibitors (TNFi), are recommended for treatment in patients with AS [18, 19]. In recently published studies, limited radiographic progression has been reported in patients with AS over long-term treatment with TNFi [20, 21]. However, majority of data on the effect of TNFi on BMD is inconclusive [21,22,23,24].
Secukinumab, a human monoclonal antibody that directly inhibits IL-17A, provided sustained improvement in patients with active AS in the pivotal Phase III MEASURE 1 study through 5 years [25, 26]. Data from this study reported a low mean progression of spinal radiographic change in secukinumab-treated patients through 4 years. However, the effect of secukinumab on BMD has not been explored yet [27,28,29]. Here, we present a post hoc analysis to evaluate the effect of secukinumab 150 mg (approved dose for AS) treatment over 2 years on BMD and bone turnover biomarkers in patients with AS from the MEASURE 1 study. A further analysis of BMD categories was performed on the following subgroups: postmenopausal women and men ≥50 years of age, and premenopausal women and men < 50 years of age.
Methods
Study design and patients
MEASURE 1 was a double-blind, randomized, placebo-controlled Phase III 2-year core study, with a 3-year extension study. The detailed study design, patients, methodology and statistical analyses have been described previously [25, 26]. Patients were initially randomized to receive intravenous (i.v.) secukinumab 10 mg/kg at baseline and at Weeks 2 and 4, followed by subcutaneous (s.c.) secukinumab 150 (IV → 150 mg) or 75 mg (IV → 75 mg) every 4 weeks thereafter. Placebo was given on the same i.v. to s.c. dosing schedule. Placebo patients were re-randomized to s.c. secukinumab 150 mg or 75 mg by Week 16 (Assessment of SpondyloArthritis International Society criteria 20 [ASAS 20] non-responders at Week 16) or Week 24 (ASAS 20 responders at Week 16). In the current post hoc analysis, data from patients originally randomized to secukinumab 150 mg (IV → 150 mg) were analyzed.
In brief, the key inclusion criteria included patients aged ≥18 years with active AS fulfilling the modified New York Criteria [30], with a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of ≥4 (on a 0–10 scale) [31] and a spinal pain score of ≥4 cm on a 10 cm visual analogue scale, despite treatment with nonsteroidal anti-inflammatory drugs. Patients who had taken not more than one previous TNFi with an inadequate response or intolerance were also allowed to enter the study. Key exclusion criteria were total spinal ankylosis, evidence of infection or cancer on chest radiography, active systemic infection within 2 weeks before baseline, and previous treatment with cell-depleting therapies or biologic agents other than TNFi agents.
This multicenter randomized clinical trial (RCT) was well designed and fulfilled the CONSORT statement checklist, which comprises a minimum standard of recommendations for reporting RCTs [32].
Outcome measures
BMD assessment
BMD (g/cm2) was measured by DXA using Hologic or GE-Lunar scanners at the lumbar spine, total hip, and femoral neck. Sites were instructed to use the same DXA scanner throughout the trial. The BMD values were standardised using a conversion formula by the central imaging laboratory by doing cross validation. Quality assurance, cross-calibration adjustment, and data processing were done centrally by a certified imaging specialist.
T-score
The T-score is the standard deviation of BMD of an individual compared to the mean BMD of young (20–29 year-old) healthy adults. According to the World Health Organization, the T-score categorizes postmenopausal women and men aged ≥50 years as follows: normal BMD: T-score ≥ − 1.0; osteopenic: − 2.5 < T-score < − 1.0; and osteoporotic: T-score ≤ − 2.5 [33, 34].
The change from baseline in T-score was calculated at Weeks 52 and 104.
Z-score
The Z-score is the BMD standard deviation of the individual compared to the mean BMD of individuals of the same gender, age, and ethnicity. Z-score values, as far as available (not available for example, Asians), were added to the study database in the preparation of this post-hoc analysis. According to the International Society for Clinical Densitometry, the Z-score categorizes premenopausal women and men aged < 50 years as below the expected range for age with BMD ≤ − 2.0 and within the expected range for age with BMD > − 2.0 [35].
