Abstract
Background
The purpose of this study was to evaluate the changes in choroidal thickness and lamina cribrosa position after nonpenetrating deep sclerectomy (NPDS) and trabeculectomy.
Methods
Twenty-three eyes with glaucoma that required filtering surgery were included (12 NDPS and 11 trabeculectomies) in this prospective observational study. OCT-enhanced depth imaging (OCT-EDI) was used to measure choroidal thickness, prelaminar tissue thickness and lamina cribrosa position before and 7 days and 1 month after surgery. All results are shown as median (interquartile range values).
Results
Intraocular pressure (IOP) was significantly lower 1 week after surgery than at baseline (7 (6/10) mmHg vs. 21 (18/26) mmHg; p < 0.001) with a mean 64% decrease. IOP remained significantly lower at 1 month with a 55% mean decrease as compared to baseline (10 (8/12) mmHg; p < 0.001). One week after surgery, the subfoveolar choroidal thickness (SFCT) significantly increased (372 (306/523) μm vs. 317 (227/413) μm; p = 0.04) and the prelaminar tissue (PLT) was significantly thicker (269 (162/360) μm vs. 138 (87/268) μm; p = 0.02) as compared to preoperative measurements. These changes were not statistically significant at one month. There were no differences concerning these parameters between the NPDS and trabeculectomy groups. During the first week, the SFCT increase was correlated with IOP reduction (r = − 0.41; p = 0.04).
Conclusions
OCT-EDI allowed the visualization of structural changes at the level of the optic nerve and choroidal vascularization during acute IOP changes. No difference was observed between NPDS and trabeculectomy concerning these structural modifications.
Similar content being viewed by others
Background
The lamina cribrosa seems to play a major role in glaucoma pathophysiology. This structure is composed of piles of connective tissue layers that are fenestrated by pores in which bundles of retinal ganglion cell (RGC) axons pass [1]. The lamina cribrosa is a dynamic structure that is modified by chronic intraocular pressure (IOP) elevation [2]. IOP rise may compress the collagen plates and affect the laminar pore area [3], with mechanical stress to RGC axons and consequently RGC apoptosis and death [4]. Although a transient IOP elevation does not seem to change lamina cribrosa position [5], this structure is thinner and more posterior compared to the Bruch membrane opening (BMO) in glaucoma patients [6, 7]. Therefore, the impact of high IOP on lamina cribrosa seems to be a gradual process.
Vascular impairment in the optic nerve head also seems to be a relevant component in the pathogenesis of glaucomatous optic neuropathy [8]. Local vasospasm, blood hypertension and nocturnal blood hypotension are associated with glaucomatous neuropathy progression [9]. Using laser Doppler flowmetry, several studies reported optic nerve head (ONH) blood flow reduction in glaucomatous eyes [10]. Hypoperfusion may induce RGC axon ischemia and oxidative stress [11]. Most recently, using optical coherence tomography angiography, some authors, including our group, detected reduced ONH blood vessel density in glaucoma patients [12, 13]. Similarly, a decrease in choroidal blood flow has been described in glaucoma patients [14], but choroidal thickness has been reported not to be affected in glaucoma [15,16,17].
Spaide et al. first described enhanced depth imaging optical coherence imaging (OCT-EDI) using the inverted image obtained when the device is closer to the eye [18]. This method provides higher image resolution on layers located under the retinal pigment epithelium. It is now possible to visualize the lamina cribrosa anterior surface and the scleral inner surface and then to assess choroidal thickness and lamina cribrosa position [19, 20].
IOP remains the main target of glaucoma treatment and, in case of glaucomatous neuropathy progression despite maximally tolerated medications, filtering surgery can be indicated. Although trabeculectomy is considered as the standard surgical procedure to reduce IOP in glaucoma patients, nonpenetrating deep sclerectomy (NPDS) may provide comparable IOP reduction with the advantage of a lower rate of postoperative complications, particularly those related to immediate postoperative hypotony [21, 22]. Previous studies suggested that the IOP decrease following trabeculectomy causes choroidal thickening [23,24,25,26,27] and lamina cribrosa anterior displacement [28, 29]. To our knowledge, modifications of choroidal thickness and optic nerve head induced by NPDS have not been assessed yet. Therefore, the aim of the present study was to evaluate the changes in choroidal thickness and lamina cribrosa position after NPDS and trabeculectomy.
