Abstract
Background
Respectively, prostate cancer (PCa) and breast cancer (BC) are the second most and most commonly diagnosed cancer in men and women, and they account for a majority of cancer-related deaths world-wide. Cancer cells typically exhibit much-facilitated growth that necessitates upregulated glycolysis and augmented amino acid metabolism, that of glutamine and aspartate in particular, which is tightly coupled with an increased flux of the tricarboxylic acid (TCA) cycle. Epidemiological studies have exploited metabolomics to explore the etiology and found potentially effective biomarkers for early detection or progression of prostate and breast cancers. However, large randomized controlled trials (RCTs) to establish causal associations between amino acid metabolism and prostate and breast cancers have not been reported.
Objective
Utilizing two-sample Mendelian randomization (MR), we aimed to estimate how genetically predicted glutamate and aspartate levels could impact upon prostate and breast cancers development.
Methods
Single nucleotide polymorphisms (SNPs) as instrumental variables (IVs), associated with the serum levels of glutamate and aspartate were extracted from the publicly available genome-wide association studies (GWASs), which were conducted to associate genetic variations with blood metabolite levels using comprehensive metabolite profiling in 1,960 adults; and the glutamate and aspartate we have chosen were two of 644 metabolites. The summary statistics for the largest and latest GWAS datasets for prostate cancer (61,106 controls and 79,148 cases) were from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium, and datasets for breast cancer (113,789 controls and 133,384 cases) were from Breast Cancer Association Consortium (BCAC). The study was performed through two-sample MR method.
Results
Causal estimates were expressed as odds ratios (OR) and 95% confidence interval (CI) per standard deviation increment in serum level of aspartate or glutamate. Aspartate was positively associated with prostate cancer (Effect = 1.043; 95% confidence interval, 1.003 to 1.084; P = 0.034) and breast cancer (Effect = 1.033; 95% confidence interval, 1.004 to 1.063; P = 0.028); however, glutamate was neither associated with prostate cancer nor with breast cancer. The potential causal associations were robust to the sensitivity analysis.
Conclusions
Our study found that the level of serum aspartate could serve as a risk factor that contributed to the development of prostate and breast cancers. Efforts on a detailed description of the underlying biochemical mechanisms would be extremely valuable in early assessment and/or diagnosis, and strategizing clinical intervention, of both cancers.
Similar content being viewed by others
Introduction
The prostate (man) and breast cancers (woman) are almost the most frequently diagnosed cancers that also constitute a major cause of cancer-related deaths [1, 2]. In the western countries, prostate cancer is the most common form of cancer among men of 50yrs and older with a mortality-to-incidence ratio of 20% [3]. As for breast cancer, the United States alone in 2017 recorded 255,180 new cases and 41,070 deaths [4]. Thus, despite tremendous advancements over the previous decade, early detection/treatment of prostate and breast cancers is next to satisfaction.
In cells, nutrients are essential for energetics and many types of bio-mass building. Accumulating evidence from basic studies suggests that cancer cells continuously adapt to dynamic metabolic micro-environment by changing the way of nutrient utilization during malignancy development. For instance, under aerobic condition, cancer cells often exhibit upregulated glycolysis for their rapid growth [5]; and they also use amino acids, in particular glutamine and aspartate, as anaplerotic nutrients for TCA cycle that is coupled with oxidative phosphorylation [6]. Hence, cancer cells are generally vulnerable to nutrient deficiency, a feature that potentially provides new targets for cancer therapy [7]. Indeed, given altered metabolism typical of cancer tissues [8], quite an ever-growing number of epidemiological studies have exploited metabolomics to research the etiology and figure out biomarkers for early detection, or progression of prostate cancer [9].
Observationally, soy proteins, rich in glutamate and aspartate, are reported to lower the androgen levels but no large RCTs have been conducted to test their health effects; in addition, animal experiment results suggest that glutamate and aspartate can decrease the testosterone levels [10, 11], and diverse epidemiological studies suggest that consumption of soy, fruits, and vegetables are linked with reduced risk of recurrence and increased survival rate of prostate cancer and breast cancer [12,13,14,15]. In a RCT of men, D-aspartate can reduce testosterone [16]. Despite these studies, however, up to now a causal relationship between serum levels of amino acids, such as glutamate and aspartate, and prostate and breast cancers remains elusive. Furthermore, at times the metabolic studies generate results that are not always consistent due to the differences in outcome examined, metabolomics platforms exploited, and characteristics and/or sizes of study populations.
