Abstract
Background
To estimate the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance in different regions, a meta-analysis was carried out.
Methods
Several databases were searched for relevant studies up to March 3, 2019. A bivariate random-effects model was used to estimate the diagnostic accuracy.
Results
We identified 97 studies involving 26,037 samples for the diagnosis of rifampicin resistance. The pooled sensitivity, specificity and AUC of Xpert MTB/RIF for rifampicin resistance detection were 0.93 (95% CI 0.90–0.95), 0.98 (95% CI 0.96–0.98) and 0.99 (95% CI 0.97–0.99), respectively. For different regions, the pooled sensitivity were 0.94(95% CI 0.89–0.97) and 0.92 (95% CI 0.88–0.94), the pooled specificity were 0.98 (95% CI 0.94–1.00) and 0.98 (95% CI 0.96–0.99), and the AUC were 0.99 (95% CI 0.98–1.00) and 0.99 (95% CI 0.97–0.99) in high and middle/low income countries, respectively. The pooled sensitivity were 0.91 (95% CI 0.87–0.94) and 0.91 (95% CI 0.86–0.94), the pooled specificity were 0.98 (95% CI 0.96–0.99) and 0.98 (95% CI 0.96–0.99), and the AUC were 0.98 (95% CI 0.97–0.99) and 0.99 (95% CI 0.97–0.99) in high TB burden and middle/low prevalence countries, respectively.
Conclusions
The diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance detection was excellent.
Similar content being viewed by others
Background
Tuberculosis (TB) remains a major global health problem and ranks as the leading cause of death from an infectious disease worldwide. In 2017, TB infected about 10.0 million people and approximately 16% (1.6 million) of infected patients died from the disease, which was a higher global total for new TB cases and deaths than previous one. Of the 1.6 million died cases, 300,000 occurred among people infected with human immunodeficiency virus (HIV) [1].
Drug-resistant TB, including multidrug-resistant TB (MDR-TB, defined as resistance to at least isoniazid and rifampicin, the two most important first-line anti-TB drugs) and extensively drug-resistant TB (XDR-TB, defined as MDR-TB plus resistance to any fluoroquinolone, such as ofloxacin or moxifloxacin, and to at least one of three injectable second-line drugs, amikacin, capreomycin, or kanamycin) has become a serious threat to global health [2]. In 2017, approximately 460,000 people, which means 3.5% of new and 18% of previously treated TB cases, were estimated to have had MDR-TB globally. And 9.0% of them had developed to XDR-TB. Rifampicin resistance (RR) was the most common resistance drug, affected approximately 558,000 people [1].
When TB is detected and effectively treated, the disease is largely curable. However, accurate and rapid detection of TB can be difficult, as challenging sample collection from deep-seated tissues and the paucibacillary characteristics of the disease [3]. Worldwide, approximately 35% of all forms of TB and 75% of patients with MDR-TB remain undiagnosed [4]. Notablely, under 3% of people who diagnosed with TB are tested to have certain pattern of drug resistance [5]. Xpert MTB/RIF was an effective, rapid, new method to diagnose TB and RR-TB, which was recommended by WHO [1].
Traditionally, the best available reference standard for TB diagnosis is solid and/or liquid culture. However, in clinical practice, prolonged turnaround times and limited laboratory infrastructure in resource-limited settings undermine the utility of culture-based diagnosis [6]. Histology is widely used for the diagnosis of TB where the technical pathologists are available However, it is time-consuming, technically demanding, and lacks specificity [7]. In early 2011, the World Health Organization (WHO) endorsed the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, USA) [8], a novel, rapid, automated, cartridge-based nucleic acid amplification test (NAAT), for the initial diagnosis in patients with suspected pulmonary MDR-TB or HIV-associated pulmonary TB [9, 10]. It can simultaneously detect TB through detection of the DNA of Mycobacterium tuberculosis and simultaneously identify a majority of the mutations that confer rifampicin resistance (which is highly predictive of MDR-TB). A high accuracy for pulmonary TB detection (sensitivity 89%, specificity 99%) was obtained [11]. In late 2013, WHO expanded its recommendations to include the diagnosis of TB in children and some forms of extrapulmonary TB (EPTB) [1].
A series of meta-analyses were carried out to determine the diagnostic accuracy of Xpert MTB/RIF in different forms of TB [12,13,14], however, evaluation of its accuracy in rifampicin resistance is rare [11]. More importantly, no study estimated the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance in countries with different TB prevalence and income till now. To replenish this, in this review, we synthesized the available data, taking into account the accuracy of Xpert MTB/RIF in diagnosing rifampicin resistance.
Methods
Literature search strategy
We searched the MEDLINE, Cochrane library, EMBASE, and Web of Knowledge for published works without language restrictions. The key searching words were used were: “Xpert MTB/RIF”, “Xpert”, “Gene Xpert”, plus “rifampicin resistance”. Our last search was accomplished on March 3, 2019.
Study selection and data extraction
The study selection and data extraction procedures were performed by two researchers (Kaican Zong and Hui Zhou) independently. Any differences in the process were solved by discussing with a third author (Shiying Li).
Inclusion criteria and exclusion criteria
Studies included in our meta-analysis should meet the following criteria: (i) clinical trials that used Xpert MTB/RIF for the detection of rifampicin resistance; (ii) samples were body tissues or fluid from suspected TB patients; (iii) the number of cases were more than 10; (iv) original data were sufficient to calculate the true positive (TP), true negative (TN), false positive (FP), and false negative (FN); (v) drug-susceptibility testing (DST) was used as the gold standard. Studies were excluded from our meta-analysis if they were: (i) case report; (ii) abstract of any conference; (iii) non-clinical research; (iv) review.