Methods
This was a prospective observational study of consecutive patients undergoing glaucoma filtering surgery at Quinze-Vingts National Ophthalmology Hospital, Paris, France, between March 2014 and August 2015. All participants involved were informed of the purpose of this study and written consent was obtained. This study has been approved by the Ethics Committee of the French Society of Ophthalmology (IRB 00008855 Société Française d’Ophtalmologie IRB#1). Filtering surgery was provided to patients with uncontrolled IOP after maximum glaucoma medications and functional progression confirmed by visual field examination. According to European Glaucoma Society guidelines [30], we performed NPDS for primary open-angle glaucoma (POAG) patients and trabeculectomy for primary angle-closure glaucoma (PACG). Glaucoma was diagnosed on the basis of clinical examination, retinal nerve fiber layer analyses using Spectral Domain Optical Coherence Tomography (SD-OCT) and visual field examination. The exclusion criteria were age under 18, history of intraocular inflammation or any retinal abnormalities, history of nonglaucomatous optic neuropathy, optic nerve drusen, history of previous intraocular surgery (except cataract or refractive surgery) or laser therapy.
All patients underwent filtering surgery (trabeculectomy or NPDS according to the individual physician’s judgment and experience) with mitomycin-C (MMC) application followed in the postoperative period by a treatment with tobramycin/dexamethasone and unpreserved indomethacin eyedrops for at least 2 months after surgery. All glaucoma medications were stopped after surgery.
At baseline, all patients underwent ophthalmological examinations including best corrected visual acuity (BCVA), slit-lamp biomicroscopy, gonioscopy, applanation tonometry and dilated funduscopy. They also underwent a central corneal thickness (CCT) measurement with ultrasound pachymetry (pachμmeter, Haag-Streit, Bern, Switzerland), an axial length measurement with partial optical coherence interferometry (IOLMaster 500; Carl Zeiss Meditec, La Jolla, CA, USA) and a visual field examination (SITA standard 24–2, Humphrey Field Analyzer – HFA II; Carl Zeiss Meditec). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were also measured preoperatively on the upper arm by an automated device. Mean blood pressure (mBP) was calculated as the DBP plus one-third the difference between SBP and DBP. The ocular perfusion pressure (OPP) was calculated as 2/3rd of mBP minus IOP [31].
Optic nerve head and macular imaging were performed using Spectralis® SD-OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) at baseline, 1 week and 1 month after surgery. The image registration feature of the Heidelberg Spectralis® device ensured that the same area was scanned each time. OCT images were taken by the same experienced operator, with enhanced depth imaging (EDI), allowing better visualization of the anterior laminar surface, the choroid and especially the choroid–sclera interface. All measurements were taken within a limited time (8:00 am to 11:00 am) to reduce the influence of diurnal variation of choroidal thickness [32]. Four B-scans were made up during each eye examination: [1] a vertical scan closest to the optic nerve head center, which did not include central artery or vein of the retina; [2] a 360-degree 3.4-mm-diameter peripapillary circle scan centered on the optic disc; [3] a vertical macular scan centered on the fovea and [4] a horizontal macular scan centered on the fovea. The resulting images were viewed and analyzed with the Eye Explorer software (v 1.7.1.0; Spectralis®, Heidelberg Engineering GmbH, Germany). Each measurement was taken by the same operator that was masked for patient status, using the software’s caliper tool.
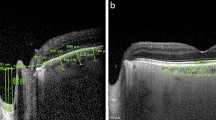
On the vertical optic nerve head scans, a reference line was drawn connecting the two terminations of the Bruch membrane. Three equidistant points were placed on this line. The distances from the reference line to the prelaminar tissue anterior surface were measured at these three points. The mean of the three measurements was defined as the cup (Fig. 1a). The distances from the reference line to the lamina cribrosa anterior surface were also measured. The mean of these measurements was defined as the lamina cribrosa depth (LCD) (Fig. 1b). Prelaminar tissue thickness (PLT) was considered as the difference between cup and LCD.
