Abstract
Background
The development of scoring systems to predict the short-term mortality and the length of hospital stay (LOS) in patients with bacteraemia is essential to improve the quality of care and reduce the occupancy variance in the hospital bed.
Methods
Adults hospitalised with community-onset bacteraemia in the coronavirus disease 2019 (COVID-19) and pre-COVID-19 eras were captured as the validation and derivation cohorts in the multicentre study, respectively. Model I incorporated all variables available on day 0, Model II incorporated all variables available on day 3, and Models III, IV, and V incorporated the variables that changed from day 0 to day 3. This study adopted the statistical and machine learning (ML) methods to jointly determine the prediction performance of these models in two study cohorts.
Results
A total of 3,639 (81.4%) and 834 (18.6%) patients were included in the derivation and validation cohorts, respectively. Model IV achieved the best performance in predicting 30-day mortality in both cohorts. The most frequently identified variables incorporated into Model IV were deteriorated consciousness from day 0 to day 3 and deteriorated respiration from day 0 to day 3. Model V achieved the best performance in predicting LOS in both cohorts. The most frequently identified variables in Model V were deteriorated consciousness from day 0 to day 3, a body temperature ≤ 36.0 °C or ≥ 39.0 °C on day 3, and a diagnosis of complicated bacteraemia.
Conclusions
For hospitalised adults with community-onset bacteraemia, clinical variables that dynamically changed from day 0 to day 3 were crucial in predicting the short-term mortality and LOS.
Similar content being viewed by others
Introduction
Despite recent advancements in haemodynamic support and antimicrobial strategies, bacteraemia remains strongly associated with high morbidity and mortality, leading to substantial healthcare costs [1]. Bacteraemia is a complex infection with varied clinical presentations and mortality rates, depending on the severity of the illness, the patient’s immune status, comorbid severity, causative microorganisms, and infection sources [2, 3]. Therefore, several scoring systems had been developed to predict short-term mortality in patients with bacteraemia to achieve the improved quality of patient care [4,5,6,7]. Regardless of whether the scoring algorithms were adopted in the emergency departments (EDs) [6] or intensive care units [4, 5], the majorities of these scoring systems were derived from clinical information at the time of bacteraemia onset. Although a new tool (i.e., the BLOOMY prediction score) both incorporating clinical data on day 0 and day 3 had been recently developed [7], a scoring system incorporating the dynamic changes in clinical data, which could reflect responses to empirical antimicrobial therapy and early resuscitation, is lacking.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first recognised in December 2019 [8]. On March 11, 2020, the World Health Organization proclaimed the coronavirus disease 2019 (COVID-19) as a worldwide pandemic [9]. The stress caused by the rapid global spread of COVID-19 has been shown to result in the unprecedented consumption of hospital resources [10, 11] and behavior changes in medical teams, such as the delayed diagnosis and treatment of bacteraemia [12]. Additionally, numerous studies detailing the difference of the incidences and causative microorganisms of bloodstream infections before and during the COVID-19 periods have been reported [13,14,15].
Accurately predicting the length of hospital stay (LOS) enables hospitals to predict the discharge dates of admitted patients and thereby improves the scheduling of elective admissions, reduces bed occupancy variance, and better predicts healthcare costs [16, 17]. Some predictive studies have analyzed the patients who underwent coronary artery bypass grafting [18, 19] and those with critical illnesses [20, 21]. However, the majority of reported predictions have been developed with clinical data gathered at the time of initial hospitalisation [20, 21] or surgery [18, 19]. Research specifically incorporating the changes in clinical data for predicting LOS was lacking among individuals with bacteraemia. Therefore, this study compared the performance of various scoring systems, using clinical information available at the time of bacteraemia onset (day 0), on day 3, and/or changes in variables from day 0 to day 3, in predicting the 30-day mortality and LOS of individuals hospitalised with community-onset bacteraemia.