Data extraction
The following data were extracted from each included study: first author, year of publication, country, study settings, gender, the number of patients, the number and type of samples, diagnostic characteristics of Xpert MTB/RIF such as TP, TN, FP and FN. We sent e-mails to the authors for more details when data of individual studies were insufficient for a meta-analysis. In the case of inability to obtain data from the authors, the studies were excluded.
Statistical analysis
MIDAS modules in the STATA statistical software (version 12.0; STATA Corporation, College Station, TX, USA) was used to perform the meta-analyses. The summary receiver operating characteristic (SROC) model and the bivariate random-effects model were used in our study to evaluate the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance detection. For each study, we calculated the sensitivity and specificity of Xpert MTB/RIF to diagnose rifampicin resistance along with 95% confidence intervals.
Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was introduced to assess the quality of each included study. The Review Manager software (version 5.3, The Nordic Cochrane Centre, Copenhagen, Denmark) was used to present the result of QUADAS assessment.
We assessed the heterogeneity between included studies by using a bivariate boxplot, which can describe the degree of interdependence including the central location and identification of any outliers with an inner oval representing the median distribution of the data points and an outer oval representing the 95% confidence bound (by visually examining the position of each individual study, within the range of boxplot suggesting more heterogeneity).
Results
Description of included studies
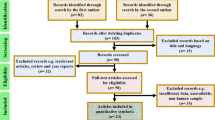
Finally, we included 97 studies in this meta-analysis [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111] (Fig. 1), including 26,037 samples for the diagnosis of rifampicin resistance. All studies were in English except five (three in Chinese [46, 64, 111] and two in Turkish [31, 79]). Twenty-six studies (26.8%) were conducted in high income countries (the World Bank income classification 2018) and 52 studies (53.6%) were in the 22 countries with a high burden of TB [1].
The median number of samples per study was 268 for rifampicin resistance detection. The samples of 56 included studies were pulmonary, such as sputum and BAL. Another 15 studies were extrapulmonary samples (e.g. body fluid, FNA, stool and blood), 16 studies included samples of both pulmonary and extrapulmonary (Tables 1 and 2).
Methodological quality of included studies
The overall methodological quality of the included studies was summarized in Fig. 2. Approximately half of the included studies collected data consecutively (n = 41; 42.2%) (Table 1) and no study used a case-control design. All studies were carried out either in tertiary care centers or reference laboratories. In index tests part, 15 studies (15.5%) were considered as unclear risk of bias. In reference standard part, 11 studies (11.3%) were considered as unclear risk of bias because the results of the reference standard were interpreted with unclear blind of the results of the index tests. In flow and timing part, 14 studies (24.7%) were considered as unclear risk of bias because not all patients were included in the analysis.
The heterogeneity of the studies included in this study was tested by a bivariate boxplot (Fig. 3a) and a Deek’s funnel plot (Fig. 3b). Most of the included studies were in the bivariate boxplot, and the slope of Deek’s funnel was almost horizontal, which all meant a good heterogeneity.
Detection of rifampicin resistance in different prevalence and income regions
The accuracy of Xpert MTB/RIF for rifampicin resistance detection was estimated in 59 studies. The pooled sensitivity, specificity and AUC of Xpert MTB/RIF for detecting rifampicin resistance were 0.93 (95% CI 0.90–0.95), 0.98 (95% CI 0.96–0.98) and 0.99 (95% CI 0.97–0.99), respectively (Fig. 4).
Of the 97 studies, 26 studies were of high income countries, 62 of middle and 9 were of low income. For TB prevalence, 52 studies were from the 22 high TB burden countries, and 45 were not. The pooled sensitivity were 0.94(95% CI 0.89–0.97) and 0.92 (95% CI 0.88–0.94), the pooled specificity were 0.98 (95% CI 0.94–1.00) and 0.98 (95% CI 0.96–0.99), and the AUC were 0.99 (95% CI 0.98–1.00) and 0.99 (95% CI 0.97–0.99) in high and middle/low income countries, respectively (Fig. 5a and Fig. 5b). The pooled sensitivity were 0.91 (95% CI 0.87–0.94) and 0.91 (95% CI 0.86–0.94), the pooled specificity were 0.98 (95% CI 0.96–0.99) and 0.98 (95% CI 0.96–0.99), and the AUC were 0.98 (95% CI 0.97–0.99) and 0.99 (95% CI 0.97–0.99) in high TB burden and middle/low prevalence countries, respectively (Fig. 5c and Fig. 5d).
The SROC plot of Xpert MTB/RIF sensitivity and specificity for rifampicin resistance detection. a High income countries, b Middle/low income countries, c High TB burden countries, d Middle/low TB prevalence countries. The points represent the sensitivity and specificity of one study; the summary point represents the summary sensitivity and specificity
Discussion
Several meta-analyses have focused on the diagnostic accuracy of Xpert MTB/RIF for pulmonary [12] or extra-pulmonary TB [13, 14] detection either on adults or children [12]. However, to our knowledge, this is the first meta-analysis for Xpert MTB/RIF diagnostic accuracy for rifampicin resistance detection in different prevalence and income regions. Our systematic review demonstrated that Xpert MTB/RIF is high sensitive diagnostic tool for rifampicin resistance detection. Firstly, the accuracy of Xpert MTB/RIF for rifampicin resistance detection was estimated in our meta-analysis. As shown in Fig. 4, the accuracy of Xpert MTB/RIF for rifampicin resistance detection was impressive. The pooled sensitivity, specificity and AUC were 0.93 (95% CI 0.90–0.95), 0.98 (95% CI 0.96–0.98) and 0.99 (95% CI 0.97–0.99), respectively. As estimated, about 75% of multi-drug resistant TB remains undiagnosed [4]. We strongly hope Xpert MTB/RIF, which provided a quick and accurate result, will contribute to early and accurate diagnosis of rifampicin resistance.