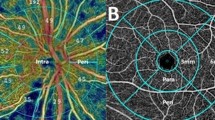
On peripapillary scans, the choroid was manually segmented and measured between the Bruch membrane hyperreflective line and the hyperreflective line of the inner surface of the sclera (Fig. 2a). The choroidal thickness was assessed in the temporal, nasal, superior and inferior sectors. The mean of these measurements was defined as mean peripapillary choroidal thickness (PCT). On vertical and horizontal macular scans, the choroidal thickness was measured using the same anatomic boundaries. Subfoveolar choroidal thickness (SFCT) was measured. We also measured choroidal thickness at 1000 μm and 3000 μm from the fovea in the nasal, temporal, superior and inferior sectors (Fig. 2b). The mean macular choroidal thickness (MCT) was defined as the mean of these nine measurements (the subfoveolar thickness plus two measurements in each quadrant). All measurements were taken at baseline, 1 week and 1 month after surgery.
Statistical analyses were performed using the SPSS software package version 20.0 (SPSS Inc., Chicago, IL, USA). Changes in IOP, SFCT, PCT and MCT were defined as baseline values minus first week or one month values. The nonparametric Mann-Whitney test was used to compare the continuous data and the χ [2] test was used to compare categorical data. As the data distribution was not normal, Spearman’s correlation was used to correlate any quantitative parameters. Statistical significance was p ≤ 0.05. All results are shown as median (interquartile range values).
Results
Twenty-three eyes of 23 glaucomatous patients requiring filtering surgery were included in this study. There were 16 men (69.5%) and seven (30.5%) women. The patients’ median age was 60 (52/74) years. At baseline, demographic and clinical characteristics were comparable between the NPDS and trabeculectomy groups (Table 1).
The mean IOP was significantly lower 1 week after surgery than at baseline (7 (6/10) mmHg vs. 21 (18/26) mmHg; p < 0.001) with a mean 64% decrease. IOP remained significantly lower at 1 month with a 55% mean decrease as compared to baseline (10 (8/12) mmHg; p < 0.001) (Table 1). IOP was significantly lower in the trabeculectomy group at 1 week (6 (4/10) mmHg vs. 8.5 (7/13) mmHg; p = 0.04), but the difference was no longer statistically significant at 1 month (p = 0.66) (Table 1).
Table 2 summarizes the main parameters assessed after surgery. The prelaminar tissue (PLT), subfoveolar choroidal thickness (SFCT) and mean macular choroidal thickness (MCT) increased significantly 1 week after surgery (p = 0.02, p = 0.04 and p = 0.04, respectively). However, this difference was not significant at 1 month (Table 2). There was no significant difference for the lamina cribrosa depth (LCD) and the mean peripapillary choroidal thickness (PCT) after surgery. PLT was thicker in the trabeculectomy group at baseline (234 (138/279) μm vs. 90.5 (58.5/201.25) μm; p = 0.01), 1 week (360 (276/379) μm vs. 163 (104.75/247.25) μm; p = 0.002) and 1 month (272 (191/347) μm vs. 152.5 (94.25/227) μm). Nevertheless, there was no difference between the two groups concerning PLT change after 1 week (p = 0.19) or 1 month (p = 0.66). Similarly, there was no difference between NPDS and trabeculectomy concerning LCD, PCT, SFCT and MCT at any time of the study.
At baseline, LCD was correlated with age (Spearman r = − 0.52; p = 0.01) and systolic blood pressure (Spearman r = − 0.47; p = 0.02) (Table 3). PCT was correlated with age (Spearman r = − 0.60; p = 0.002). SFCT was correlated with age (Spearman r = − 0.49; p = 0.018) and axial length (Spearman r = − 0.50; p = 0.016). MCT was correlated with age (Spearman r = − 0.56; p = 0.005) and axial length (Spearman r = − 0.53; p = 0.009) (Table 4). Tables 3 and 4 show the relationships between LCD, PLT, PCT, SFCT, MCT and clinical characteristics at baseline.
One week after surgery, the changes in SFCT and MCT were negatively correlated with the change in IOP (Spearman r = − 0.41; p = 0.04 and Spearman r = − 0.46; p = 0.02), (Fig. 3).
Figures 4, 5, 6 and 7 show examples of lamina cribrosa shape and choroidal changes after filtering surgery (Figs. 4, 5, 6 and 7).