Methods
Study design
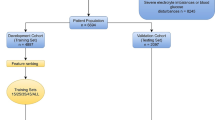
This 5-year, multicentre retrospective cohort study was conducted in the EDs of three hospitals in southern Taiwan. The hospitals included one university-affiliated medical centre with 1,200 beds and two teaching hospitals with 460 and 380 beds, respectively. The study enrolled adult patients (age ≥ 18 years) hospitalised with community-onset bacteraemia. The derivation cohort was enrolled from January 2017 to December 2020; the validation cohort was enrolled from January 2021 to December 2021, during the COVID-19 pandemic in Taiwan. The primary and secondary outcomes were the crude mortality rate within 30 days after bacteraemia onset and the LOS, respectively. The scoring systems were established using a joint approach both by conventional regression models and machine learning (ML) methods. The study followed the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology Initiative.
Patient selections
During the study period, the results of blood cultures sampled from ED patients were screened for bacterial growth using the electronic medical charts. The inclusion criteria were adults with bacterial growth on blood cultures. For patients with multiple bacteraemic episodes, only the first episode was included. First, this study excluded patients with contaminated blood cultures or bacteraemia diagnosed prior to the ED visits to identify individuals with community-onset bacteraemia. In addition, the study excluded non-hospitalised individuals and those with undetermined mortality or LOS prior to the study endpoint (such as those who had been hospitalised less than 30 days and did not revisit the study hospital). The remaining patients were deemed eligible for study.
Data collection
A predetermined record form was adopted to capture the patient demographic and clinical characteristics of bacteraemia. All information was independently gathered by a board-certified ED physician and an infectious disease physician who were both trained in medical chart reviews; the physicians were blinded to the aim and hypotheses of the present study, and any recording discrepancies were resolved through discussion between the authors. For comprehensive analyses, the clinical data obtained from medical charts were grouped into the following four components: i (unchanging variables on day 0), ii (unchanging variables on day 3), iii (changeable variables on day 0), and iv (changeable variables on day 3). The variables grouped in these components are listed in Supplemental Table 1. Furthermore, component v included alterations in the changeable variables from day 0 to day 3; the alteration descriptions are listed in Supplemental Table 2. The components of Models I, II, III, IV, and V are presented in Fig. 1.
Definitions
Bacteraemia, the presence of bacteria in the bloodstream, is generally diagnosed with blood cultures after the exclusion of sample contamination. As previously defined [22], community-onset bacteraemia indicated that the episode first identified <48 hours following ED arrival, which included healthcare facility- and community-associated bacteraemia. According to the previous criteria [23], blood cultures with the growth of potentially contaminating pathogens, such as coagulase-negative staphylococci (CoNS), micrococci, Bacillus species, Propionibacterium species, and Gram-positive bacilli, are considered to be contaminated. The isolation of more than one microbial species from a single bacteraemia episode was classified as polymicrobial bacteraemia. According to the international guideline of the Surviving Sepsis Campaign [24], complicated bacteraemia was defined if a patient fits one of the following criteria: (1) the presence of endocarditis, (2) infections of implanted prostheses, (3) bacterial growth from follow-up blood cultures taken 2-4 days after the initial set, (4) no defervescence at 72 hours after the initiation of appropriate antibiotic treatment, and (5) the presence of metastatic infections.
The Pitt bacteraemia score (PBS) was employed to assess the severity of illness; the score components are vital signs, mental status, use of vasopressor agents, receipt of mechanical ventilation, and cardiac arrest [25]. The comorbid severity was assessed by a previously established classification (McCabe classification) [26]. The overall length of the hospitalisation and ED stay was measured as the LOS. Crude mortality was equated with death from all causes.
Sampling of blood cultures and microbiological methods
Blood sampling was performed by nurses or physicians in EDs, and two sets of blood cultures were routinely done from different peripheral veins or arteries with at least 30 minutes between the two samplings. A set of blood cultures is routinely composed of one bottle of aerobic culture and another of anaerobic culture, with approximately 10 mL of blood in each bottle. Immediately, blood cultures were incubated in a BACTEC 9240 instrument (Becton Dickinson Diagnostic Systems, Sparks, MD, USA) for 5 days at 35ºC. Bacteraemic isolates were identified by the matrix-assisted laser desorption ionization time-of-flight mass spectrometry.