The change from baseline in Z-score was calculated at Weeks 52 and 104. The age-matched Z-scores for 3 skeletal sites (lumbar spine, total hip, and femoral neck) at Weeks 52 and 104 were also calculated.
Bone turnover biomarkers
Bone turnover biomarkers such as the anabolic or bone formation biomarkers [osteocalcin (μg/L), procollagen type 1 N-terminal propeptide (μg/L), procollagen type 1 carboxy-terminal peptide (μg/L), bone specific alkaline phosphatase (U/L)], the bone resorption biomarker type I collagen C-telopeptides (μg/L), and other biomarkers (bone turnover regulators) such as osteoprotegerin (pmol/L) and sclerostin (pmol/L) were assessed by a validated enzyme-linked immunosorbent assay (ELISA) method with a lower limit of quantification of 80 ng/mL. These bone turnover biomarkers reflect the rate of bone formation and bone resorption over the treatment phase [14, 16].
Serum samples for biomarker analysis were collected before 11 am after an overnight fast of at least 8 h at selected visits. Changes from baseline in bone turnover biomarkers were summarized at Weeks 52 and 104.
Radiographic assessment
The cervical and the lumbar spine radiographs (lateral view) were obtained at baseline and Week 104. Images were digitized centrally and to ensure blinding, patient identifiers and temporal indicators were removed [28]. Structural damage and radiographic progression was assessed by central readers using modified Stoke Ankylosing Spondylitis Spine Score (mSASSS; score range 0–72, higher score indicates greater structural damage) [36]. In radiographs scored using mSASSS, a lateral view of the anterior parts of lumbar spine is evaluated for the presence of squaring, erosions and/or sclerosis (1 point), syndesmophytes (2 points) and bridging syndesmophytes (3 points) [37]. mSASSS score was assessed in relation to BMD change, taking baseline syndesmophytes into consideration, with baseline syndesmophytes being robust predictors of radiographic progression [38]. Presence of baseline syndesmophytes was derived from mSASSS: according to the definition of levels of mSASSS, baseline syndesmophytes were considered present if a patient had at least one vertebra with a score of 2 or 3 [36, 39]. For the present analysis, ‘notable radiographic progression’ was set as an increase in mSASSS ≥2 units over 2 years of treatment.
Statistical analysis
Analyses are post hoc and descriptive (data presented as observed). All analyses are presented for patients originally randomized to the secukinumab 150 mg group (IV → 150 mg). Analyses included patients with non-missing baseline BMD value and at least one post-baseline lumbar spine BMD value at Weeks 52 or 104 from the full analysis set. The baseline value is defined as the last observation on the day of or before the first dose of study drug.
To assess normalization of bone turnover markers, the course over time in the high and low quartile subgroups (at baseline) was explored by boxplots. A scatter plot was generated to evaluate the changes in lumbar spine BMD from baseline to Week 104 in relation to change in mSASSS from baseline to Week 104; patients with and without baseline syndesmophytes were represented by different symbols, and horizontal and vertical lines indicated the change in lumbar spine BMD of zero and change in mSASSS of 2, respectively, as depicted in Fig. 4.
Results
Patients
Baseline demographic and clinical characteristics in patients with AS from MEASURE 1 have been reported previously [25]. A total of 104 patients (originally randomized to secukinumab 150 mg) were included in this post hoc analysis; postmenopausal women and men ≥50 years of age, 21.1% (n = 22) and premenopausal women and men < 50 years of age, 78.8% (n = 82). Patients (n = 78) with a non-missing baseline BMD value and at least one post-baseline lumbar spine BMD value at Weeks 52 or 104 from the full analysis set were considered for further analysis of normal BMD, osteopenia, and osteoporosis. Among the 78 patients, 22 patients were postmenopausal women and men, and 56 patients were premenopausal women and men < 50 years of age.
