Abstract
Background
New intraocular lenses (IOLs) have emerged since the originally coined monofocal and multifocal IOLs. The extended depth of focus (EDoF) and enhanced monofocal IOLs (mono-EDoF) that have appeared in the last decade have caused some confusion in their classification. The aim of this review was to summarize the outcomes provided by mono-EDOF IOLs and to determine which of the endpoints, described by the American National Standard (ANSI) for EDoF IOLs, are fulfilled.
Methods
The MEDLINE, EMBASE, and WEB OF SCIENCE databases were searched. Two independent reviewers screened the studies for inclusion and data extraction. The search strategy was limited to studies published between 2020 and 2022, but not by language. The results are presented as a narrative summary accompanied by tables, in alignment with the objectives of this scoping review. Compliance with the endpoints for clinical outcomes described in the American National Standard Z80.35–2018 (ANSI) for EDoF lenses was checked and additional endpoints were defined.
Results
Two systematic reviews, 13 laboratory, 21 clinical, and two mixed studies were included. Tecnis Eyhance was the mono-EDOF with the highest volume of evidence to date. Although laboratory studies included other IOLs, clinical evidence for them is still scarce, with only one study of IsoPure compared to a standard monofocal IOL. Evidence in comparison to EDoF lenses is also scarce, even for Tecnis Eyhance, with only three studies including this lens in comparison to an EDoF lens. After evaluation of the ANSI criteria, agreement was found in the failure for the increase in depth of field equal to or greater than 0.5 D for a visual acuity (VA) level of 0.2 logMAR and none of the studies supported that the median monocular VA at intermediate distance was at least 0.2 logMAR.
Conclusions
Additional clinical evidence is required for other mono-EDOF IOLs beyond Tecnis Eyhance. Until the arrival of a standard classification, mono-EDOF should be better still classified as monofocal because the ANSI standards were not fully met.
Similar content being viewed by others
Introduction
A monofocal intraocular lens (IOL) is a medical device implanted in the eye to restore distant vision in eyes with cataract. Beyond monofocal IOLs, other technologies such as simultaneous vision lenses (SVL) have also been developed to restore intermediate and/or near vision. SVL have been historically classified as multifocal IOLs, for which the light is split into multiple foci, and extended depth of focus lenses (EDoF), for which the far-distance focus is extended [1, 2].
A new technology named Tecnis Eyhance (Johnson & Johnson) was introduced in 2019 which as EDoF lenses extend the far distance focus [3]. Since this technology extends the depth of focus in a similar way to EDoF lenses, some confusion emerged among anterior segment surgeons, some declaring this lens as EDoF even though the IOL was launched as a new generation of monofocal intraocular lens. Subsequently, other IOLs with hypothetical similar visual performance were launched, and a new category popularly known as plus monofocal, mono-EDoF, or enhanced monofocals appeared. The new generation of monofocal IOLs that enhance intermediate vision might include Tecnis Eyhance (Johnson & Johnson), IsoPure (Physiol), Xact (Santen), Zoe (OphthalmoPro GmbH), RayOne EMV (Rayner), Lentis Quantum (Teleon Surgical), Evolux (Sifi), Vivinex Impress (Hoya), and Extend HP (Hanita Lenses).
Standards have not yet considered these lenses as a new category of SVL; therefore, they should still be classified as monofocal or EDoF lenses. The definition of EDoF has been reported by the American National Standard Z80.35–2018 (ANSI) [4], and describes up to four effectiveness end-points that should be met in full for classifying an IOL as EDoF, between them: (1) to demonstrate a statistical superiority over a control monofocal group on mean, monocular photopic distance-corrected intermediate visual acuity at 66 cm; (2) to demonstrate at least 0.5 D greater monocular photopic negative lens induced distance-corrected depth of focus compared to the monofocal control IOL at 0.2 logMAR visual acuity threshold; (3) the median, monocular distance-corrected photopic intermediate visual acuity at 66 cm is at least 0.2 logMAR, and (4) the mean, monocular photopic best corrected distance acuity for the EDoF IOL is statistically non-inferior to the control using a non-inferiority margin of 0.1 logMAR [4].
Unfortunately, the standard described by the ANSI requires comparison with a monofocal IOL in a randomized clinical trial, [4] and common published post-marketing studies are case series without a control lens. A case series without a control group does not allow for the evaluation of requirements 1, 2, and 4, as described by the ANSI. In addition, the descriptive statistic of the median described in the third requirement is not always reported since clinical studies usually offer a mean value. The inclusion of other absolute end-points instead of a relative comparison with a monofocal intraocular lens would help to describe the functional performance of the IOL from case series studies without a control group. Furthermore, these absolute values are expected to be included in future standard updates [5].