Machine learning
Five ML methods, in terms of random forest (RF), support vector machine (SVM), extreme gradient boosting (XGBoost), gradient boost, and light gradient boosting machine (Light GBM), were adopted by ML packages (i.e., scikit-learn, XGBoost, and Light GBM) of Python v3.8 for data preprocessing and building supervised learning models. In the data preprocessing, the method of Multivariate Imputation by Chained Equations (MICE) is used to fill in the missing values. Through multiple regressions over random data, samples get closer to the real dataset. In the process of predictive modeling, classification models and regression models were established using the above five ML methods, along with default hyperparameter settings provided by scikit-learn, XGBoost, and light GBM, for predicting 30-day mortality and LOS, respectively. These ML models were implemented in the following processing: creating an estimator, fitting the training set to the estimator, and predicting new values or class labels for the testing samples. Besides, both classification and regression tasks were implemented on Model I -V to compare their performance.
Statistical analyses
SAS version 9.4 software (SAS Institute, Cary, NC, USA) was used for statistical analyse. To identify the independent predictors of 30-day mortality, all variables identified as having P values < .05 by univariate analyses were included in the backward stepwise logistic regression model. This study developed a scoring algorithm consisting of independent predictive variables to predict 30-day mortality. The area under the receiver operating characteristic (ROC) curve was calculated for all MLs and statistical methods to estimate their accuracy in predicting 30-day mortality.
For predicting LOS, generalized linear models (GLMs) with three different distributions (i.e., normal, negative binomial, and Poisson) were used to recognise the best-fitting model, by the model performance with stepwise selection and an P value of <0.05 included variables in the model. The calibration of GLMs was assessed by plotting predicted versus the observed LOS averaged over patients with identical predicted values. The ideal calibration would be indicated by values close to the 45° line on a plot. For the ML models and GLMs, the mean square error (MSE) and root mean square error (RMSE) were employed to evaluate performance in predicting LOS.
Results
Patient demographics in the overall cohort
Of the 6,344 individuals hospitalised with positive blood cultures, 4,473 patients met the study inclusion criteria. The derivation and validation cohorts contained 3,639 (81.4%) and 834 (18.6%) patients, respectively (Fig. 2). Model I was established and validated in the overall cohort (6,344 patients). In this cohort, the median (interquartile range [IQR]) patient age was 69 (57–80) years; 52.4% (2,347 patients) of the patients were male. The LOS after bacteraemia onset ranged from 1 to 293 days, with a median (IQR) of 10 (6–18) days. Of the overall cohort, the patients deemed critically ill (PBS ≥ 4) at the onset of bacteraemia accounted for 23.5% (1,049 patients); the 3-day and 30-day crude mortality rates were 5.7% (256) and 17.5% (784), respectively.
After excluding 296 (6.6%) patients who died within 3 days after bacteraemia onset, 4,217 patients were included in the derivation (3,479 patients, 82.5%) and validation (738 patients, 17.5%) cohorts, respectively, for further analyses using Models II, III, IV, and V. In this cohort, the median (IQR) LOS after bacteraemia onset was 11 (7–19) days, and the 30-day crude mortality rate was 12.5% (527 patients).
Clinical characteristics and outcomes between the derivation (pre-COVID-19 era) and validation (COVID-19 era) cohorts
Differences between the derivation and validation cohorts with respect to patient demographic characteristics, bacteraemia characteristics, and clinical outcomes are presented in Table 1. Compared with those in the derivation cohort, fewer patients in the validation cohort were bedridden, had previous hospitalisations or invasive procedures, or had the causative microorganisms of E. coli, Streptococcus species, or Pseudomonas species. Conversely, the lower body mass index, the shorter LOS, as well as the few patients with previous chemotherapy, complicated bacteraemia, causative microorganisms of Klebsiella species, and comorbidities of diabetes mellitus or chronic kidney diseases were exhibited in the validation cohort. Notably, the validation cohort contained more patients with critical illness at the time of onset and more patients with higher rates of 3-day and 30-day crude mortality compared with patients in the derivation cohort.
ML or logistic regression in predicting 30-day mortality
The independent predictors of 30-day mortality identified in Models I, II, III, IV, and V were presented in Supplemental Tables 3, 4, 5, 6 and 7. The performance of six methods (i.e., logistic regression, RF, SVM, XGBoost, gradient boosting, and Light GBM) in predicting 30-day mortality as determined using the area under the ROC curve are listed in Table 2. Of the five models established for the derivation cohort, Model IV had the highest area using the logistic regression, SVM, and Light GBM techniques; Model V had the highest area through the RF and gradient boosting techniques; and Model III had the highest area through the XGBoost technique. Furthermore, for the validation cohort, Model IV consistently had the highest area using the logistic regression and five of ML methods (namely the SVM, XGBoost, gradient boosting, and Light GBM techniques).