MR, as a newly approach, gets information from genome-wide association studies (GWAS) to evaluate the causal relationship between exposures and phenotype without any potentially harmful intervention [17, 18]. Briefly, MR analysis widely utilizes the power of genetic variants as IVs to evaluate the causal associations between risk factors and disease outcomes [19]. Because genetic variants are inherently inherited at conception, an MR analysis can avoid potential bias along with misinterpretation of results by removing confounding factors in traditional observational studies typically associated with socio-economic status, lifestyle (alcohol and smoking) and health status. Mendel’s second law dictates that each pair of alleles undergoes independent assortment without interference from environmental factors. Two-sample MR analysis requires summary-level data from two independent GWASs for putative exposures and outcomes [20]. Here, exploiting genetically instrumented glutamate and aspartate from GWAS [21] and large case–control studies of prostate and breast cancers with extensive genotyping [22, 23], an MR study was performed to estimate the causal effects of serum glutamate and aspartate levels and the development of prostate and breast cancers.
Subjects and methods
Study design and data sources
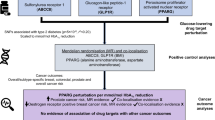
As shown in Fig. 1, a two-sample Mendelian Randomization approach was designed in this study. It is based on the assumption that instrumental variables are related to serum levels of glutamate and aspartate, but independent of the risk of cancer and cofounders.
The IVs were extracted from the publicly available genome-wide association studies, which were a common, low-frequency and rare variants GWASs, and conducted in 1,960 adults to associate genetic variations with blood metabolites by comprehensive metabolite profiling [21]. The glutamate and aspartate chosen in our study were two of the whole 644 metabolites according three longitudinal data collections. SNPs were removed as the call rate was less than 95%, the P value was above 10−6 and the minor allele frequency was less than 1%. Information of data on the association of SNPs with serum glutamate and aspartate and the association of SNPs with breast cancer and prostate cancer were obtained from the GWAS database [22, 23].
Genetic instruments for glutamate and aspartate
Genetic signatures, such as SNPs, associated with glutamate or aspartate were obtained from a large GWAS study [21], in which the participants were of European origin. SNPs as IVs, were not confounded by socio-economic status and lifestyle factors (alcohol and smoking). Different genetic variants were obtained with different cutoffs for significance, genome-wide association significance (5 × 10−8) or a less stringent significance (5 × 10−6). The strength of each SNP was evaluated using the F-statistic, calculated using a well-established formula [24]. A cutoff of 10 as a "rule of thumb" was used to distinguish between strong and weak instruments [25]. Weak instruments can bias the findings. An MR approach is based on the hypothesis that genetic exposure influences the outcome directly [26], otherwise, it is not suitable to perform the analysis in the presence of pleiotropy. Thus, to validate that the SNPs were associated with prostate or breast cancer solely via glutamate or aspartate, pleiotropy was checked, i.e., genetic associations with prostate or breast cancer via estrogen-related factors such as breast density and use of hormone replacement therapy [27, 28] and high body mass index (BMI) [29], with PhenoScanner V2 website (www.phenoscanner.medschl.cam.ac.uk). It is a comprehensively curated genetic cross-reference system and provides all well-established associations of known SNPs with their phenotypes, including subgenome-wide associations [30]. In order to make sure the independent contribution of selected SNPs, i.e., the correlation between the selected SNPs, LD-link website (https://ldlink.nci.nih.gov/, population: CEU) was used to perform a linkage disequilibrium (LD) test, which was a suite of web-based applications designed to easily and efficiently interrogate LD in population groups. For SNPs (r2 < 0.8), the SNPs (5 × 10−8) were used. For less strongly associated SNPs (5 × 10−6), only the uncorrelated SNPs (r2 < 0.01) were chosen.
Genetic associations with prostate and breast cancers
Summary data for prostate cancer was extracted from the largest GWAS meta-analysis including 79,148 cases and 61,106 controls of European ancestry from the PRACTICAL consortium (http://practical.icr.ac.uk/blog/) [23]. Summary statistics for breast cancer was extracted from the latest and largest meta-GWAS from BCAC including 133,384 cases and 113,789 controls (http://bcac.ccge.medschl.cam.ac.uk/) [22]. The participants were women of European ancestry. The written consent of listed participants was provided, and all the studies from which we extracted data for our MR analysis were supported by the ethical review boards.