A preliminary search was conducted and two systematic reviews on the topic were identified [6, 7]. The purpose these reviews was to assess the efficacy, spectacle independence, patient satisfaction, and adverse event rates in comparison with monofocal IOLs. These systematic reviews concluded that new enhanced monofocal IOLs increased the intermediate and near vision in comparison to conventional monofocal IOLs, [6, 7] without compromising the contrast sensitivity or inducing photic phenomena [6]. These reviews required a narrow scope including only an intervention IOL (Tecnis Eyhance) in comparison of other monofocal IOLs, [6] or even a particular monofocal model [7]. Conversely, this is the first scoping review covering a wide scope that explores not only the clinical results of enhanced monofocal IOLs in comparison to standard monofocal IOLs, but also in comparison to EDoF lenses and laboratory studies. In the absence of a standard definition for these new lenses and to avoid confusion with EDoF lenses, the aim of this scoping review was to summarize the clinical and laboratory outcomes provided by enhanced monofocal IOLs (Concept) and to determine which of the endpoints, described by the ANSI for EDoF lenses and some additional endpoints that might be included in future standards (Context), are fulfilled in patients undergoing cataract surgery (Population).
Review question
The following questions were addressed in this scoping review.
-
What are the optical quality and profiles described in laboratory studies of enhanced monofocal lenses?
-
What are the functional outcomes of patients implanted with enhanced monofocal IOLs for cataract surgery?
-
Which efficacy endpoints, described by the standard (ANSI) and additional ones, are fulfilled for enhanced monofocals?
Keywords
Enhanced monofocal, monofocal plus, mono-EDoF, EDoF, simultaneous vision.
Eligibility criteria
Participants
This scoping review considered studies involving patients who underwent cataract surgery with bilateral implantation of an enhanced monofocal IOL in the capsular bag, and studies evaluating IOLs in the laboratory through the measurement of optical quality or optical profile. The following exclusion criteria were applied for the clinical studies: age < 19 years, comorbidities, history of corneal laser refractive surgery, postoperative complications as the main study purpose, eyes requiring toric IOL implantation or additional corneal incisions (astigmatism > 1.5 D), combining different targets (micro-monovision), or different IOL models (mix-and-match) between eyes.
Concept
The main purpose of the eligible clinical studies was to provide monocular and binocular postoperative clinical results considering the following outcomes: visual acuity at several distances, defocus curves, contrast sensitivity function, and patient-reported outcomes such as the percentage of patients achieving spectacle independence, satisfaction, positive photic phenomena perception, positive dysphotopsia, and recommendation or decision to undergo the same IOL implantation. Eligible laboratory studies reported the results of optical quality or surface profiles.
Context
At least one of the following IOLs was included in the study: Tecnis Eyhance (Johnson & Johnson), IsoPure (Physiol), Xact (Santen), Zoe (OphthalmoPro GmbH), RayOne EMV (Rayner), Lentis Quantum (Teleon Surgical), Evolux (Sifi), Vivinex Impress (Hoya), and Extend HP (Hanita Lenses).
Types of Sources
This scoping review considered original studies, either case series (CS) or randomized clinical trials (RCT), systematic reviews, meta-analyses, case reports, and comments. The sources of gray literature were excluded. No language restrictions were applied.
Methods
The scoping review was conducted in accordance with the JBI methodology for scoping reviews, [8] and following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [9]. This review was conducted in accordance with the a priori protocol registered in the Open Science Framework (https://osf.io/m7wcu). A stakeholder and experts in IOLs were consulted when preparing the study protocol and when discussing the scoping review results as recommended by the JBI Manual for Evidence Synthesis [10]. The following deviations occurred from the a priori protocol: inclusion of the Extend HP (Hanita Lenses) in the Context, the addition of new key references found through snowballing techniques for manuscript published from the search date to the data extraction date starting on 3th of November 2022, and a third independent reviewer was included to resolve discrepancies.