Regarding the variables incorporated into Model IV, the 10 most powerful predictors of 30-day mortality are identified using logistic regression and the RF, XGBoost, gradient boosting, and Light GBM techniques (Table 3). Of these predictors, the most frequently identified were deteriorated consciousness from day 0 to day 3 (5/50) and deteriorated respiration from day 0 to day 3 (5/50); the other frequently identified variables included stationary shock from day 0 to day 3 (3/50), stationary consciousness from day 0 to day 3 (3/50), improved consciousness from day 0 to day 3 (3/50), and haemoglobin on day 0 (3/50).
ML or GLM methods in predicting the LOS
The calibration curves of GLMs for predicting the LOS for the derivation and validation cohorts are presented in Supplemental Figs. 1 and 2, respectively. The performances of six methods (i.e., logistic regression, RF, SVM, XGBoost, gradient boosting, and Light GBM) in predicting LOS, evaluated using MSE and RMSE, are presented in Table 4. Of the five models constructed for the derivation cohort, Model V had the lowest value for logistic regression and the RF, SVM, and Light GBM techniques; Models II and III had the lowest values for the gradient boost and XGBoost techniques, respectively. Regarding the validation cohort, Model V had the lowest values for the RF, SVM, XGBoost, and gradient boost techniques; Models II and IV had the lowest values for the logistic regression and Light GBM techniques, respectively.
For the variables integrated into Model V, the 10 most powerful predictors of LOS were identified using GLMs with one of three distributions and the RF, XGBoost, gradient boost, and Light GBM techniques (Table 5). Of these predictors, the most frequently identified variables included deteriorated consciousness from day 0 to day 3 (7/70), a body temperature ≤ 36.0 °C or ≥ 39.0 °C on day 3 (7/70), and a diagnosis of complicated bacteraemia (7/70); other frequently identified variables were blood urea nitrogen on day 3 (5/70), bacteraemia caused by bone and joint infections (5/70), bacteraemia with multiple points of entry (4/70), stationary consciousness from day 0 to day 3 (3/70), ventilation dependence from day 0 to day 3 (3/70), and the receipt of mechanical ventilation on day 3 (3/70).
Discussion
Frontline physicians commonly encounter patients with community-onset bacteraemia, because of its annual incidence of up to 0.15% in the community and the case-fatality rate of highly up to 17% [1] . Therefore, several scoring systems have been developed to predict short-term mortality in patients with bacteraemia to achieve higher quality of care [4,5,6,7]. Traditionally, the majorities of these scores were derived from clinical data obtained at the time of bacteraemia onset. Of the models established in the current study, the best performance in predicting 30-day mortality was Model IV, which consisted of unchanging variables on day 3, changeable variables on day 0, and the alterations of changeable variables from day 0 to day 3. Consistent with the BLOOMY score [7], the clinical condition on day 0 and day 3 (as demonstrated in Model IV) had been evidenced as the crucial determinates of short-term fatality. Moreover, similar to updated reports that highlighted the importance of dynamic vital signs and laboratory data in predicting short-term mortality among septic or bacteraemic individuals [27, 28], the changed variables form day 0 to day 3 (as the component in Model IV) were recognised as the powerful determinants of 30-day mortality, in terms of the changes in the conscious level, respiratory condition, and hemodynamic status, which can be recognised as the responses to prompt antimicrobial therapy and early resuscitation.
Accurately predicting LOS at the onset of bacteraemia enables to improve the usage of medical resource and the quality of patient care [16, 17]. In the present study, Model V demonstrated the highest accuracy in predicting LOS by incorporating both unchanging and changeable variables on day 3, along with the changes in changeable variables between day 0 and day 3. In the literature, this is the novel finding emphasized the importance of variables on day 3 and their dynamic changes, incorporated in Model V, as the powerful determinant in predicting LOS, instead of variables at the onset of bacteraemia. Of these determinants, the conscious and respiratory status from day 0 to day 3, blood urea nitrogen and body temperature on day 3, and specific characteristics of bacteraemia (complicated bacteraemia and bacteraemia with multiple ports of entry) were particularly recognised. More importantly, irrespective of whether predicting short-term mortality or LOS, the changes in changeable variables from day 0 to day 3 remained as a crucial determinant in the current study.