The overall sensitivity of Xpert MTB/RIF for rifampicin resistance detection were almost the same between high TB prevalence countries and middle/low ones (0.91, 95% CI 0.87–0.94 versus 0.91, 95% CI 0.86–0.94). And for different income levels, the sensitivities of high income ones was also similar with the ones of middle/low income (0.94, 95% CI 0.89–0.97 versus 0.92, 95% CI 0.88–0.94). We can see, taking the different levels of TB prevalence and country income into account, no significant differences were found between subgroups, either in sensitivities, specificities and AUCs.
TB remains one of the world’s deadliest communicable diseases. However, it is intensively distributed in several high burden countries. In 2017, more than half of the new TB was developed in the South-East Asia and Western Pacific Regions. To be specific, one quarter were in the African Region. India and China alone accounted for 24 and 13% of the total cases, respectively [4]. Interestingly, the tendency of TB prevalence was consisted with the economic development at some degree. The income levels of the 22 high TB burden countries all were all middle or low, except one (Russian) [4]. Therefore, it is of significant meanings to estimate the diagnostic accuracy of Xpert MTB/RIF in countries with different levels of TB prevalence and income. Some researchers discovered that the Xpert MTB/RIF showed a higher sensitivity of TB detection in lower TB prevalence countries, which could significantly help the physicians to make clinical decisions [112]. However, our result, from another aspect, showed the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance detection was not differed between countries with different TB prevalence and incomes.
Advantages of this review were the use of a standard protocol, a bivariate random-effects model used for meta-analysis, and independent reviewers. The data set involved comprehensive searching to identify studies as well as repeated correspondence with authors of study to obtain additional data on the studies.
While there were still some limitations in our analysis. We may have missed some studies despite the comprehensive search. Secondly, sample processing was highly variable across and within studies, as there was no recommendation available on how to process non-respiratory samples from the manufacturer or the WHO.
Conclusions
In conclusion, based on our meta-analysis, the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance detection was excellent. The overall sensitivity of Xpert MTB/RIF for rifampicin resistance detection in different TB prevalence and income countries were not significant different. We believe that the information obtained from this study will aid the decision making of physicians who take care of patients with possible resistant tuberculosis infection.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BA:
-
Bronchial aspirate
- BAL:
-
Bronchoalveolar lavage
- CCRS:
-
Composite clinical reference standard
- CSF:
-
Cerebrospinal fluid
- DST:
-
Drug-susceptibility testing
- EPTB:
-
Extra-pulmonary tuberculosis
- FN:
-
False negative
- FNA:
-
Fine needle aspirate
- FP:
-
False positive
- HIV:
-
Human immunodeficiency virus
- IQR:
-
Interquartile range
- LN:
-
Lymph node
- MDR-TB:
-
multidrug-resistant TB
- NAAT:
-
Nucleic acid amplification test
- QUADAS:
-
Quality assessment of diagnostic accuracy studies
- RR:
-
Rifampicin resistance
- SROC:
-
Summary receiver operating characteristic
- TA:
-
Tracheal aspirate
- TB:
-
Tuberculosis
- TN:
-
True negative
- TP:
-
True positive
- WHO:
-
World Health Organization
- XDR-TB:
-
Extensively drug-resistant TB
References
World Health Organization (2018) Global Tuberculosis Report 2018. Geneva.
Zumla A, Abubakar I, Raviglione M, Hoelscher M, Ditiu L, McHugh TD, Squire SB, Cox H, Ford N, McNerney R, Marais B, Grobusch M, Lawn SD, Migliori GB, Mwaba P, O'Grady J, Pletschette M, Ramsay A, Chakaya J, Schito M, Swaminathan S, Memish Z, Maeurer M, Atun R. Drug-resistant tuberculosis—current dilemmas, unanswered questions, challenges, and priority needs. J Infect Dis. 2012;205(Suppl 2):S228–40.
Harries A. How does the diagnosis of tuberculosis in persons infected with HIV differ from diagnosis in persons not infected with HIV? In: Frieden T, editor. Toman’s tuberculosis: case detection, treatment, and monitoring-questions and answers. WHO/HTM/TB/2004.334. Geneva: World Health Organization; 2004. p. 80–3.
Zumla A, George A, Sharma V, Herbert N. Baroness Masham of Ilton. WHO’s 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet. 2013;382(9907):1765–7.
Chaisson RE, Nuermberger EL. Confronting multidrugresistant tuberculosis. N Engl J Med. 2012;366(23):2223–4.
World Health Organization. Policy framework for implementing new tuberculosis diagnostics. Geneva: World Health Organization; 2011.
Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005;43:4357–62.
World Health Organization. Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO/HTM/TB/2011.4. Geneva: World Health Organization; 2011.
Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, Cuevas LE, McHugh TD, Zijenah L, Kapata N, Abubakar I, McNerney R, Hoelscher M, Memish ZA, Migliori GB, Kim P, Maeurer M, Schito M, Zumla A. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–61.
Automated Real-Time Nucleic Acid Amplification Technology for Rapid and SimultaneousDetection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update. Geneva: World Health Organization; 2013.
Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
Detjen AK, DiNardo AR, Leyden J, Steingart KR, Menzies D, Schiller I, Dendukuri N, Mandalakas AM. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(6):451–61.
Penz E, Boffa J, Roberts DJ, Fisher D, Cooper R, Ronksley PE, James MT. Diagnostic accuracy of the Xpert® MTB/RIF assay for extra-pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2015;19(3):278–84 i-iii.
Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44(2):435–46.
Al-Ateah SM, Al-Dowaidi MM, El-Khizzi NA. Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non-respiratory clinical specimens using the Cepheid Gene Xpert® system. Saudi Med J. 2012;33(10):1100–5.