Search strategy
The search strategy and the initial and secondary searches were conducted by one of the reviewers using the 2Dsearch tool (UXLabs Limited) and checked for adequacy by a second reviewer. The search strategy aimed to locate only published studies. An initial limited search of MEDLINE (PubMed) and Google Scholar for the first 200 references was performed by using several keywords to identify relevant and irrelevant studies. A keyword search was performed to identify commonly used words in titles and abstracts, and the index terms assigned to these references were explored using PubReMiner (Jan Koster (AMC)). In the second stage, the scope of the search was optimized to maximize sensitivity such that all previously identified relevant references were retrieved, and the major possible number of irrelevant references was omitted. The text words contained in the titles and abstracts of the articles and the index terms used to describe the articles were used to develop a full search strategy for PubMed on October 2, 2022. The search strategy, including all the identified keywords and index terms, was adapted for EMBASE (Elsevier) and Web of Science (Isi Web of Knowledge). Specific year ranges were applied to retrieve studies on enhanced monofocal IOLs from 2020 to 2022. Language filtering was not applied. A detailed search strategy is presented in supplemental file A. New references published from the systematic search to the date of data extraction on November 3, 2022, were identified through snowballing search techniques, executing the search algorithm again, and looking for those published in the last month.
Study/source of evidence selection
Following the search, all identified citations were collated and uploaded to the Rayyan platform (Qatar Computing Research Institute, Doha, Qatar) for screening. Two independent reviewers screened titles and abstracts to assess the inclusion and exclusion criteria. The full text of the selected citations was assessed in detail against the inclusion criteria by the same two independent reviewers. The reasons for excluding sources of evidence at first and full-text screenings that did not meet the inclusion criteria were recorded and reported in this scoping review. Any disagreements between the reviewers at each stage of the selection process were resolved by a third independent reviewer.
Data extraction
Data were extracted from the papers included in the scoping review by two independent reviewers using a data extraction tool developed by the reviewers and described in the protocol. The extracted data included specific details about the participants, concept, context, study methods, and key findings relevant to the review questions. For studies that included plots for reporting results, as is habitual for defocus curves and contrast sensitivity, data were extracted from the images by one of the reviewers using the WebPlotDigitizer (Ankit Rohatgi) tool.
A new draft data extraction tool, not included in the protocol, was developed during the course of the review for laboratory studies because of the non-uniformity of the reported results for this concept. Any disagreements between the reviewers in data extraction were resolved by discussion. Missing data was filled as “Not Available” (NA), and no requests were made to authors for missing or additional data. Critical appraisal was not conducted, because it was not required for scoping reviews.
Data analysis and presentation
Tables and figures were used to present the data and illustrate the scoping review findings, as described in the protocol for clinical studies. A narrative summary describes how the results relate to the objectives and review questions. Different metrics were reported in the laboratory studies; therefore, the organization for presenting these data was decided during scoping review writing, instead of the protocol. The criteria for selecting the indices shown in the results section were the capability of the information to be summarized in single indices and the uniformity shown in the included manuscripts to describe common indices.
Patient and public involvement
There was no patient or public involvement in this scoping review.
Results
Study inclusion
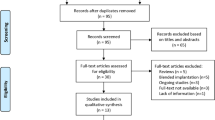
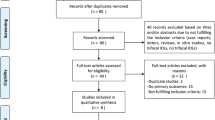
Ninety-four papers were identified in the database search after duplicate removal and 56 were excluded after screening by title and abstract. Thirty of them were unrelated to the study aim and were obtained from a Web of Science database search, which had less specificity. Twenty-four were excluded because none of the IOLs described in the context were included, and only two papers were excluded because of comorbidities or different study types from those described in the inclusion criteria. After the full text evaluation, three clinical studies were excluded since the inclusion criteria was not accomplished, [11,12,13] and one mixed was maintained (partly excluded) since the criteria was accomplished in the laboratory study context but not in the clinical [14]. During the search update in the moment of data extraction, three papers were included, two not indexed in the databases and found through snowballing techniques, [15, 16] and 1 new after search update [17]. Finally, 38 studies were included in the data extraction process (Fig. 1).
Characteristics of included studies
Two systematic reviews, 13 laboratory studies, 21 clinical studies (five RCT and two CS), and two mixed studies were included. For one of the mixed studies, only the laboratory data was extracted since the inclusion criteria was not accomplished for the clinical data [14].