Demographics and baseline BMD values for patients originally randomized to secukinumab 150 mg (IV → 150 mg) group (N = 104) are presented in Table 1. Within this population, 66.3% of patients were male, with a mean (SD) age at baseline of 40.4 (12.3) years. Overall, the mean time since diagnosis of AS was 6.4 years (Table 1). The mean (SD) baseline BMD (g/cm2) was 1.026 (0.2) at the lumbar spine, 0.902 (0.2) for total hip, and 0.819 (0.2) at the femoral neck at baseline. The mean baseline BMD T-scores in this population for lumbar spine (N = 104), total hip (N = 96) and femoral neck (N = 96) were − 0.6 (1.6), − 0.5 (1.2) and − 0.8 (1.1), respectively. The mean baseline BMD Z-scores for lumbar spine (N = 66), total hip (N = 84) and femoral neck (N = 84) were − 0.8 (1.6), − 0.6 (1.1) and − 0.6 (1.0), respectively (Table 1). In this analysis, taken together there were four patients with concomitant osteoporosis treatment including zoledronic acid and risedronate. However, due to small n numbers no further sub-group analysis was carried out in these patients.
Efficacy
Efficacy data is reported only for patients originally randomized to secukinumab 150 mg (IV → 150 mg) group. The mean of individual BMD percent changes at Week 52 were 2.6% for lumbar spine, 0.9% for total hip, and 0.8% for femoral neck. Corresponding percent changes at Week 104 were 4.7% for lumbar spine, 0.5% for total hip, and 0.2% for femoral neck.
For postmenopausal women and men ≥50 years, the proportion of patients with normal BMD, osteopenia and osteoporosis was 54.5, 31.8 and 13.6%, respectively at baseline. The proportion of patients with normal BMD, osteopenia and osteoporosis remained consistent from baseline to Week 52 (54.5%/36.4%/9.1%) and Week 104 (54.5%/36.4%/9.1%) for the lumbar spine. A similar trend was observed for total hip and femoral neck where no considerable change in the number of patients in each group was observed over time (Fig. 1). In premenopausal women and men < 50 years of age, the proportion of patients having BMD below the expected range for age at baseline was 25.0, 11.3, and 9.9% for the lumbar spine, total hip, and femoral neck, respectively. Consistent with the results, the proportion of patients having BMD below the expected range for age was 21.2, 17.8, and 8.9% for the lumbar spine, total hip, and femoral neck, respectively at Week 104 (Fig. 2), indicating no substantial shift in the distribution of patients towards abnormal BMD through 2 years of treatment.
Normal BMD, Osteopenia, and Osteoporosis Rates in Postmenopausal Women and Men ≥50 years of age through Week 104. Data presented as observed. Normal BMD, T-score ≥ − 1.0; Osteopenia, − 1.0 > T-score > − 2.5; and Osteoporosis, T-score ≤ − 2.5. N, total number of patients; n, number of evaluable patients
BMD Below and Within Expected Range in Premenopausal Women and Men < 50 Years of age through Week 104. Data presented as observed. BMD within the expected range for age (‘Within Range’) = Z score > − 2.0, and below the expected range for age (‘Below Range’) = Z score ≤ − 2.0. BMD, bone mineral density; N, total number of patients; n, number of evaluable patients
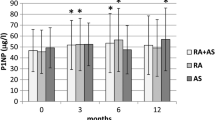
The time course in the high and low quartile subgroups (at baseline) for various bone turnover biomarkers are presented as boxplots in Fig. 3. Independent of high or low baseline values, bone turnover biomarkers remained in the same range overall, following a consistent pattern through 2 years of treatment. The mean changes from baseline in other biomarkers through Week 104 are listed in Table 2. Amongst the bone turnover (formation and resorption) markers, in active AS patients after 2 years of secukinumab therapy, no significant changes at Weeks 52 and 104 were observed.