Antonenka U, Hofmann-Thiel S, Turaev L, Esenalieva A, Abdulloeva M, Sahalchyk E, Alnour T, Hoffmann H. Comparison of Xpert MTB/RIF with ProbeTec ET DTB and COBAS TaqMan MTB for direct detection of M. tuberculosis complex in respiratory specimens. BMC Infect Dis. 2013;13:280.
Balcells ME, García P, Chanqueo L, Bahamondes L, Lasso M, Gallardo AM, Cifuentes L. Rapid molecular detection of pulmonary tuberculosis in HIV-infected patients in Santiago, Chile. Int J Tuberc Lung Dis. 2012;16(10):1349–53.
Barmankulova A, Higuchi M, Sarker MA, Alim MA, Hamajima N. Tuberculosis and rifampicin resistance among migrants in kyrgyzstan: detection by a new diagnostic test. Nagoya J Med Sci. 2015;77(1–2):41–9.
Barnard M, Gey van Pittius NC, van Helden PD, Bosman M, Coetzee G, Warren RM. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J Clin Microbiol. 2012;50(11):3712–6.
Bates M, O’Grady J, Maeurer M, Tembo J, Chilukutu L, Chabala C, Kasonde R, Mulota P, Mzyece J, Chomba M, Mukonda L, Mumba M, Kapata N, Rachow A, Clowes P, Hoelscher M, Mwaba P, Zumla A. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect Dis. 2013;13(1):36–42.
Biadglegne F, Mulu A, Rodloff AC, Sack U. Diagnostic performance of the Xpert MTB/RIF assay for tuberculous lymphadenitis on fine needle aspirates from Ethiopia. Tuberculosis (Edinb). 2014;94(5):502–5.
Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48(7):2495–501.
Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–15.
Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–505.
Bowles EC, Freyée B, van Ingen J, Mulder B, Boeree MJ, van Soolingen D. Xpert MTB/RIF®, a novel automated polymerase chain reaction-based tool for the diagnosis oftuberculosis. Int J Tuberc Lung Dis. 2011;15(7):988–9.
Carriquiry G, Otero L, González-Lagos E, Zamudio C, Sánchez E, Nabeta P, Campos M, Echevarría J, Seas C, Gotuzzo E. A diagnostic accuracy study of Xpert®MTB/RIF in HIV-positive patients with high clinical suspicion of pulmonary tuberculosis in Lima, Peru. PLoS One. 2012;7(9):e44626.
Cayci YT, Bilgin K, Coban AY, Birinci A, Durupınar B. An evaluation of false-positive rifampicin resistance on the Xpert MTB/RIF. Mem Inst Oswaldo Cruz. 2017;112(11):756–9.
Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, Banada PP, Deshpande S, Shenai S, Gall A, Glass J, Krieswirth B, Schumacher SG, Nabeta P, Tukvadze N, Rodrigues C, Skrahina A, Tagliani E, Cirillo DM, Davidow A, Denkinger CM, Persing D, Kwiatkowski R, Jones M, Alland D. The new xpert MTB/RIF ultra: improving detection of mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. MBio. 20179;8(4).
Chiang TY, Fan S, Jou R. Performance of an Xpert-based diagnostic algorithm for the rapid detection of drug-resistant tuberculosis among high-risk populations in a low-incidence setting. PLoS One. 2018;13(7):e0200755.
Chikaonda T, Nguluwe N, Barnett B, Gokhale RH, Krysiak R, Thengolose I, Rosenberg NE, Stanley C, Mpunga J, Hoffman IF, Hosseinipour M, Scott L, Stevens W. Performance of Xpert® MTB/RIF among tuberculosis outpatients in Lilongwe, Malawi. Afr J Lab Med. 2017;6(2):464.
Ciftçi IH, Aslan MH, Aşık G. Evaluation of Xpert MTB/RIF results for the detection of Mycobacterium tuberculosis in clinical samples. Mikrobiyol Bul. 2011;45(1):43–7 Turkish.
Deggim V, Somoskovi A, Voit A, Böttger EC, Bloemberg GV. Integrating the Xpert MTB/RIF assay into a diagnostic workflow for rapid detection of Mycobacteriumtuberculosis in a low-prevalence area. J Clin Microbiol. 2013;51(7):2396–9.
Dharan NJ, Blakemore R, Sloutsky A, Kaur D, Alexander RC, Ghajar M, Musser KA, Escuyer VE, Rowlinson MC, Crowe S, Laniado-Laborin R, Valli E, Nabeta P, Johnson P, Alland D. Performance of the G4 Xpert® MTB/RIF assay for the detection of mycobacterium tuberculosis and rifampin resistance: a retrospective case-control study of analytical and clinical samples from high- and low-tuberculosis prevalence settings. BMC Infect Dis. 2016;16(1):764.
Dorman SE, Chihota VN, Lewis JJ, Shah M, Clark D, Grant AD, Churchyard GJ, Fielding KL. Performance characteristics of the Cepheid Xpert MTB/RIF test in a tuberculosis prevalence survey. PLoS One. 2012;7(8):e43307.
Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, Hall SL, Chakravorty S, Cirillo DM, Tukvadze N, Bablishvili N, Stevens W, Scott L, Rodrigues C, Kazi MI, Joloba M, Nakiyingi L, Nicol MP, Ghebrekristos Y, Anyango I, Murithi W, Dietze R, Lyrio Peres R, Skrahina A, Auchynka V, Chopra KK, Hanif M, Liu X, Yuan X, Boehme CC, Ellner JJ, Denkinger CM, Study Team. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84.
Du J, Huang Z, Luo Q, Xiong G, Xu X, Li W, Liu X, Li J. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. J Res Med Sci. 2015;20(1):26–31.