Laboratory studies
With regard optical quality metrics, the depth of focus achieved for predicted visual acuity of 0.2 logMAR and the increase range in comparison to a standard monofocal was retrieved for 4 manuscripts (Table 1) [15, 18,19,20]. Three papers showed the influence of the IOL centration on the modulation transfer function (MTF) at 50 lp/mm (Table 2) [21,22,23]. The wavefront spherical aberration was also reported in 3 studies (Table 3), [14, 19, 24] and the data provided by four manuscripts was not able to be compared since the MTF was normalized, single indices were not used or the data was presented for specific wavelengths or no common metrics [25,26,27,28]. Thus the findings of these studies are reported directly in the text as follows. Only one study reported the relative light intensity at 3 mm showing the Depth of Focus (DoF) achieved for an EDoF and three enhanced monofocal IOLs [27]. The major loss of energy at far distance was related to the increase of DoF in the following order: EDoF, Vivinex Impress, Xact, Tecnis Eyhance, and IsoPure [27]. These results were on agreement with other metrics such as the through focus MTF at 50 lp/mm which showed half MTF at far distance of the EDoF IOLs but a higher increase of the DoF in comparison to the RayOne EMV and the Tecnis Eyhance [25]. Only one study reported the chromatic performance of an enhanced monofocal, particularly the Xact [26]. The halo size was measured through the PSF-cross section showing similar profiles of enhanced monofocals Tecnis Eyhance, Zoe and IsoPure in comparison to the standard monofocal [18,19,20].
With regard to surface profiles, two manuscripts presented the profile of the Tecnis Eyhance measured with the Talysurf CLI 1000 and Zeta Z300 profilometers and in comparison to standard monofocal and refractive EDOF lenses [31, 32]. These studies demonstrated variations between the maximum and minimum elevations of the profiles below ± 1 µm for the standard monofocal, ± 2 µm for the enhanced monofocal, and ± 4 µm for the refractive EDoF.
Clinical studies
Three clinical studies compared enhanced monofocals with EDoF lenses, [33,34,35] 14 compared the clinical results with a standard monofocal, [3, 17, 28, 36,37,38,39,40,41,42,43,44,45,46] and three case series without a comparison group were identified [16, 47, 48] Only one study included both monofocal and EDoF lenses in comparison to Tecnis Eyhance [49]. Supplemental file B contains all the data extracted from clinical studies.
Table 4 shows the endpoints described in each of the enhanced monofocal studies, and the Supplemental A file contains all data extracted from these studies. All the studies comparing EDoF lenses (Symfony, Eyecril SERT and Vivity) with enhanced monofocal lenses were conducted with the Tecnis Eyhance [33,34,35, 49]. General agreement was reported about a higher depth of focus range for EDOF lenses and statistically significant differences only at near vision [33,34,35, 49]. Despite of the higher range of the depth of focus showed in the defocus curve and the statistically significant differences at near, no significant differences were reported for visual acuities at intermediate vision [33,34,35]. The comparison with the standard monofocals is presented in the next section.
EDoF criteria
Although four randomized clinical trials (RCT) were identified, [39, 43, 45, 46] none accomplished all the requirements described by the ANSI for evaluating the EDOF criteria. Table 5 summarizes the criteria for classifying an intraocular lens as an EDOF according to the ANSI standard. Three possible answers were covered in the table, “yes” and “not” when the criteria were or not accomplished, respectively, according to the reviewed paper, and “unclear” when not enough information was provided by the study to provide an answer. Only complete agreement was obtained for ANSI-1 and ANSI-4, indicating no inferiority at distance and better visual acuity at intermediate with the best distance correction in comparison to the standard monofocal. Controversy, but with a higher number of studies reporting no accomplishment of this criteria, was found for the ANSI-2 which describes that the depth of focus was not generally increased above 0.5 D for a visual acuity of 0.2 logMAR. Furthermore, no studies reported the accomplishment of the ANSI-3 criteria that was generally not reported or not accomplished according to two studies [3, 49]. Additional end-points that were not included in the standards are described in Table 5.
Discussion
In this scoping review, the results reported by laboratory and clinical evidence for enhanced monofocal IOLs have been explored. Two systematic reviews, 13 laboratory studies, 21 clinical studies, and two mixed studies were included. The systematic reviews only included evidence for the Tecnis Eyhance IOL, which was for both laboratory and clinical studies, the enhanced monofocal IOL with the highest volume of evidence to date. Laboratory studies have reported optical quality of other enhanced monofocal IOLs such as RayOne EMV [20, 23,24,25], IsoPure [20, 27], Zoe [20], Acunex Quantum [21], Lentis Quantum [22], and Vivinex Impress [27], but optical profiles have been only documented for Tecnis Eyhance [31, 32]. However, laboratory or clinical studies have not been conducted for other announced enhanced monofocal IOLs such as Evolux and Extend HP.