Example of morphological changes in optic nerve head after NPDS. Preoperatively, (a) lamina cribrosa depth was 445 μm and prelaminar tissue thickness was 42 μm. At 1 week (b), lamina cribrosa depth was 303 μm and prelaminar tissue thickness was 53 μm. At 1 month (c), lamina cribrosa depth was 354 μm and prelaminar tissue thickness was 53 μm
Discussion
Excepting angle anatomy, the two groups were comparable at baseline. This was consistent with Beijing eye study results, angle opening degree seems not the affect choroidal thickness [33]. This also corroborates with Lee et al. findings, angle anatomy seems not to affect lamina cribrosa position [34]. Then, we consider that post operative differences between the two groups are mainly due to IOP reduction difference during the first week.
Consistent with previous studies analyzing similar parameters after trabeculectomy [23, 26, 35], we found overall choroidal thickening 1 week after surgery. This finding was not significant at 1 month and may correspond to an early stage of hypotony maculopathy during the first postoperative days. Since we did not find any difference concerning choroidal thickness between the two groups, we hypothesize that early hypotony maculopathy risk is not lower after NPDS as compared to that of trabeculectomy. Choroidal thickening is related to IOP reduction, but its exact pathophysiology remains uncertain. According to Saeedi et al., choroidal thickness increases as the force of IOP on the choroid decreases [25] and as the axial length decreases due to scleral relaxation [36]. According to Kara et al., choroidal thickening is related to an OPP increase induced by IOP reduction [24]. This corresponds to the retrobulbar blood flow rising observed after trabeculectomy [37]. Nickla and Wallman presumed that the choroidal thickness increase stemmed from two mechanisms [38]: first the synthesis of osmotically active proteoglycans, which help pull water into the choroid; second, to the increase in the size or number of the choriocapillaris vessels. Recently, Zhang et al. showed that the choroidal thicknening observed when decreasing IOP occurred both in the large choroidal vessels and the interstitium of the choroid [39]. This corroborates with the osmotic mechanism hypothesis. Mermoud et al. assessed the efficacy and postoperative complications of NPDS. They observed a lower rate of choroidal detachement after NPDS than after trabeculectomy (5% vs. 20%; p = 0.05) [40]. This complication, which usually occurs during the first weeks after filtering surgery seems to be correlated with lower IOP and may impair visual function [41, 42]. Given that choroidal thickening is directly related to IOP changes, the absence of very low IOP in the trabeculectomy group in our study might explain the absence of a difference between the two groups.
One week after surgery, we also found a significant PLT increase. This result can be compared to that reported by Lee et al., who observed significant PLT thickening at postoperative month 6 (95.77 ± 40.97 μm at baseline vs. 101.71 ± 42.06 μm postoperatively; p = 0.048) [29]. However, in the present study, this change was not correlated with IOP reduction and there were no significant differences between NPDS and trabeculectomy groups. Explanation for this thickening remains uncertain. Previous studies hypothesized that the vessel size could increase due to a rise in ocular blood flow [37]. Reis et al. also suggested that this modification is caused by size changes in RGC axons and astrocytes after IOP reduction [28]. Our results corroborate the findings of Agoumi et al., who observed that a quick IOP rise induced prelaminar tissue compression [5].
We did not find any significant difference concerning lamina cribrosa position before and after filtering surgery. Moreover, there was no difference between NPDS and trabeculectomy groups for this parameter. In a study evaluating changes in lamina cribrosa position after trabeculectomy in glaucoma patients, Reis et al. reported anterior lamina cribrosa displacement. However, their results were only significant at 6 months after surgery [28]. Since the lamina cribrosa is mainly composed of collagen [43, 44], it can be hypothesized that it is less compliant to quick IOP reduction, as observed in the early postoperative period, than the prelaminar tissue, which is composed of vascular and nervous elements [45]. This may explain the lack of laminar displacement in our study conducted during the 1st month after surgery. However, we cannot exclude that laminar displacement may occur later after surgery. Furthermore, OCT-EDI allows the analysis of the anterior surface of the lamina cribrosa, but visualization of the posterior surface of the lamina cribrosa remains difficult with this technique. Consequently, an overall displacement of the lamina cribrosa or a thickening of this structure could not be differentiated. The impact of an anterior lamina cribrosa displacement on glaucomatous neuropathy remains unclear. The axoplasmic transport obstruction previously described could be related to the signifiant pressure gradient on RGC axons as they pass through the lamina cribrosa. A chronic IOP elevation may raise this pressure gradient, impacting RGC axons and causing disease progression [46]. Anterior laminar displacement induced by filtering surgery may correspond to a reversal of the mechanical stress on RGC axons. The prelaminar tissue thickening might correspond to an increase in blood flow and a shift in axoplasmic fluid induced by this decompression.