Statistical analysis
In order to evaluate the casual effect between glutamine and aspartate and cancers, MR methods including simple median, weighted median (WM), penalized weighted median, the inverse-variance-weighted (IVW), penalized IVW, robust IVW, penalized robust IVW, MR-Egger, penalized MR-Egger, robust MR-Egger, and penalized robust MR Egger are selected for analysis. Penalized analyses would give consistent estimates if a plurality of the instrumental variables are valid [31,32,33]. Among them, IVW is the major analyses method. For multiple independent genetic variants, IVW could weigh the average of these single causal estimates using the inverse of their approximate variances as weights [34]. The weighted median method [35] and MR Egger [34] were conducted in the sensitivity analysis to account for potential bias from unknown pleiotropy. The WM method could be considered to account for differences in the precision of estimates and could provide consistent estimates even if 50% of the information comes from invalid SNPs [35]. The simple median estimator is calculated as the median of the Wald ratio estimates [ratio of SNP on outcome to SNP on glutamate and aspartate]. The estimate obtained by WM method were validated as IVs were greater than or equal to three SNPs [36]. Otherwise, the Wald ratio was directly used as there was one SNP. Causal estimate was also obtained from MR-Egger, which was based on the assumption that the pleiotropic effects were independently distributed from the genetic associations with the exposure [34]. The intercept from MR Egger was checked whether it was nonzero because this indicates that some of the genetic predictors might be acting other than via the exposure (i.e., directionally pleiotropic) [34], invalidating the IVW estimates. The MR Egger estimate is less precise than that from IVW, because the variance of the MR Egger estimate additionally depends on the variability between the genetic associations with the exposure, and it is much larger than that from IVW [34]. Supplementing these more widely used approaches, a robust adjusted profile score (RAPS), that is robust to idiosyncratic pleiotropy [37], and improved MR methods including the robust option, penalized option, and the penalized option of the weighted median, IVW, and MR-Egger [38] were recently developed MR methods.
Heterogeneity test was performed using Cochran’s Q-test to identify whether the MR results were biased by the potential heterogenic factors. A leave-one-out permutation test was performed to assess whether the IVW estimate was biased by the influence of particular SNPs. Causal estimates between glutamate and aspartate levels and prostate and breast cancers risk were expressed as odds ratios (OR) and 95% confidence interval (CI) per standard deviation increment in plasma glutamate or aspartate level.
All the analyses with P < 0.05 were considered statistically significant. All statistical analyses were performed using the R Studio (R version 4.0.2) software and the R package “Mendelian Randomization”.
Results
Genetic instruments for glutamate and aspartate
The SNPs as the potential IVs obtained from the large GWASs of European ancestry [21], were not confounded by socio-economic status and lifestyle factors (alcohol and smoking). By using different cutoffs for significance, the different genetic variants were selected. With genome-wide association significance (P < 5 × 10−8), 5 SNPs were selected, including 1 SNP for glutamate and 4 SNPs for aspartate. However, the result of the linkage disequilibrium test on the LD-link website showed that rs139051778 and rs33966350 associated with aspartate were not independent. In fact, these two SNPs were within a same gene; thus, to get more reliable results, rs139051778 were excluded for analysis, as rs33966350 were with lower P value. With a less stringent significance (P < 5 × 10−6), 14 genetic variants were associated with serum glutamate or aspartate levels. Finally, the characteristics of these 18 genetic variants in total were shown in Table 1.
The selected SNPs were from genes thought to be functionally relevant to the exposures and none of the SNPs were associated with key confounders. The SNP rs113141482 is on the gene GPR158, which encodes G protein-coupled receptor 158 (GPR158). GPR158 is a newly characterized cell surface protein that plays the same role, as other G-protein coupled receptors (GPCRs), on promoting prostate cancer (PCa) malignancy. Indeed, currently, the glutamate family member GPR158 is a therapeutic target for PCa [39]. The SNP rs33966350 is a locus on the gene ENPEP associated with blood pressure [35] and is related to aspartate, given that the gene is also relevant to the metabolism of aspartate in function: ENPEP encodes glutamyl aminopeptidase, catalyzing the cleavage of glutamate and aspartate from the N-terminal polypeptides.