Regarding the laboratory evidence, the elevation changes for refractive EDoF lenses were not achieved for the Tecnis Eyhance [31, 32], and the optical quality studies that included both, enhanced monofocals and EDoF lenses, showed a higher depth of focus range with poorer distance optical quality for EDoF lenses in comparison to enhanced monofocals [25, 27]. These laboratory results were in agreement with clinical evidence of studies including enhanced monofocals and EDoF lenses, demonstrating a higher range of focus and better DCNVA for the latter [33,34,35, 49]. Interestingly, no significant differences were reported for visual acuities at intermediate vision [33,34,35]. These results suggest that enhanced monofocal IOLs might provide comparable visual acuity at intermediate vision in comparison to the EDoF and the most important differences might be found at near vision. However, further RCT comparing enhanced monofocals and EDoF lenses are required to conduct a future systematic review that provides evidence to answer this question.
Although enhanced monofocals have demonstrated superior intermediate vision in comparison to standard monofocals, the EDoF criteria checked in this scoping review evidence that the increase in the range of vision might not be enough to classify these lenses as EDoF lenses. According to the ANSI, for a lens to be classified as EDoF, it must to meet each and every one of the four criteria included in Table 5. Unfortunately, published studies rarely present the results of these four criteria, in many cases, because the authors chose to report the results binocularly rather than monocularly or the mean rather than the median, as described by the ANSI. For this reason, although not described by ANSI, we extended the comparison using five additional criteria that would help to better understand lens performance when ANSI criteria have not been reported (Table 5).
Strengths and limitations
The objective of this scoping review was to map the laboratory and clinical evidence of popularly known enhanced monofocal IOLs. A systematic approach was conducted following the recommendations for this review type, which is a major strength compared to non-systematic approaches such as narrative reviews. On the other hand, the wide scope does not allow the evaluation of effectiveness as in systematic reviews and meta-analysis, but recent meta-analyses have been already published answering to the efficacy question [6, 7]. These meta-analysis also included case series, with a higher number of references in the Wan et al. [6] meta-analysis.
Our scoping review also included three cases series that were not included in the previous ones, [17, 37, 44] but the results provided in these three references were in agreement with the conclusions reported by Wan et al. [6]. This scoping review also included a comparison study for IsoPure [44] IOL in contrast to previous systematic reviews that only included Tecnis Eyhance. It is also important to note that Wan et al. [6] incorrectly included a study cited as Alió 2021 in some endpoints of their meta-analysis, whereas Tecnis Eyhance was not included in Alió’s study. A major strength of this scoping review in comparison with the previously published meta-analysis is the inclusion of lenses beyond Tecnis Eyhance, the inclusion of studies comparing results with EDoF lenses, and the exploration of the accomplishment of the EDoF criteria for ANSI standards. Unfortunately, a major limitation is that there is no standard definition of enhanced monofocal IOLs; therefore, the lenses included in the context are those that, according to the reviewers, might be qualified as enhanced monofocals. The findings summarized in this scoping review could help the reader to compare the results between the reviewed IOLs, but until future standards consider the inclusion of a new category and define the criteria for this category, all these lenses can be considered as monofocal lenses, considering that associating the term “enhanced” to the lenses might be arbitrary because of the lack of standard criteria.
Conclusion
This review identified 39 studies divided into two systematic reviews, 13 laboratory studies, 21 clinical studies, and two mixed studies. Up to 11 laboratory studies and 17 clinical studies included optical, surface profile, and clinical results for Tecnis Eyhance, which means that approximately 74% of the current evidence of enhanced monofocals comes from this particular IOL. Conversely, although laboratory evidence can be found from other enhanced monofocal IOLs, only IsoPure and Xact IOLs provided clinical evidence in four studies, three case series without a comparison group, and only one case series comparing a standard monofocal IOL with the IsoPure IOL. Sufficient clinical evidence in comparison to standard monofocals has been provided by Tecnis Eyhance for the assessment of its efficacy in two published meta-analyses; however, for the remaining IOLs, clinical evidence is still very scarce. All the studies for which the ANSI criteria for EDoF lens classification could be checked corresponded to Tecnis Eyhance, except one study for IsoPure. For the four criteria that should be met to qualify these IOLs as EDoFs, ANSI-2 was not generally met, and no studies supported that the ANSI-3 criteria were met. In comparison with EDoF lenses, evidence is scarce, even for Tecnis Eyhance. Only four studies compared enhanced monofocals with EdoF lenses, particularly for Tecnis Eyhance, and only one study included three groups with monofocal, enhanced monofocal, and EDoF lenses. Tecnis Eyhance was positioned in this study with an increase in depth of focus between the standard monofocal and EDoF. Because of the lack of a standard classification for these lenses and because the ANSI standards were not fully met, these lenses should be better classified as monofocal until the international standards consider a new classification between monofocal and EDoF lenses.