The possibility of a loss of central vision, or “wipe-out syndrome,” has been described after trabeculectomy in patients with advanced glaucomatous optic neuropathy. In the studies that evaluated the rate of severe loss of central vision after trabeculectomy, the prevalence of this complication has been reported in 0–7.7% of these patients [41, 42, 47], and it might be related to profound hypotony during the first postoperative days [42]. According to Law et al., patients with high preoperative IOP and a sudden substantial IOP reduction are most likely to undergo this complication [41]. To our knowledge, this sudden loss of central vision has not been described after NPDS [48]. The reversal of the pressure gradient on RGC axons might be involved in wipe-out syndrome pathophysiology with the sudden axon decompression being noxious and inducing cell death. This physiopathological mechanism seems to be similar to the one described as free radical-mediated reperfusion injury. Reversal of ischemia provides acute free radical release with a chain reaction and structural changes in endothelial cells [49]. Vessel permeability rises and leads to intersitial edema, which can aggravate ischemia by a compressive mechanism. In the present study, there was no difference in prelaminar tissue thickening between the trabeculectomy and the NPDS groups. This may suggest that decompression risk is not lower after NPDS despite a lower IOP reduction. In the present study, or serous choroidal detachment during the follow up; consequently, the lack of a difference between the two groups concerning prelaminar tissue may be related to the absence of severe hypotony.
This study has some limitations including the absence of regression analysis and the small number of patients analyzed. Although, the number of patients in the trabeculectomy group was smaller than previous studies, including NPDS population it is larger than three of the four studies analyzing these parameters after trabeculectomy [23, 25, 35]. Moreover, the results after NPDS are coherent and correlated with a lower IOP reduction during the 1st postoperative week. Nevertheless, some differences between NPDS and trabeculectomy groups might remain undetected because of a lack of power due to the small number of eyes included in each group. Secondly, we measured the changes in choroid and optic nerve head that occurred during the 1st month after surgery. Even if it would have been interesting to analyze the changes after a longer period following surgery, previous similar studies found no difference between 1 month and later results (3–6 months) [25, 28, 29]. Thirdly, each measurement was taken manually using the Eye Explorer software. This technique has shown good interobserver repeatability [18, 19] and to our knowledge, there is currently no automated software to measure these parameters automatically. Finally, as these structural changes might also be related to vascular changes, it would be interesting in future studies to assess vessel density modifications using OCT-angiography.
To our knowledge, this study is the first to evaluate choroidal thickness, prelaminar tissue thickness and lamina cribrosa position changes after NPDS, and to compare the results to the effects of trabeculectomy. OCT-EDI allowed us to visualize the choroidal thickness increase and prelaminar tissue thickening during acute IOP changes following filtering surgery. These changes were greater during the 1st week after surgery and correlated with IOP reduction. No difference was observed between NPDS and trabeculectomy concerning these structural modifications. Such evaluations using a modern OCT technology adapted to deep tissues and structures may help better understand certain severe complications of filtering surgery such as the wipe-out syndrome and hypotony maculopathy.
Abbreviations
- BCVA:
-
Best Corrected Visual Acuity
- BMO:
-
Bruch Membrane Opening
- CCT:
-
Central Corneal Thickness
- DBP:
-
Diastolic Blood Pressure
- EDI:
-
Enhanced Depth Imaging
- IOP:
-
Intraocular Pressure
- LCD:
-
Lamina Cribrosa Depth
- mBP:
-
Mean Blood Pressure
- MCT:
-
Mean Macular Choroidal Thickness
- MMC:
-
Mitomycin-C
- NPDS:
-
Nonpenetrating Deep Sclerectomy
- OCT-EDI:
-
Enhanced Depth Imaging Optical Coherence Imaging
- ONH:
-
Optic Nerve Head
- OPP:
-
Ocular Perfusion Pressure
- PACG:
-
Primary Angle-closure Glaucoma
- PCT:
-
mean Peripapillary Choroidal Thickness
- PLT:
-
Prelaminar Tissue Thickness
- POAG:
-
Primary Open-angle Glaucoma
- RGC:
-
Retinal Ganglion Cell
- SBP:
-
Systolic Blood Pressure
- SD-OCT:
-
Spectral Domain Optical Coherence Tomography
- SFCT:
-
Subfoveolar Choroidal Thickness
References
Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Ophthalmol. 1992;113:447–52.