Feliciano CS, Namburete EI, Rodrigues Plaça J, Peronni K, Dippenaar A, Warren RM, Silva WA Jr, Bollela VR. Accuracy of whole genome sequencing versus phenotypic (MGIT) and commercial molecular tests for detection of drug-resistant Mycobacterium tuberculosis isolated from patients in Brazil and Mozambique. Tuberculosis (Edinb). 2018;110:59–67.
do Giang C, Duong TN, Ha DT, Nhan HT, Wolbers M, Nhu NT, Heemskerk D, Quang ND, Phuong DT, Hang PT, Loc TH, Lan NT, Dung NH, Farrar J, Caws M. Prospective evaluation of GeneXpert for the diagnosis of HIV- negative pediatric TB cases. BMC Infect Dis. 2015;15:70.
Gu Y, Wang G, Dong W, Li Y, Ma Y, Shang Y, Qin S, Huang H. Xpert MTB/RIF and GenoType MTBDRplus assays for the rapid diagnosis of bone and jointtuberculosis. Int J Infect Dis. 2015;36:27–30.
Guenaoui K, Harir N, Ouardi A, Zeggai S, Sellam F, Bekri F, Cherif TS. Use of GeneXpert mycobacterium tuberculosis/rifampicin for rapid detection of rifampicin resistant mycobacterium tuberculosis strains of clinically suspected multi-drug resistance tuberculosis cases. Ann Transl Med. 2016;4(9):168.
Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-López EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48(1):229–37.
Hillemann D, Rüsch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49(4):1202–5.
Huang H, Zhang Y, Li S, Wang J, Chen J, Pan Z, Gan H. Rifampicin resistance and multidrug-resistant tuberculosis detection using Xpert MTB/RIF in Wuhan, China: a retrospective study. Microb Drug Resist. 2018;24(5):675–9.
Huh HJ, Jeong BH, Jeon K, Koh WJ, Ki CS, Lee NY. Performance evaluation of the Xpert MTB/RIF assay according to its clinical application. BMC Infect Dis. 2014;14:589.
Hu P, Bai L, Liu F, Ou X, Zhang Z, Yi S, Chen Z, Gong D, Liu B, Guo J, Tan Y. Evaluation of the Xpert MTB/RIF assay for diagnosis of tuberculosis and rifampin resistance in county-level laboratories in Hunan province, China. Chin Med J (Engl). 2014;127(21):3744–50.
Jin YH, Shi SY, Zheng Q, Shen J, Ying XZ, Wang YF. Application value of Xpert MTB/RIF in diagnosis of spinal tuberculosis and detection of rifampin resistance. Zhongguo Gu Shang. 2017;30(9):787–91 Article in Chinese.
Kawkitinarong K, Suwanpimolkul G, Kateruttanakul P, Manosuthi W, Ubolyam S, Sophonphan J, Avihingsanon A, Ruxrungtham K. Real-Life Clinical Practice of Using the Xpert MTB/RIF Assay in Thailand. Clin Infect Dis. 2017;64(suppl_2):S171–8.
Khalil KF, Butt T. Diagnostic yield of Bronchoalveolar Lavage gene Xpert in smear-negative and sputum-scarce pulmonary tuberculosis. J Coll Physicians Surg Pak. 2015;25(2):115–8.
Kim CH, Woo H, Hyun IG, Kim C, Choi JH, Jang SH, Park SM, Kim DG, Lee MG, Jung KS, Hyun J, Kim HS. A comparison between the efficiency of the Xpert MTB/RIF assay and nested PCR in identifying Mycobacterium tuberculosis during routine clinical practice. J Thorac Dis. 2014;6(6):625–31.
Kim CH, Hyun IG, Hwang YI, Kim DG, Lee CY, Lee MG, Jung KS, Woo H, Hyun J, Kim HS, Park MJ. Identification of Mycobacterium tuberculosis and rifampin resistance in clinical specimens using theXpert MTB/RIF assay. Ann Clin Lab Sci. 2015 Winter;45(1):32–8.
Kim MJ, Nam YS, Cho SY, Park TS, Lee HJ. Comparison of the Xpert MTB/RIF Assay and Real-time PCR for the Detection of Mycobacteriumtuberculosis. Ann Clin Lab Sci. 2015 Spring;45(3):327–32.
Kim SY, Kim H, Kim SY, Ra EK, Joo SI, Shin S, Seong MW, Yoo CG, Kim EC, Park SS. The Xpert® MTB/RIF assay evaluation in South Korea, a country with an intermediate tuberculosisburden. Int J Tuberc Lung Dis. 2012;16(11):1471–6.
Kim YW, Kwak N, Seong MW, Kim EC, Yoo CG, Kim YW, Han SK, Yim JJ. Accuracy of the Xpert® MTB/RIF assay for the diagnosis of extra-pulmonary tuberculosis in South Korea. Int J Tuberc Lung Dis. 2015;19(1):81–6.
Kim YW, Seong MW, Kim TS, Yoo CG, Kim YW, Han SK, Yim JJ. Evaluation of Xpert(®) MTB/RIF assay: diagnosis and treatment outcomes in rifampicin-resistant tuberculosis. Int J Tuberc Lung Dis. 2015;19(10):1216–21.
Kokuto H, Sasaki Y, Yoshimatsu S, Mizuno K, Yi L, Mitarai S. Detection of Mycobacterium tuberculosis (MTB) in Fecal Specimens From Adults Diagnosed With Pulmonary Tuberculosis Using the Xpert MTB/Rifampicin Test. Open Forum Infect Dis. 2015;2(2):ofv074.
Kostera J, Leckie G, Abravaya K, Wang H. Performance of the Abbott RealTime MTB RIF/INH resistance assay when used to test mycobacterium tuberculosis specimens from Bangladesh. Infect Drug Resist. 2018;11:695–9.