Availability of data and materials
Data extracted is provided in Tables and Supplemental File.
References
Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. Acrysof IQ panoptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: A clinical overview. Asia-Pacific J Ophthalmol. 2019;8:335–49.
Kanclerz P, Toto F, Grzybowski A, Alio JL. Extended Depth-of-Field Intraocular Lenses: An Update. Asia-Pacific J Ophthalmol (Philadelphia, Pa). 2020;9:194–202.
Mencucci R, Cennamo M, Venturi D, Vignapiano R, Favuzza E. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J Cataract Refract Surg. 2020;46:378–87.
American National Standard for Ophthalmics. ANSI Z80.35-2018: extended depth of focus intraocular lenses. 2018. Available at: https://webstore.ansi.org/standards/vc%20(asc%20z80)/ansiz80352018. Accessed 3 Mar 2023.
Eydelman M. AECOS European Symposium. In: Session 5. Lens Speak 2022: FDA Perspective. Belgium; 2022.
Wan KH, Au ACK, Kua WN, Ng ALK, Cheng GPM, Lam NM, et al. Enhanced Monofocal Versus Conventional Monofocal Intraocular Lens in Cataract Surgery: A Meta-analysis. J Refract Surg. 2022;38:538–46.
Redruello-Guerrero P, Rivera-Izquierdo M, Jiménez-Gutiérrez C, Láinez-Ramos-Bossini AJ, Yela R, López-Marín I. Improvement of intermediate vision with new monofocal intraocular lenses: A systematic review and meta-analysis. Eur J Ophthalmol. 2022:11206721221127075. https://doi.org/10.1177/11206721221127075. Epub ahead of print.
Peters M, Godfrey C, McInerney P, Munn Z, Trico A, Khalil H. Chapter 11: Scoping Reviews (2020 version). In: E Aromataris, Z Munn, editors. JBI Manual for Evidence Synthesis. JBI; 2020.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–73.
Francis E. 11.1.3 The scoping review framework. JBI Manual for Evidence Synthesis. 2022. https://jbi-global-wiki.refined.site/space/MANUAL/4687752/11.1.3+The+scoping+review+framework. Accessed 10 Nov 2022.
Blancafort Alias S, Del Campo CZ, Salvador-Miras I, Luna Mariné S, Gómez Prieto MJ, Liñán Martín F, et al. Exploring Vision-Related Quality of Life: A Qualitative Study Comparing Patients’ Experience of Cataract Surgery with a Standard Monofocal IOL and an Enhanced Monofocal IOL. Clin Ophthalmol. 2022;16:1641–52.
Ungewiss J, Röck T, Wörner M, Wetzel D, Bartz-Schmidt KU, Schiefer U. Benchmarking Visual Performance with Monofocal Intraocular Lenses with and without Enhanced Optical Properties in a Nighttime Driving Simulator Environment: A Proof-of-Concept Study. Klin Monbl Augenheilkd. 2022;239:996–1004.
Karuppiah P, Varman NA, Varman A, Balakumar D. Comparison of clinical outcomes of trifocal intraocular lens (AT LISA, Eyecryl SERT trifocal) versus extended depth of focus intraocular lens (Eyhance, Eyecryl SERT EDOF). Indian J Ophthalmol. 2022;70:2867.
Fernández-Vega-Cueto L, Vega F, Guerra-Velasco R, Millán MS, Madrid-Costa D, Alfonso JF. Optical and Clinical Outcomes of an Enhanced Monofocal Intraocular Lens for High Hyperopia. J Refract Surg. 2022;38:572–9.
Azor JA, Vega F, Armengol J, Millan MS. Optical Assessment and Expected Visual Quality of Four Extended Range of Vision Intraocular Lenses. J Refract Surg. 2022;38:688–97.
Stodulka P, Slovak M. Visual Performance of a Polynomial Extended Depth of Focus Intraocular Lens. Open J Ophthalmol. 2021;11:214–28.
Steinmüller LN, Greve D, Rua Amaro D, Bertelmann E, von Sonnleithner C. Analysis of higher-order aberrations in relation to the clinical outcome of an enhanced monofocal IOL. Eur J Ophthalmol. 2022:11206721221134171. https://doi.org/10.1177/11206721221134171. Online ahead of print.