Although the SARS-CoV-2 was first detected in late 2019 [8], Taiwan’s response to the COVID-19 pandemic effectively halted the domestic spread of the virus; the government mandated the rapid closure of borders and immediate home quarantines for international arrivals and increased mask manufacturing [29]. These public policies combined with social behaviours initially proved effective in controlling COVID-19, with only 522 recorded cases during 2020 [30]. Unfortunately, SARS-CoV-2 spread rapidly across Taiwan in May 2021, with case numbers rising to 8,924 within one month [30]. Accordingly, the year 2021 was reasonably regarded as a period of the COVID-19 pandemic in the present study.
The global spread of SARS-CoV-2 resulted in the unprecedented demand for hospital resources, mechanical ventilators, beds, personal protective equipment, and medical personnel [10, 11]. Increased demands on healthcare workers could led to the delayed diagnosis and/or treatment of bloodstream infections [12]. Furthermore, the COVID-19 pandemic impacted the incidences and causative microorganisms of bacteraemia [13,14,15], and the incidence discrepancy and bacteraemia variation resulted from COVID-19-related stress in community individuals and medical teams were highly speculated; this stress in medical teams might agree with a previous investigation indicating a high contamination rate of blood culture in hospitalised patients during the COVID-19 era [31]. Consequently, delayed treatment, bacteraemia variations, and COVID-19-related stresses might result in unfavourable prognoses during the COVID-19 era, as demonstrated in the present study. Consistent with previous studies [14, 15], low incidence of E. coli bacteraemia in the COVID-19 era were disclosed in the present study. Dissimilar to previous studies that examined overall types of bacteraemia [14, 15], the altered incidence of Pseudomonas and CoNS bacteraemia between the COVID-19 and non-COVID-19 periods was not disclosed because the present study specifically focused on community-onset bacteraemia. In sum, the differences in bacteraemia characteristics and short-term prognoses between the non-COVID-19 and COVID-19 eras was reasonably demonstrated, and thus the COVID-19 era had been appropriately chosen as the validation period in the present study.
Numerous studies have compared the performance of ML models and traditional logistic regression models in predicting mortality [32, 33]. Furthermore, studies have adopted numerous ML methods to predict LOS in the literature [18,19,20,21]. For predicting short-term mortality in the non-COVID-19 and COVID-19 eras, Model IV was consistently identified as having the best predictive performance using the majorities of adopted methods in the current study. For predicting LOS in the non-COVID-19 and COVID-19 eras, Model V was consistently identified as having the best predictive performance through the majorities of adopted methods in the present study. Of importance, this study was the first to incorporate changeable data into ML or GLM methods to predict LOS. Consequently, we reasonably demonstrate the crucial role of data that dynamically changed from day 0 to day 3 and the importance of integrating data on day 3 in predicting the LOS and short-term mortality in adults with community-onset bacteraemia.
This study has several possible limitations and multiple strengths. First, the retrospective nature of this study made it prone to the selection and information bias during data collection. To reduce the information bias, all clinical information was randomly and independently retrieved by two physicians who were blind to the hypothesis and they inspected medical records together to solve discrepancies. Second, because of the multicenter design in the present study, the few proportions of patients with uncertain mortality or incomplete clinical information were excluded from analyses, and thereby the selection bias should be negligible. Third, bacteraemia severity and laboratory data had been designed for collection on day 3 because the microbiology reports in blood cultures were generally received by clinicians in the study hospitals on that day; in addition, monitoring of patients from day 0 to day 3 revealed the responses to empirical antimicrobial therapy and early resuscitation. Therefore, the information bias caused by the data missing on day 3 should be trivial in the current study. Finally, because all study hospitals were located in southern Taiwan, the findings in this study may be limited for generalization to other populations, which may have varying causative microorganisms, bacteraemia severity, or severity of comorbidities. However, the present study was the first to provide the external validation of the predicting model on bacteraemia patients in the COVID-19 era.