Kurbaniyazova G, Joncevska M, Kalon S, Kalmambetova G, Mohr T, Toktogonova A, Takieva K, Islam KM, Luelmo F. Results of Xpert® MTB/RIF implementation in Kyrgyzstan. Int J Tuberc Lung Dis. 2017;21(3):333–7.
Kurbatova EV, Kaminski DA, Erokhin VV, Volchenkov GV, Andreevskaya SN, Chernousova LN, Demikhova OV, Ershova JV, Kaunetis NV, Kuznetsova TA, Larionova EE, Smirnova TG, Somova TR, Vasilieva IA, Vorobieva AV, Zolkina SS, Cegielski JP. Performance of Cepheid ® Xpert MTB/RIF ® and TB-Biochip ® MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2013;32(6):735–43.
Kwak N, Choi SM, Lee J, Park YS, Lee CH, Lee SM, Yoo CG, Kim YW, Han SK, Yim JJ. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS One. 2013;8(10):e77456.
Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, Bekker LG, Wood R. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8(7):e1001067.
Lee HY, Seong MW, Park SS, Hwang SS, Lee J, Park YS, Lee CH, Lee SM, Yoo CG, Kim YW, Han SK, Yim JJ. Diagnostic accuracy of Xpert® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013;17(7):917–21.
Li Q, Bao XD, Liu Y, Ou XC, Pang Y, Zhao YL. Comparison of two molecular assays for detecting smear negative pulmonary tuberculosis. Biomed Environ Sci. 2016;29(4):248–53.
Li Y, Pang Y, Zhang T, Xian X, Wang X, Yang J, Wang R, Chen M, Chen W. Rapid diagnosis of extrapulmonary tuberculosis with Xpert mycobacterium tuberculosis/rifampicin assay. J Med Microbiol. 2017;66(7):910–4.
Liu X, Huang Z, Du J. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38(10):741–5 Article in Chinese.
Lorent N, Kong C, Kim T, Sam S, Thai S, Colebunders R, Rigouts L, Lynen L. Systematic screening for drug-resistant tuberculosis with Xpert(®) MTB/RIF in a referral hospital in Cambodia. Int J Tuberc Lung Dis. 2015;19(12):1528–35.
Luetkemeyer AF, Firnhaber C, Kendall MA, Wu X, Mazurek GH, Benator DA, Arduino R, Fernandez M, Guy E, Johnson P, Metchock B, Sattler F, Telzak E, Wang YF, Weiner M, Swindells S, Sanne IM, Havlir DV, Grinsztejn B, Alland D. AIDS Clinical Trials Group A5295 and Tuberculosis Trials Consortium Study 34 Teams. Evaluation of Xpert MTB/RIF versus AFB smear and culture to Identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081–8.
Metcalfe JZ, Makumbirofa S, Makamure B, Sandy C, Bara W, Mason P, Hopewell PC. Xpert(®) MTB/RIF detection of rifampin resistance and time to treatment initiation in Harare, Zimbabwe. Int J Tuberc Lung Dis. 2016;20(7):882–9.
Mokaddas E, Ahmad S, Eldeen HS, Al-Mutairi N. Discordance between Xpert MTB/RIF assay and Bactec MGIT 960 Culture System for detection of rifampin-resistant Mycobacterium tuberculosis isolates in a country with a low tuberculosis (TB) incidence. J Clin Microbiol. 2015;53(4):1351–4.
Moon HW, Hur M, Kim JY, Yun YM. Comparison of three molecular assays for the detection of rifampin resistance in Mycobacterium tuberculosis. J Clin Lab Anal. 2015;29(2):142–5.
Moure R, Muñoz L, Torres M, Santin M, Martín R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011;49(3):1137–9.
Mwanza W, Milimo D, Chilufya MM, Kasese N, Lengwe MC, Munkondya S, de Haas P, Ayles H, Muyoyeta M. Diagnosis of rifampicin-resistant tuberculosis: discordant results by diagnostic methods. Afr J Lab Med. 2018;7(2):806.
Myneedu VP, Behera D, Verma AK, Bhalla M, Singh N, Arora J, Singhal R, Mathur M, Lal P, Sarin R. Xpert(®) MTB/RIF assay for tuberculosis diagnosis: evaluation in an Indian setting. Int J Tuberc Lung Dis. 2014;18(8):958–60.
N’guessan K, Assi JS, Ouassa T, Ahui-Brou JM, Tehe A, Keita Sow M, Guei A, Kouakou J, Dosso M. Assessment of the genotype MTBDRplus assay for rifampin and isoniazid resistance detection on sputum samples in Cote d'Ivoire. Eur J Microbiol Immunol (Bp). 2014;4(3):166–73.
N'Guessan K, Ouassa T, Dean AS, Alagna R, Adagra GD, Ibode V, Cirillo DM, Kouakou J. Multidrug-resistant tuberculosis in Côte d'Ivoire from 1995 to 2016: results of National Surveys. Eur J Microbiol Immunol (Bp). 2018;8(3):91–4.
Nikolayevskyy V, Kontsevaya I, Nikolaevskaya E, Surkova E, Samchenko S, Esipenko S. Diagnostic performance and impact of routinely implemented Xpert® MTB/RIF assay in a setting of high incidence of drug-resistant TB in Odessa Oblast, Ukraine. Clin Microbiol Infect. 2019;25(8):1040.e1-1040.e6.
Nicol MP, Workman L, Isaacs W, Munro J, Black F, Eley B, Boehme CC, Zemanay W, Zar HJ. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis. 2011;11(11):819–24.
O’Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, Mukonda L, Mumba M, Tembo J, Chomba M, Kapata N, Maeurer M, Rachow A, Clowes P, Hoelscher M, Mwaba P, Zumla A. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting wheretuberculosis and HIV infection are highly endemic. Clin Infect Dis. 2012;55(9):1171–8.