Bone turnover biomarkers course over time for highest and lowest quartile subgroups (at baseline). Symbol 1 – lowest quartile group of parameter at baseline. Symbol 2 – highest quartile group of parameter at baseline. For each subgroup: The box represents the upper and lower quartiles and the whiskers (10th and 90th percentiles) represents the minimum and maximum values. The center horizontal line represents the median and * represents the mean. Circles outside the boxes represent outliers
The changes in the lumbar spine BMD from baseline to Week 104 in relation to change in mSASSS from baseline to Week 104 for patients with or without baseline syndesmophytes are presented in the form of a scatter plot to assess an increase in BMD in relation to radiographic progression (Fig. 4). A higher change in mSASSS score (≥ 2) and a higher probability for disease progression was observed in patients with baseline syndesmophytes compared to patients without baseline syndesmophytes.
Scatter plot of change in lumbar spine BMD and mSASSS in patients with/without baseline syndesmophytes. BMD, bone mineral density; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score. Horizontal line indicates change in the lumbar spine BMD of zero, and vertical line is an indicator of change in mSASSS of 2
Further, there was no notable change from baseline in mSASSS score (≥2) and no radiographic progression over time was observed in secukinumab-treated patients. As such, no correlation was seen between the increase in lumbar spine BMD and radiographic progression (syndesmophyte formation).
Discussion
The effect of secukinumab treatment in patients with AS from the MEASURE 1 study through 5 years have been reported previously [25, 26]. Almost 45% of patients included in the MEASURE 1 study originally randomized to secukinumab 150 mg had a low BMD at the anteroposterior lumbar spine L1–L4 and almost 58% at the femoral neck, with osteoporotic values in more than 10% of patients (postmenopausal women and men ≥50 years), consistent with previous studies [5, 40, 41]. The proportion of patients with low BMD in premenopausal women and men < 50 years of age was 25% at the lumbar spine.
To the best of our knowledge, this post hoc analysis in secukinumab 150 mg (IV → 150 mg) group is the first study assessing the effects of an IL-17 inhibitor on BMD at different sites and in relation to radiographic progression, in combination with the evaluation of bone turnover markers. According to the demographic and baseline clinical characteristics, the assessment of BMD was analyzed based on T-score and Z-score due to the heterogeneity of the study population.
Increase in BMD from baseline in the spine (P < 0.001) and hip (P = 0.040) at Week 102 following treatment with the TNFi infliximab (ASSERT trial) have been previously reported in patients with ankylosing spondylitis [42]. In this post hoc analysis, BMD was maintained or increased over 2 years in both men and women treated with secukinumab (an IL-17A inhibitor), with a pronounced increase observed in the lumbar spine BMD, based on the mean of individual BMD percent changes. Thus, as seen with TNFi, secukinumab preserves BMD in patients with AS [24, 43]. However, the measurement of lumbar spine BMD with DXA in patients with AS is often problematic because BMD is falsely elevated due to bone proliferation or new bone formation, which is a characteristic feature of AS [44].
In MEASURE 1, low mean progression of spinal radiographic change at Week 104 was demonstrated with secukinumab [28, 29]. In the current post hoc analysis, the scatter plot showed no notable change or comparatively low change in mSASSS score through 2 years, despite the increase in BMD at the lumbar spine. Most of the patients who had a change in lumber spine BMD did not have a change in mSASSS. Hence, the increase in bone density/BMD was not due to increase in mSASSS score which is considered as a new bone formation due to disease worsening. This observation suggests that there is no association between lumbar spine BMD increase and syndesmophytes formation, as measured by mSASSS score and further substantiating the radiographic data reported over 2 years in the MEASURE 1 core study. Hence, a false elevation of BMD values based on syndesmophyte formation is not suggested from this analysis.
The prevalence of syndesmophytes is related to new bone formation, as is the case for BMD increase. However, syndesmophytes are the result of vertebra fusion (bridging of intervertebral spaces) with reduced bone quality than normal. The altered biomechanics and bone quality are thought to be responsible for the increased risk of vertebral fractures in patients with AS. Any increase in DXA measured BMD, which is not accompanied by an increase in radiographic progression, could be interpreted as evidence for improved bone quality and reduced fracture risk. However, other factors such as aortic calcifications, progressive degenerative changes in the lumbar spine not assessed in the mSASSS, osteoblastic lesions, or new vertebral fractures can also lead to increased BMD but would not improve bone quality or fracture risk. Only more advanced imaging analyses including quantitative computed tomography (QCT) or finite element analysis and/or prospective trials powered for vertebral fracture reduction could examine the hypothesis that the observed improvement in DXA measured BMD results in better bone strength and reduced fracture risk.