Alarcon A, Cánovas C, Koopman B, Weeber H, Auffarth GU, Piers PA. Enhancing the Intermediate Vision of Monofocal Intraocular Lenses Using a Higher Order Aspheric Optic. J Refract Surg. 2020;36:520–7.
Vega F, Millán MS, Gil MA, Garzón N. Optical performance of a monofocal intraocular lens designed to extend depth of focus. J Refract Surg. 2020;36:625–32.
Łabuz G, Son HS, Naujokaitis T, Yildirim TM, Khoramnia R, Auffarth GU. Laboratory Investigation of Preclinical Visual-Quality Metrics and Halo-Size in Enhanced Monofocal Intraocular Lenses. Ophthalmol Ther. 2021;10:1093–104.
Borkenstein AF, Borkenstein EM, Schmid R. Analysis of a novel hydrophobic acrylic enhanced monofocal intraocular lenscompared to its standard monofocal type on the optical bench. BMC Ophthalmol. 2022;22(1):356.
Borkenstein AF, Borkenstein E-M, Schmid R. Evaluating Optical Quality of a New Hydrophilic Enhanced Monofocal Intraocular Lens and Comparison to the Monofocal Counterpart: An Optical Bench Analysis. Ophthalmol Ther. 2022. https://doi.org/10.1007/s40123-022-00561-4.
Schmid R, Luedtke H, Borkenstein AF. Effect of decentration and tilt on four novel extended range of vision intraocular lenses regarding far distance. Eur J Ophthalmol. 2022:11206721221128864. https://doi.org/10.1177/11206721221128864. Online ahead of print.
Schmid R, Borkenstein AF. Analysis of higher order aberrations in recently developed wavefront-shaped IOLs. Graefe’s Arch Clin Exp Ophthalmol. 2022;260:609–20.
Schmid R, Fuchs C, Luedtke H, Borkenstein AF. Depth of focus of four novel extended range of vision intraocular lenses. Eur J Ophthalmol. 2023;33(1):257–61.
Montagud-Martínez D, Ferrando V, Martínez-Espert A, Garcia-Delpech S, Monsoriu JA, Furlan WD, et al. In Vitro Chromatic Performance of Three Presbyopia-Correcting Intraocular Lenses with Different Optical Designs. J Clin Med. 2022;11(5):1212.
Pieh S, Artmayr C, Pai V, Schartmüller D, Kriechbaum K. Through-Focus Response of Extended Depth of Focus Intraocular Lenses. J Refract Surg. 2022;38:497–501.
Huh J, Eom Y, Yang SK, Choi Y, Kim HM, Song JS. A comparison of clinical outcomes and optical performance between monofocal and new monofocal with enhanced intermediate function intraocular lenses: a case-control study. BMC Ophthalmol. 2021;21:365.
Vega F, Millán MS, Garzón N, Altemir I, Poyales F, Larrosa JM. Visual acuity of pseudophakic patients predicted from in-vitro measurements of intraocular lenses with different design. Biomed Opt Express. 2018;9:4893–906.
Alarcon A, Canovas C, Rosen R, Weeber H, Tsai L, Hileman K, et al. Preclinical metrics to predict through-focus visual acuity for pseudophakic patients. Biomed Opt Express. 2016;7:1877.
Tognetto D, Cecchini P, Giglio R, Turco G. Surface profiles of new-generation IOLs with improved intermediate vision. J Cataract Refract Surg. 2020;46:902–6.
Miret JJ, Camps VJ, García C, Caballero MT, Gonzalez-Leal JM. Analysis and comparison of monofocal, extended depth of focus and trifocal intraocular lens profiles. Sci Rep. 2022;12:1–8.
Sabur H, Unsal U. Visual outcomes of non-diffractive extended-depth-of-focus and enhanced monofocal intraocular lenses: a case-control study. Eur J Ophthalmol. 2023;33(1):262–8.
Lee JH, Moon SY, Chung HS, Park SY, Lee H, Kim JY, et al. Clinical outcomes of a monofocal intraocular lens with enhanced intermediate function compared with an extended depth-of-focus intraocular lens. J Cataract Refract Surg. 2022;48:61–6.
Jeon YJ, Yoon Y, Kim T, Koh K. Comparison Between an Intraocular Lens With Extended Depth of Focus (Tecnis Symfony ZXR00) and a New Monofocal Intraocular Lens With Enhanced Intermediate Vision (Tecnis Eyhance ICB00). Asia-Pacific J Ophthalmol. 2021;10:542–7.