Conclusions
The COVID-19 pandemic altered the bacteraemia characteristics and patient demographics among adults with community-onset bacteraemia. Irrespective of the pre-COVID-19 and COVID-19 eras, the importance of dynamic variables changed from day 0 to day 3 (i.e., the indicator in response to empirical antimicrobial therapy and early support care), in predicting the short-term outcomes or LOS was crucially emphasized through the traditional statistic and ML methods in the present study. Accordingly, the principal findings in the current study may contribute to the development of an advanced predictive algorithm and help reduce the disease burden in the nearly future.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bates DW, Pruess KE, Lee TH. How bad are bacteremia and sepsis? Outcomes in a cohort with suspected bacteremia. Arch Intern Med. 1995;155(6):593–8.
Martinez RM, Wolk DM. Bloodstream infections Microbiology spectrum. 2016;4(4):4.4. 42.
Hung YP. LEE CC, Ko WC: Effects of Inappropriate Administration of Empirical Antibiotics on Mortality in Adults With Bacteraemia: Systematic Review and Meta-Analysis. Front med. 2022;9:869822.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart C, Suter P, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. In.: Springer-Verlag; 1996.
Zhang Z, Chen K, Chen L. APACHE III outcome prediction in patients admitted to the intensive care unit with sepsis associated acute lung injury. PLoS ONE. 2015;10(9): e0139374.
Bewersdorf JP, Hautmann O, Kofink D, Khalil AA, Abidin IZ, Loch A. The SPEED (sepsis patient evaluation in the emergency department) score: a risk stratification and outcome prediction tool. Eur J Emerg Med. 2017;24(3):170.
Tacconelli E, Göpel S, Gladstone BP, Eisenbeis S, Hölzl F, Buhl M, Górska A, Cattaneo C, Mischnik A, Rieg S. Development and validation of BLOOMY prediction scores for 14-day and 6-month mortality in hospitalised adults with bloodstream infections: a multicentre, prospective, cohort study. Lancet Infect Dis. 2022;22(5):731–41.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The lancet. 2020;395(10223):507–13.
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020;91(1):157.
Newton PN, Bond KC, Adeyeye M, Antignac M, Ashenef A, Awab GR, Bannenberg WJ, Bower J, Breman J, Brock A. COVID-19 and risks to the supply and quality of tests, drugs, and vaccines. Lancet Glob Health. 2020;8(6):e754–5.
Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382(18): e41.
Miyagami T, Uehara Y, Harada T, Watari T, Shimizu T, Nakamura A, Ogura N, Kushiro S, Masuyama K, Kanai Y. Delayed treatment of bacteremia during the COVID-19 pandemic. Diagnosis. 2021;8(3):327–32.
Bayo SM, Ruíz MPP, Hijazo MM, Usón MCV. Bacteremia during COVID-19 pandemic in a tertiary hospital in Spain. Enferm Infecc Microbiol Clin. 2022;40(4):183–6.
Denny S, Rawson TM, Hart P, Satta G, Abdulaal A, Hughes S, Gilchrist M, Mughal N, Moore LS. Bacteraemia variation during the COVID-19 pandemic; a multi-centre UK secondary care ecological analysis. BMC Infect Dis. 2021;21(1):556.
Ng QX, Ong NY, Lee DYX, Yau CE, Lim YL, Kwa ALH, Tan BH. Trends in Pseudomonas aeruginosa (P. aeruginosa) bacteremia during the COVID-19 pandemic: a systematic review. Antibiotics. 2023;12(2):409.
Robinson GH, Davis LE, Leifer RP. Prediction of hospital length of stay. Health Serv Res. 1966;1(3):287.
Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clinical Infectious Diseases. 2006;42(Supplement_2):S82–9.
Triana AJ, Vyas R, Shah AS, Tiwari V. Predicting length of stay of coronary artery bypass grafting patients using machine learning. J Surg Res. 2021;264:68–75.
Alshakhs F, Alharthi H, Aslam N, Khan IU, Elasheri M. Predicting postoperative length of stay for isolated coronary artery bypass graft patients using machine learning. International Journal of General Medicine. 2020;13:751.