Jung KI, Jung Y, Park KT, Park CK. Factors affecting plastic lamina cribrosa displacement in glaucoma patients. Invest Ophthalmol Vis Sci. 2014.
Akagi T, et al. In vivo imaging of lamina cribrosa pores by adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2012;53:4111–9.
Burgoyne CF, Downs JC, Bellezza AJ, Suh J-KF, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73.
Agoumi Y, et al. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology. 2011;118:52–9.
Park H-YL, Park CK. Diagnostic capability of lamina cribrosa thickness by enhanced depth imaging and factors affecting thickness in patients with glaucoma. Ophthalmology. 2013;120:745–52.
Furlanetto RL, et al. Posterior displacement of the lamina cribrosa in glaucoma: in vivo interindividual and intereye comparisons. Invest Ophthalmol Vis Sci. 2013;54:4836–42.
Calvo P, et al. Predictive value of retrobulbar blood flow velocities in glaucoma suspects. Invest Ophthalmol Vis Sci. 2012;53:3875–84.
Prünte C, Orgül S, Flammer J. Abnormalities of microcirculation in glaucoma: facts and hints. Curr Opin Ophthalmol. 1998;9:50–5.
Grunwald JE, Piltz J, Hariprasad SM, DuPont J. Optic nerve and choroidal circulation in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2329–36.
Cioffi GA, et al. Chronic ischemia induces regional axonal damage in experimental primate optic neuropathy. Arch Ophthalmol. 2004;122:1517–25.
Jia Y, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121:1322–32.
Lévêque P-M, Zéboulon P, Brasnu E, Baudouin C, Labbé A. Optic disc vascularization in Glaucoma: value of spectral-domain optical coherence tomography angiography. J Ophthalmol. 2016;6956717(2016).
Marangoni D, et al. Subfoveal choroidal blood flow and central retinal function in early glaucoma. Acta Ophthalmol Copenh. 2012;90:e288–94.
Maul EA, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118:1571–9.
Mwanza J-C, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3430–5.
Park H-YL, Lee N-Y, Shin H-Y, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014;23:225–31.
Spaide RF, Koizumi H, Pozzoni MC, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5.
Lee EJ, et al. Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152:87–95.e1.
Cheng J, Ma X, Wei R. Efficacy of non-penetrating trabecular surgery for open angle glaucoma: a meta-analysis. Chin Med J. 2004;117(1006–1010).
El Sayyad F, Helal M, El-Kholify H, Khalil M, El-Maghraby A. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology. 2000;107:1671–4.
Chen S, et al. Changes in choroidal thickness after trabeculectomy in primary angle closure glaucoma. Invest Ophthalmol Vis Sci. 2014;55:2608–13.
Kara N, et al. Changes in choroidal thickness, axial length, and ocular perfusion pressure accompanying successful glaucoma filtration surgery. Eye Lond Engl. 2013;27:940–5.
Saeedi O, et al. Change in choroidal thickness and axial length with change in intraocular pressure after trabeculectomy. Br J Ophthalmol. 2014;98:976–9.
Kadziauskiene A, Kuoliene K, Asoklis R, Lesinskas E, Schmetterer L. Changes in choroidal thickness after intraocular pressure reduction following trabeculectomy. Acta Ophthalmol. 2016;94:586–91.
Yoshikawa M, et al. Longitudinal change in choroidal thickness after trabeculectomy in primary open-angle glaucoma patients. Jpn J Ophthalmol. 2017;61:105–12.
Reis ASC, et al. Laminar displacement and prelaminar tissue thickness change after glaucoma surgery imaged with optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:5819–26.