Ou X, Xia H, Li Q, Pang Y, Wang S, Zhao B, Song Y, Zhou Y, Zheng Y, Zhang Z, Zhang Z, Li J, Dong H, Chi J, Zhang J, Kam KM, Huan S, Jun Y, Chin DP, Zhao Y. A feasibility study of the Xpert MTB/RIF test at the peripheral level laboratory in China. Int J Infect Dis. 2015;31:41–6.
Ozkutuk N, Surucüoglu S. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonarytuberculosis in an intermediate-prevalence setting. Mikrobiyol Bul. 2014;48(2):223–32 Turkish.
Pan X, Yang S, Deighton MA, Qu Y, Hong L, Su F. A comprehensive evaluation of Xpert MTB/RIF assay with Bronchoalveolar lavage fluid as a single test or combined with conventional assays for diagnosis of pulmonary tuberculosis in China: a two-center prospective study. Front Microbiol. 2018;9:444.
Pang Y, Wang Y, Zhao S, Liu J, Zhao Y, Li H. Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis. Pediatr Infect Dis J. 2014;33(10):1047–51.
Park KS, Kim JY, Lee JW, Hwang YY, Jeon K, Koh WJ, Ki CS, Lee NY. Comparison of the Xpert MTB/RIF and Cobas TaqMan MTB assays for detection of Mycobacteriumtuberculosis in respiratory specimens. J Clin Microbiol. 2013;51(10):3225–7.
Pimkina E, Zablockis R, Nikolayevskyy V, Danila E, Davidaviciene E. The Xpert® MTB/RIF assay in routine diagnosis of pulmonary tuberculosis: a multicentre study in Lithuania. Respir Med. 2015;109(11):1484–9.
Pinyopornpanish K, Chaiwarith R, Pantip C, Keawvichit R, Wongworapat K, Khamnoi P, Supparatpinyo K, Sirisanthana T. Comparison of Xpert MTB/RIF Assay and the Conventional Sputum Microscopy in Detecting Mycobacterium tuberculosis in Northern Thailand. Tuberc Res Treat. 2015;2015:571782.
Rachow A, Zumla A, Heinrich N, Rojas-Ponce G, Mtafya B, Reither K, Ntinginya EN, O'Grady J, Huggett J, Dheda K, Boehme C, Perkins M, Saathoff E, Hoelscher M. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid XpertMTB/RIF assay--a clinical validation study. PLoS One. 2011;6(6):e20458.
Rahman A, Sahrin M, Afrin S, Earley K, Ahmed S, Rahman SM, Banu S. Comparison of Xpert MTB/RIF assay and GenoType MTBDRplus DNA probes for detection of mutations associated with rifampicin resistance in mycobacterium tuberculosis. PLoS One. 2016;11(4):e0152694.
Raizada N, Sachdeva KS, Nair SA, Kulsange S, Gupta RS, Thakur R, Parmar M, Gray C, Ramachandran R, Vadera B, Ekka S, Dhawan S, Babre A, Ghedia M, Alavadi U, Dewan P, Khetrapal M, Khanna A, Boehme C, Paramsivan CN. Enhancing TB case detection: experience in offering upfront Xpert MTB/RIF testing to pediatric presumptive TB and DR TB cases for early rapid diagnosis of drug sensitive and drug resistant TB. PLoS One. 2014;9(8):e105346.
Reither K, Manyama C, Clowes P, Rachow A, Mapamba D, Steiner A, Ross A, Mfinanga E, Sasamalo M, Nsubuga M, Aloi F, Cirillo D, Jugheli L, Lwilla F. Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in children: a prospective, multi-centre evaluation. J Infect. 2015;70(4):392–9.
Rice JP, Seifert M, Moser KS, Rodwell TC. Performance of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis and rifampin resistance in a low-incidence, high-resource setting. PLoS One. 2017;12(10):e0186139.
Sharma SK, Kohli M, Yadav RN, Chaubey J, Bhasin D, Sreenivas V, Sharma R, Singh BK. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLoS One. 2015;10(10):e0141011.
Sharma SK, Chaubey J, Singh BK, Sharma R, Mittal A, Sharma A. Drug resistance patterns among extra-pulmonary tuberculosis cases in a tertiary care Centre in North India. Int J Tuberc Lung Dis. 2017;21(10):1112–7.
Singh UB, Pandey P, Mehta G, Bhatnagar AK, Mohan A, Goyal V, Ahuja V, Ramachandran R, Sachdeva KS, Samantaray JC. Genotypic, phenotypic and clinical validation of GeneXpert in extra-pulmonary and pulmonary tuberculosis in India. PLoS One. 2016;11(2):e0149258.
Soeroto AY, Lestari BW, Santoso P, Chaidir L, Andriyoko B, Alisjahbana B, van Crevel R, Hill PC. Evaluation of Xpert MTB-RIF guided diagnosis and treatment of rifampicin-resistant tuberculosis in Indonesia: a retrospective cohort study. PLoS One. 2019;14(2):e0213017.
Ssengooba W, Nakiyingi L, Armstrong DT, Cobelens FG, Alland D, Manabe YC, Dorman SE, Ellner JJ, Joloba ML. Clinical utility of a novel molecular assay in various combination strategies with existing methods for diagnosis of HIV-related tuberculosis in Uganda. PLoS One. 2014;9(9):e107595.
Strydom K, Ismail F, Matabane MM, Onwuegbuna O, Omar SV, Ismail N. Comparison of Three Commercial Molecular Assays for Detection of Rifampin and Isoniazid Resistance among Mycobacterium tuberculosis Isolates in a High-HIV-Prevalence Setting. J Clin Microbiol. 2015;53(9):3032–4.