Bone turnover biomarkers, released during bone remodeling process, including bone formation and resorption, could have the potential to provide prognostic information on fracture risk to supplement radiographic measures of bone mass [45]. However, measurement of bone turnover biomarkers is often challenging as these markers are influenced by several pre-analytical and disease-related confounding factors (e.g., time of day when sampled, underlying renal function) [17]. As the MEASURE 1 study was not powered to detect differences in these often highly variable biomarkers, these results need to be interpreted with caution. It was observed that independent of higher and lower baseline values, bone turnover biomarkers (anabolic, bone resorption and other biomarkers) remained in the same range overall, following a consistent pattern through 2 years of secukinumab treatment. These results indicated no detrimental effect of secukinumab treatment on the bone metabolism.
Further limitations of this analysis include the unavailability of individual mSASSS data for cervical and lumbar spine, lack of a control group and a limited sample size. Any differences in BMD, T-score, or Z-score at the lumbar spine in patients with and without radiographic spinal progression was not analysed as a part of this analysis due to small patient numbers. The lack of vertebral fracture assessment and correlation analysis for bone turnover markers with BMD further limits the evaluation of BMD and bone turnover biomarker changes in relation to bone quality, as well as the overall interpretation of the findings.
Conclusions
The results of this post hoc analysis from the MEASURE 1 study showed that after 2 years of treatment with secukinumab in patients with AS, BMD of the lumbar spine, femoral neck and total hip was stable or increased, with the most striking findings at the lumbar spine. Importantly, these increases in BMD were not correlated with radiographic mSASSS progression or biomarker changes.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers’ access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the study in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.
Abbreviations
- AS:
-
Ankylosing Spondylitis
- axSpA:
-
Axial Spondyloarthritis
- BASDAI:
-
Bath Ankylosing Spondylitis Disease Activity Index
- BMD:
-
Bone Mineral Density
- DXA:
-
Dual-energy X-ray Absorptiometry
- IL:
-
Interleukin
- i.v.:
-
Intravenous
- mSASSS:
-
Modified Stoke Ankylosing Spondylitis Spine Score
- QCT:
-
Quantitative computed tomography
- s.c.:
-
Subcutaneous
- SD:
-
Standard Deviation
- TNFi:
-
Tumor necrosis factor inhibitors
References
Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379–90.
Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84.
Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(1):65–73.
Davey-Ranasinghe N, Deodhar A. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2013;25(4):509–16.
Klingberg E, Lorentzon M, Mellstrom D, Geijer M, Gothlin J, Hilme E, et al. Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14(3):R108.
Lories RJ, Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin N Am. 2012;38(3):555–67.
Tan S, Wang RS, Ward MM. Syndesmophyte growth in ankylosing spondylitis. Curr Opin Rheumatol. 2015;27(4):326–32.
Singh HJ, Nimarpreet K, Ashima DS, Kumar A, Prakash S. Study of bone mineral density in patients with ankylosing spondylitis. J Clin Diagn Res. 2013;7(12):2832–5.
Malochet-Guinamand S, Pereira B, Tatar Z, Tournadre A, Molto A, Dougados M, et al. Prevalence and risk factors of low bone mineral density in spondyloarthritis and prevalence of vertebral fractures. BMC Musculoskelet Disord. 2017;18(1):357.
Ulu MA, Batmaz I, Dilek B, Cevik R. Prevalence of osteoporosis and vertebral fractures and related factors in patients with ankylosing spondylitis. Chin Med J. 2014;127(15):2740–7.
Munoz-Ortego J, Vestergaard P, Rubio JB, Wordsworth P, Judge A, Javaid MK, et al. Ankylosing spondylitis is associated with an increased risk of vertebral and nonvertebral clinical fractures: a population-based cohort study. J Bone Miner Res. 2014;29(8):1770–6.