Associations with prostate and breast cancers
Based on the single genome-wide significant SNPs, the result of IVW analysis (Figs. 2 and S1) was shown that the genetically instrumented aspartate was positively associated with prostate and breast cancers. Importantly, when less strongly associated SNPs was included, the results from the improved the MR methods including the robust IVW and penalized robust IVW methods also showed a significant association with prostate cancer (Table S1) and breast cancer (Table S2). Interestingly, the intercept of MR Egger, penalized MR-Egger, robust MR-Egger and penalized robust MR-Egger were not equal to zero and there was no pleiotropy. In addition, the P values of MR Egger, penalized MR-Egger, robust MR-Egger and penalized robust MR-Egger were less than 0.05. Therefore, the causal association in the Figs. 2 and S1 was further supported. However, the association between aspartate and breast cancer did not remain in the result of IVW analysis when including less strongly associated SNPs (Figs. S2 and S4).
Genetically instrumented glutamate was not significantly associated with breast cancer and prostate cancer based on the single genome-wide significant SNPs (Fig. 3). These associations were generally robust to different SNP selections as less strongly associated SNPs were included (Figs. S3, S5, Tables S3 and S4).
The summary information of GWASs on outcomes was displayed in Table S5. And the genetic associations between serum levels of glutamate and aspartate and the outcomes were shown in Table 1.
Sensitivity analysis
As IVs were greater than or equal to three SNPs, the MR-Egger intercept test was performed to examine the pleiotropy. The intercept values from MR Egger analysis were nonzero, suggesting that the MR Egger estimate may have greater validity and the pleiotropy did not bias the results.
The single genome-wide significant SNPs in the Tables 2 and 3 and less strongly associated SNPs in the Tables S1 and S2 were to predict serum level of aspartate and the result of MR-Egger intercept test indicated that the intercept of the MR-Egger did not significantly differ from zero. Therefore, no signs of directional pleiotropy among these instruments were discovered. Less strongly associated SNPs in Tables S3 and S4 were to predict serum level of glutamate and the similar result of MR-Egger intercept test was shown. There was only one SNP at genome-wide association significance to predict glutamate, the MR-Egger intercept test could not be performed (Table 4). Additionally, robust adjusted profile score (RAPS) was also used to test the idiosyncratic pleiotropy, which further confirm the IVW findings (Tables 2, 3, 4 and Tables S1, S2, S3, S4). The single MR estimates were provided from each of the genetic variants using MR-Egger method (Fig. S9). The above results showed that all 18 variants in Table 1 were valid instrumental variables and could be used in the MR analysis.
Furthermore, there was no sign of heterogenetic effects between the genetic effects and risk of prostate and breast cancers in the Cochran’s Q statistics test (Tables 2, 3 and Tables S1, S2, S3 and S4) and It was identified that the IVW estimate was not biased by the influence of particular SNPs in the leave-one-out sensitivity analysis, based on SNPs with genome-wide significance and more SNPs with less stringent significance (Figs. S6, S7 and S8).
Discussion
Balanced diet/nutrition intake constitutes a preventive strategy for cancer incidences that in turn may impede the development and progression of cancer. Amino acids, glutamine and aspartate in particular, are vital alternative nutrients for cellular energetics and biomass synthesis apart from glucose. With consistency of the implication of evolutionary biology theory, our finding suggested that aspartate, with the potential to affect the endocrine system [40], was a underlying risk factor for prostate and breast cancers.
To the best of our knowledge, it is the first MR study to examine the potential causal effects of glutamate and aspartate on prostate and breast cancers. Genetically instrumented glutamate and aspartate can remove potential confounding factors in observational studies and make a difference of the effects of these two dietary programs, which are correlated and co-occur. And at the same time it can also minimize the measurement error in nutrition studies from self-reported dietary consumption [21]. Furthermore, it is cost-efficient depending on large GWASs and case–control studies with extensive genotyping [41]. The samples for MR analysis were from two completely separate GWASs, one sample for genetic variants on exposures (glutamate and aspartate) [42] and the other sample for genetic variants on outcomes (prostate cancer [23] and breast cancer [22]), which means any correlation in the sample with the exposures is unlikely to be replicated in the sample with the clinical outcomes.