Lee EJ, Kim T-W, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012;119:1359–66.
[No authors listed]. European Glaucoma society terminology and guidelines for Glaucoma, 4th edition - chapter 3: treatment principles and options supported by the EGS Foundation: part 1: foreword; introduction; glossary; chapter 3 treatment principles and options. Br J Ophthalmol 101, 130–195 (2017).
Barbosa CP, et al. Intraocular pressure and ocular perfusion during hemodialysis. Arq Bras Oftalmol. 2011;74:106–9.
Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–6.
Wei WB, et al. Subfoveal choroidal thickness: the Beijing eye study. Ophthalmology. 2013;120:175–80.
Lee EJ, Kim T-W, Weinreb RN, Suh MH, Kim H. Lamina cribrosa thickness is not correlated with central corneal thickness or axial length in healthy eyes: central corneal thickness, axial length, and lamina cribrosa thickness. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Für Klin Exp Ophthalmol. 2013;251:847–54.
Usui S, et al. Changes in axial length and choroidal thickness after intraocular pressure reduction resulting from trabeculectomy. Clin. Ophthalmol. Auckl NZ. 2013;7:1155–61.
Silver DM, Geyer O. Pressure-volume relation for the living human eye. Curr Eye Res. 2000;20:115–20.
Yamazaki Y, Hayamizu F. Effect of trabeculectomy on retrobulbar circulation and visual field progression in patients with primary open-angle glaucoma. Clin Ophthalmol Auckl NZ. 2012;6:1539–45.
Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68.
Zhang X, et al. The effect of change in intraocular pressure on choroidal structure in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2017;58:3278–85.
Mermoud A, et al. Comparison of deep sclerectomy with collagen implant and trabeculectomy in open-angle glaucoma. J Cataract Refract Surg. 1999;25:323–31.
Law SK, Nguyen AM, Coleman AL, Caprioli J. Severe loss of central vision in patients with advanced glaucoma undergoing trabeculectomy. Arch Ophthalmol Chic Ill. 2007;1960(125):1044–50.
Costa VP, Smith M, Spaeth GL, Gandham S, Markovitz B. Loss of visual acuity after trabeculectomy. Ophthalmology. 1993;100:599–612.
Caparas VL, Cintron C, Hernandez-Neufeld MR. Immunohistochemistry of proteoglycans in human lamina cribrosa. Am J Ophthalmol. 1991;112:489–95.
Hernandez MR, Luo XX, Igoe F, Neufeld AH. Extracellular matrix of the human lamina cribrosa. Am J Ophthalmol. 1987;104:567–76.
Jonas JB, Müller-Bergh JA, Schlötzer-Schrehardt UM, Naumann GO. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. 1990;31:736–44.
Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma II The site of injury and susceptibility to damage. Arch Ophthalmol Chic Ill. 1981;1960(99):635–49.
Martinez JA, Brown RH, Lynch MG, Caplan MB. Risk of postoperative visual loss in advanced glaucoma. Am J Ophthalmol. 1993;115:332–7.
Ke M, Guo J, Qian Z. Meta analysis of non-penetrating trabecular surgery versus trabeculectomy for the treatment of open angle glaucoma. J Huazhong Univ Sci Technol Med Sci Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. 2011;31:264–70.
Bulkley GB. Reactive oxygen metabolites and reperfusion injury: aberrant triggering of reticuloendothelial function. Lancet Lond Engl. 1994;344:934–6.
Acknowledgements
Not applicable.
Funding
The authors declare that they have no sources of funding.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. AB and AP performed optic nerve head and macular imaging. AB analyzed and interpreted the patient data. EBO, EB, CB and AL performed filtering surgery. AP, EBO, EB, CB and AL has been involved in manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants involved were informed of the purpose of this study and written consent was obtained. This study has been approved by the Ethics Committee of the French Society of Ophthalmology (IRB 00008855 Société Française d’Ophtalmologie IRB#1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bouillot, A., Pierru, A., Blumen-Ohana, E. et al. Changes in choroidal thickness and optic nerve head morphology after filtering surgery: nonpenetrating deep sclerectomy versus trabeculectomy. BMC Ophthalmol 19, 24 (2019). https://doi.org/10.1186/s12886-019-1031-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-019-1031-3