Hinze AM, Louie GH. Osteoporosis Management in Ankylosing Spondylitis. Curr Treatm Opt Rheumatol. 2016;2(4):271–82.
Magrey M, Khan MA. Osteoporosis in ankylosing spondylitis. Curr Rheumatol Rep. 2010;12(5):332–6.
Klingberg E, Nurkkala M, Carlsten H, Forsblad-d'Elia H. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol. 2014;41(7):1349–56.
Klingberg E, Lorentzon M, Gothlin J, Mellstrom D, Geijer M, Ohlsson C, et al. Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures, and syndesmophytes. Arthritis Res Ther. 2013;15(6):R179.
Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017;5:18.
Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20(6):846–52.
van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–91.
Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599–613.
Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65(10):2645–54.
Maas F, Arends S, Wink FR, Bos R, Bootsma H, Brouwer E, et al. Ankylosing spondylitis patients at risk of poor radiographic outcome show diminishing spinal radiographic progression during long-term treatment with TNF-alpha inhibitors. PLoS One. 2017;12(6):e0177231.
Li H, Li Q, Chen X, Ji C, Gu J. Anti-tumor necrosis factor therapy increased spine and femoral neck bone mineral density of patients with active ankylosing spondylitis with low bone mineral density. J Rheumatol. 2015;42(8):1413–7.
van der Weijden MA, van Denderen JC, Lems WF, Nurmohamed MT, Dijkmans BA, van der Horst-Bruinsma IE. Etanercept increases bone mineral density in ankylosing spondylitis, but does not prevent vertebral fractures: results of a prospective observational cohort study. J Rheumatol. 2016;43(4):758–64.
Haroon NN, Sriganthan J, Al Ghanim N, Inman RD, Cheung AM. Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):155–61.
Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an Interleukin-17A Inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–48.
Baraliakos X, Braun J, Deodhar A, Poddubnyy D, Kivitz A, Tahir H, et al. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD Open. 2019;5(2):e001005.
Baraliakos X, Borah B, Braun J, Baeten D, Laurent D, Sieper J, et al. Long-term effects of secukinumab on MRI findings in relation to clinical efficacy in subjects with active ankylosing spondylitis: an observational study. Ann Rheum Dis. 2016;75(2):408–12.
Braun J, Baraliakos X, Deodhar A, Baeten D, Sieper J, Emery P, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76(6):1070–7.
Braun J, Baraliakos X, Deodhar A, Poddubnyy D, Emery P, Delicha EM, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology (Oxford). 2019;58(5):859–68.
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–8.
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath ankylosing spondylitis disease activity index. J Rheumatol. 1994;21(12):2286–91.
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18.
Consensus Development Conference on Osteoporosis. Hong Kong, April 1-2, 1993. Am J Med. 1993;95(5A):1S–78S.
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–76.
Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone. 2008;43(6):1115–21.
Creemers MC, Franssen MJ, van't Hof MA, Gribnau FW, van de Putte LB, van Riel PL. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 2005;64(1):127–9.
van der Heijde D, Braun J, Deodhar A, Baraliakos X, Landewé R, Richards HB, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology (Oxford). 2019;58(3):388–400.
Kim HR, Hong YS, Park S-H, Ju JH, Kang KY. Low bone mineral density predicts the formation of new syndesmophytes in patients with axial spondyloarthritis. Arthritis Res Ther. 2018;20(1):231.
Baraliakos X, Listing J, Rudwaleit M, Haibel H, Brandt J, Sieper J, et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis. 2007;66(7):910–5.
van der Weijden MA, Claushuis TA, Nazari T, Lems WF, Dijkmans BA, van der Horst-Bruinsma IE. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol. 2012;31(11):1529–35.
Lee SG, Park YE, Park SH, Kim TK, Choi HJ, Lee SJ, et al. Increased frequency of osteoporosis and BMD below the expected range for age among South Korean women with rheumatoid arthritis. Int J Rheum Dis. 2012;15(3):289–96.
Visvanathan S, van der Heijde D, Deodhar A, Wagner C, Baker DG, Han J, et al. Effects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(2):175–82.