Alsinglawi B, Alnajjar F, Mubin O, Novoa M, Alorjani M, Karajeh O, Darwish O. Predicting length of stay for cardiovascular hospitalizations in the intensive care unit: Machine learning approach. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). Montreal: IEEE; 2020. p. 5442–45.
Wu J, Lin Y, Li P, Hu Y, Zhang L, Kong G. Predicting Prolonged Length of ICU Stay through Machine Learning. Diagnostics. 2021;11(12):2242.
Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–64.
Lee CC, Lin WJ, Shih HI, Wu CJ, Chen PL, Lee HC, Lee NY, Chang CM, Wang LR, Ko WC. Clinical significance of potential contaminants in blood cultures among patients in a medical center. J Microbiol Immunol Infect. 2007;40(5):438–44.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Lee CC, Lee CH, Yang CY, Hsieh CC, Tang HJ, Ko WC. Beneficial effects of early empirical administration of appropriate antimicrobials on survival and defervescence in adults with community-onset bacteremia. Crit Care. 2019;23(1):363.
McCabe WR. Gram-negative bacteremia. Adv Intern Med. 1974;19:135–58.
Douglas-Louis R, Lou M, Lee B, Minejima E, Bubeck-Wardenburg J, Wong-Beringer A. Prognostic significance of early platelet dynamics in Staphylococcus aureus bacteremia. BMC Infect Dis. 2023;23(1):82.
Cheng CY, Kung CT, Chen FC, Chiu IM, Lin CHR, Chu CC, Kung CF, Su CM. Machine learning models for predicting in-hospital mortality in patient with sepsis: Analysis of vital sign dynamics. Front Med. 2022;9: 964667.
Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323(14):1341–2.
Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, MacDonald B, Giattino C: Taiwan: Coronavirus Pandemic Country Profile. Our World in Data Global Change Data Lab Available online at: https://ourworldindata.org/coronavirus/country/taiwan.
Sepulveda J, Westblade LF, Whittier S, Satlin MJ, Greendyke WG, Aaron JG, Zucker J, Dietz D, Sobieszczyk M, Choi JJ. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J Clin Microbiol. 2020;58(8):e00875-e820.
Churpek MM, Yuen TC, Winslow C, Meltzer DO, Kattan MW, Edelson DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368.
Taylor RA, Pare JR, Venkatesh AK, Mowafi H, Melnick ER, Fleischman W, Hall MK. Prediction of in-hospital mortality in emergency department patients with sepsis: a local big data–driven, machine learning approach. Acad Emerg Med. 2016;23(3):269–78.
Acknowledgements
We would like to thank for Ms. Tzu-Jung Chuang for providing statistical consulting.
Funding
This study was partially supported by research grants from the National Science and Technology Council (NSTC 110–2314-B-006–068, NSTC 111–2314-B-675–001-MY3, NSTC 110–2221-E-006–136-MY3, NSTC 111–2221-E-006–001, and NSTC 111–2634-F-002–022), the Ministry of Health and Welfare (MOHW109-TDU-B-211–114003), and National Cheng Kung University Hospital (NCKUH-11104005, NCKUH-11204026, and NCKUH-11210024), Tainan, Taiwan.
Author information
Authors and Affiliations
Contributions
CCL, CTL, and WK conceived the study idea and designed the study. YH, CCH, and CYuH supervised the data collection and chart reviews. CCL provided the data of microbiologic analyses. CCL, CYaH, and CTL provided methodological and statistical advice on study design and data analysis. CCL and WK provided expertise in infectious disease. CCL drafted this manuscript. CTL and WK revised it carefully from a professional point of view. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (IRB) of National Cheng Kung University Hospital (B-ER-109–144), the IRB of Madou Sin-Lau Hospital (SLH 9919–108-006), and the IRB of Tainan Sin-Lau Hospital (SLH-111-B-003). The informed consent was waived by the IRBs of National Cheng Kung University Hospital, Madou Sin-Lau Hospital, and Tainan Sin-Lau Hospital, because of the retrospective nature of the study. All designed methods were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, CC., Hung, YP., Hsieh, CC. et al. Predictive models for short-term mortality and length of hospital stay among adults with community-onset bacteraemia before and during the COVID-19 pandemic: application of early data dynamics. BMC Infect Dis 23, 605 (2023). https://doi.org/10.1186/s12879-023-08547-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08547-8