Yangzes S, Kamble N, Grewal S, Grewal SPS. Comparison of an aspheric monofocal intraocular lens with the new generation monofocal lens using defocus curve. Indian J Ophthalmol. 2020;68:3025–9.
Ucar F, Cetinkaya S. The Evaluation of a New IOL with Extended Depth of Focus to Increase Visual Acuity for Intermediate Distance. SN Compr Clin Med. 2021;3:2285–91.
Unsal U, Sabur H. Comparison of new monofocal innovative and standard monofocal intraocular lens after phacoemulsification. Int Ophthalmol. 2021;41:273–82.
Nanavaty MA, Ashena Z, Gallagher S, Borkum S, Frattaroli P, Barbon E. Visual Acuity, Wavefront Aberrations, and Defocus Curves with an Enhanced Monofocal and a Monofocal Intraocular Lens: A Prospective. Randomized Study J Refract Surg. 2022;38:10–20.
Lopes D, Loureiro T, Carreira R, Rodrigues Barros S, Cardoso JN, Campos P, et al. Comparative evaluation of visual outcomes after bilateral implantation of an advanced or conventional monofocal intraocular lens. Eur J Ophthalmol. 2022;32:229–34.
Kang KH, Song MY, Kim KY, Hwang K-Y, Kwon Y-A, Koh K. Visual Performance and Optical Quality after Implantation of a New Generation Monofocal Intraocular Lens. Korean J Ophthalmol. 2021;35:112–9.
Esat C, Hülya B, Gokhan E, Berna Y, Fatih A, Murat F, et al. Vision outcomes with a new monofocal IOL. Int Ophthalmol. 2021;41:491–8.
de Luis Eguileor B, Martínez-Indart L, Martínez Alday N, Sacristán Egüén C, Cuadros SC. Differences in intermediate vision: monofocal intraocular lenses vs. monofocal extended depth of focus intraocular lenses. Arch Soc Esp Oftalmol. 2020;95:523–7.
Bova A, Vita S. Clinical and aberrometric evaluation of a new monofocal IOL with intermediate vision improvement. J Ophthalmol. 2022;2022:4119698.
Auffarth GU, Gerl M, Tsai L, Janakiraman DP, Jackson B, Alarcon A, et al. Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J Cataract Refract Surg. 2021;47:184–91.
Garzón N, Poyales F, Albarrán-Diego C, et al. Visual and optical quality of enhanced intermediate monofocal versus standard monofocal intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2022;260:3617–25.
Baur ID, Khoramnia R, Weindler J, Naujokaitis T, Poompokawat P, Auffarth GU. Clinical outcomes of a new hybrid monofocal IOL with extended depth of focus. J Refract Surg. 2021;37:601–8.
Tañá-Sanz P, Rodríguez-Carrillo MD, Elvira-Giner B, Ruiz-Santos M, Montés-Micó R, Tañá-Rivero P. Enhanced monofocal extended depth of focus IOL with a diffractive surface design. J Refract Surg. 2021;37:595–600.
Corbelli E, Iuliano L, Bandello F, Fasce F. Comparative analysis of visual outcome with 3 intraocular lenses: monofocal, enhanced monofocal, and extended depth of focus. J Cataract Refract Surg. 2022;48:67–74.
Acknowledgements
None.
Funding
This work was financially supported by Johnson and Johnson (Grant ID:1316). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
JF and MR-V have contributed to the conception and design of the study. CR-d-L, FZ-M and MR-V have contributed to the screening for inclusion and data extraction. Article drafting has been performed by JF and MR-V. CR-d-L, FZ-M and MR-C-d-M have made contributions to the revising of the article. All authors have approved the final version to be published and its accuracy and integrity.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Scoping review does not involve human participants, therefore Ethics Committee approval was not required.
Consent for publication
Not applicable.
Competing interests
Indaloftal SL (Qvision) received a research grant from Johnson & Johnson for the current study. Outside this work, Dr. Fernández is a consultant from Medicontur and has participated as a speaker in the activities of Oculus, Zeiss, and Bausch and Lomb. Remaining authors have nothing to disclose.
Additional information
Publisher’ s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fernández, J., Rocha-de-Lossada, C., Zamorano-Martín, F. et al. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth of focus lenses: a scoping review. BMC Ophthalmol 23, 101 (2023). https://doi.org/10.1186/s12886-023-02844-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-023-02844-1