Ashany D, Stein EM, Goto R, Goodman SM. The effect of TNF inhibition on bone density and fracture risk and of IL17 inhibition on radiographic progression and bone density in patients with axial spondyloarthritis: a systematic literature review. Curr Rheumatol Rep. 2019;21(5):20.
Deminger A, Klingberg E, Lorentzon M, Geijer M, Gothlin J, Hedberg M, et al. Which measuring site in ankylosing spondylitis is best to detect bone loss and what predicts the decline: results from a 5-year prospective study. Arthritis Res Ther. 2017;19(1):273.
Ferreira A, Alho I, Casimiro S, Costa L. Bone remodeling markers and bone metastases: from cancer research to clinical implications. Bonekey Rep. 2015;4:668.
Acknowledgements
The authors thank the patients who participated in this study and the study investigators for their contributions. John Gallagher (Novartis UK) provided medical and editorial guidance. Medical writing support, under the guidance of the authors, was provided by Gurleen Kaur and Priyanka Malla, Senior Scientific writers (Novartis, India). Study was funded by Novartis Pharma AG in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3)
Funding
The study was sponsored by Novartis Pharma AG and designed by the scientific steering committee and Novartis personnel in collaboration with the authors. The institutional review board at each participating centre approved the protocol. Data were collected in accordance with Good Clinical Practice guidelines by the study investigators and were analysed by the sponsor. All the authors contributed to the interpretation of the data and had access to the full data sets. The statistical analyses were performed by statisticians employed by the sponsor and were reviewed by all the authors. Agreements between the sponsor and the investigators included provisions relating to confidentiality of the study data. The writing support for the manuscript was provided by a medical writer from Novartis, India, and funded by the sponsor. All the authors vouch for the accuracy and completeness of the data and analyses, as well as for the fidelity of this report to the trial protocol, which are available from the funder.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, were involved in the drafting and critical review of the manuscript and approved the final version for submission. All authors agree to be accountable for all aspects of the work and attest to the accuracy and integrity of the work. All the authors have read and approved the final version of the manuscript to be published.
Study conception and design: JB, XB, BP, and SH contributed to the study design and conception.
Acquisition of data: BP and SH contributed to the acquisition of the data.
Analysis and interpretation of data: JB, BB, XB, LSG, BP, EQF, and SH J contributed to the analysis and interpretation of the data.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All clinical studies were conducted in compliance with the Declaration of Helsinki, International Council for Harmonization Guidelines for Good Clinical Practice and local country regulations and were approved by the institutional review board or independent ethics committee (Supplementary Table S1) at each participating centre. All patients provided written informed consent to participate in the respective studies.
Consent for publication
Not required.
Competing interests
JB: Grant/research support from: Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB, Eli Lilly consultant for: Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB, Eli Lilly and received speakers bureau fees from: Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB pharma, Eli Lilly.
BB: Grant/research support from: Kinemed, Extendicare Foundation, Lilly, GE/Lunar and MSD; consultant for: GE/Lunar, Gilead, Theramex, UCB; received speakers bureau fees from UCB, MSD, Biogen, Sanofi Genzyme; Travel support from Janssen, UCB, Gilead, AbbVie.
XB: Grant/research support from: AbbVie, Novartis, Consultant for: AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB, Werfen, Speakers bureau: AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB.
LSG: Grant/research support from: Novartis, Pfizer and UCB, Consultant for: AbbVie, Eli Lilly, GSK, Novartis and UCB.
BP: Employee of Novartis and owns Novartis stock.
EQF: Employee of Novartis and owns Novartis stock.
SH: Employee of Novartis and owns Novartis stock.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. List of ethical approval reference numbers for each participating center (MEASURE 1 study).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Braun, J., Buehring, B., Baraliakos, X. et al. Effects of secukinumab on bone mineral density and bone turnover biomarkers in patients with ankylosing spondylitis: 2-year data from a phase 3 study, MEASURE 1. BMC Musculoskelet Disord 22, 1037 (2021). https://doi.org/10.1186/s12891-021-04930-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-021-04930-1