Tahseen S, Qadeer E, Khanzada FM, Rizvi AH, Dean A, Van Deun A, Zignol M. Use of Xpert(®) MTB/RIF assay in the first national anti-tuberculosis drug resistance survey in Pakistan. Int J Tuberc Lung Dis. 2016;20(4):448–55.
Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, Dawson R, Whitelaw A, Hoelscher M, Sharma S, Pai M, Warren R, Dheda K. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184(1):132–40.
Tsuyuguchi K, Nagai H, Ogawa K, Matsumoto T, Morimoto K, Takaki A, Mitarai S. Performance evaluation of Xpert MTB/RIF in a moderate tuberculosis incidence compared with TaqMan MTB and TRCRapid M.TB. J Infect Chemother. 2017;23(2):101–6.
Ullah I, Javaid A, Masud H, Ali M, Basit A, Ahmad W, Younis F, Yasmin R, Khan A, Jabbar A, Husain M, Butt ZA. Rapid detection of mycobacterium tuberculosis and rifampicin resistance in extrapulmonary tuberculosis and sputum smear-negative pulmonary suspects using Xpert MTB/RIF. J Med Microbiol. 2017;66(4):412–8.
Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49(7):2540–5.
van Kampen SC, Tursynbayeva A, Koptleuova A, Murzabekova Z, Bigalieva L, Aubakirova M, Pak S, van den Hof S. Effect of Introducing Xpert MTB/RIF to Test and Treat Individuals at Risk of Multidrug-ResistantTuberculosis in Kazakhstan: A Prospective Cohort Study. PLoS One. 2015;10(7):e0132514.
van Kampen SC, Susanto NH, Simon S, Astiti SD, Chandra R, Burhan E, Farid MN, Chittenden K, Mustikawati DE, Alisjahbana B. Effects of introducing Xpert MTB/RIF on diagnosis and treatment of drug-resistant tuberculosispatients in Indonesia: A pre-post intervention study. PLoS One. 2015;10(6):e0123536.
Wang G, Wang S, Jiang G, Fu Y, Shang Y, Huang H. Incremental cost-effectiveness of the second Xpert MTB/RIF assay to detect mycobacterium tuberculosis. J Thorac Dis. 2018;10(3):1689–95.
Wang G, Wang S, Jiang G, Yang X, Huang M, Huo F, Ma Y, Dai G, Li W, Chen X, Huang H. Xpert MTB/RIF ultra improved the diagnosis of paucibacillary tuberculosis: a prospective cohort study. J Infect. 2019;78(4):311–6.
Williamson DA, Basu I, Bower J, Freeman JT, Henderson G, Roberts SA. An evaluation of the Xpert MTB/RIF assay and detection of false-positive rifampicin resistance in Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2012;74(2):207–9.
Yin QQ, Jiao WW, Han R, Jiao AX, Sun L, Tian JL, Ma YY, Rao XC, Shen C, Li QJ, Shen AD. Rapid diagnosis of childhood pulmonary tuberculosis by Xpert MTB/RIF assay using bronchoalveolar lavage fluid. Biomed Res Int. 2014;2014:310194.
Yuan M, Lyu Y, Chen ST, Cai C, Li Y, Zhang ZG, Li YX, Dong LL, Fu YH, Huang HR, Gao JM, Li WM. Evaluation of Xpert MTB/RIF for the diagnosis of Extrapulmonary tuberculosis in China. Biomed Environ Sci. 2016;29(8):599–602.
Zar HJ, Workman L, Isaacs W, Munro J, Black F, Eley B, Allen V, Boehme CC, Zemanay W, Nicol MP. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clin Infect Dis. 2012;55(8):1088–95 Epub 2012 Jul 2.
Zar HJ, Workman L, Isaacs W, Dheda K, Zemanay W, Nicol MP. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use ofXpert MTB/RIF on respiratory specimens: a prospective study. Lancet Glob Health. 2013;1(2):e97–104.
Zetola NM, Shin SS, Tumedi KA, Moeti K, Ncube R, Nicol M, Collman RG, Klausner JD, Modongo C. Mixed Mycobacterium tuberculosis complex infections and false-negative results for rifampin resistance by GeneXpert MTB/RIF are associated with poor clinical outcomes. J Clin Microbiol. 2014;52(7):2422–9.
Zhang AM, Li F, Liu XH, Xia L, Lu SH. Application of Gene Xpert Mycobacterium tuberculosis DNA and resistance to rifampicin assay in the rapid detection of tuberculosis in children. Zhonghua Er Ke Za Zhi. 2016;54(5):370–4 Article in Chinese.
Li S, Liu B, Peng M, Chen M, Yin W, Tang H, Luo Y, Hu P, Ren H. Diagnostic accuracy of Xpert MTB/RIF for tuberculosis detection in different regions with different endemic burden: a systematic review and meta-analysis. PLoS One. 2017;12(7):e0180725.
Acknowledgements
None.
Funding
This work was supported by the National Science Foundation of China (No. 81801990).
Author information
Authors and Affiliations
Contributions
ZKC and LSY conceived the study. ZKC and JYZ carried out the literature selection, data extraction and statistical analysis. LC accomplished the manuscript draft. ZH and JYZ participated in the analysis. The final manuscript was approved by all the authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was established according to the ethical guidelines of the Helsinki Declaration and approved by the Human Ethics Committee of Department of Respiratory Disease, The Seventh People’s hospital of Chongqing. Written informed consent was obtained from individual participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zong, K., Luo, C., Zhou, H. et al. Xpert MTB/RIF assay for the diagnosis of rifampicin resistance in different regions: a meta-analysis. BMC Microbiol 19, 177 (2019). https://doi.org/10.1186/s12866-019-1516-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-019-1516-5