Despite above-claimed strength, this study has several limitations. First, our findings on serum aspartate were seemingly inconsistent with anti-cancer effect of aspartate in food, such as soy [14, 15, 43,44,45]. A possible explanation is that the effects of serum glutamate and aspartate reflected endogenous exposures that may distinguish with exogenous dietary exposures; but levels of serum glutamate and aspartate are likely affected by dietary consumption [46]. Second, MR requires the genetic instruments associated with the exposures and the genetic variants are no pleiotropy. There are no confounders in the causal association [47]. As a result, here only 1 SNP associated with glutamate met the requirements with genome-wide significance threshold (P < 5 × 10−8). Thus, the exposure glutamate was dropped in the sensitivity analysis, which might lead to the decreased reliability of the result without MR-Egger and WM analysis. Therefore, more less strongly associated SNPs were included for analysis. However, the inconsistencies between the results of IVW and MR Egger analyses could also be caused by weak instrument bias and the potential differences in validities of all the selected SNPs. Third, the genetic associations in our study were from studies largely conducted in European descent with genomic control [21, 48]. Some genetic variants may be different from other populations, which was caused by “population bottlenecks” [49]. Thus, the results in our study might not be applied to other populations in other parts of the world, although the allele frequency of major SNP was similar in ethnic groups. GWAS datasets from other populations should be collected to replicate and confirm the findings. Fourth, it could not be assessed whether in our estimates the effects of glutamate and aspartate on cancers vary by sex or age. The stratified MR analysis should be performed. Fifth, to reduce the possibility of false positive results, a Bonferroni correction (corrected P: 0.05/4 = 0.0125) of multiple independent tests (tests for associations of two metabolites with two types of cancer, respectively) should be used. Therefore, our findings were deemed suggestive evidence of possible associations (0.0125 < P < 0.05). Hence, this necessitates further studies to replicate our findings and get more conclusive results. Last but not the least, the underlying pathways of the causal effects remained to be clarified.
Aspartate and glutamate belong to the arginine family, along with asparagine, glutamine and arginine itself. They are inter-convertible via complex metabolism in most mammals. In our findings, aspartate was the risk factor for prostate cancer and breast cancer development. Emerging evidence reveals that glutamine and interlinked asparagine metabolism may be critical for endothelial cell (EC) metabolism, as a regulator of angiogenesis [50]. Therefore, the fact that the serum levels of aspartate and glutamine serving as a risk factor might be exerted via their relevant metabolites given that asparagine and glutamine are known to promote cancer cell proliferation and vessel sprouting. Furthermore, in one breast cancer model, asparagine bioavailability impacts the ratios of epithelial-to-mesenchymal-like tumor cells and tumor progression [51]. In the epithelial‐mesenchymal transition (EMT) and PCa progression, aspartate is generally a recognized contributor, its metabolism when elevated is accompanied with high levels of adenylosuccinate, arginosuccinate, malate, asparagine known to be correlated with tumor progression [52].
Another plausible mechanism underlying our findings is a link between aspartate and arginine via the urea cycle. The urea cycle detoxifies free ammonia in the livers of mammals, in which arginine is synthesized in two steps: citrulline and aspartate are used to synthesize argininosuccinate which is then converted to arginine. Arginine is a non-essential amino acid in adults but is necessary for fast-growing cells such as cancer cells. Currently Graboa et al. [53] have reported that arginine is crucial during malignancy development. Arginine deprivation has been a novel and promising approach to treat tumors that are not hepatocyte-derived thus unable to self-suffice for arginine owing to a lack of the urea cycle [54]. However, the effects of glutamate and aspartate on human health are very complex. Some studies show that nutritional supplements, aspartate and glutamate, possess beneficial health and anti-oxidative effects. For example, aspartate can improve liver metabolism [55], and glutamate can modulate the body weight [56], regulate the release of hormones [57] and lipid metabolism [58], probably owing to its impact upon the TCA cycle and ATP production [59]. Aspartate might also operate by lowering androgens [10], and high level of circulating androgens is a risk factor for prostate cancer, a notion for which there is, however limited, evidence in human studies [16].
Currently, the technique of ultra-high performance liquid chromatography-tandem mass spectrometry measuring levels of amino acids such as aspartate and glutamate has been well validated [60]. It will be worthwhile to exploit more relevant genetic instruments if available. Our work, based on MR studies that constitute a tool for testing causation, cannot dictate the exact size/degree of causal effects [61] nor can replace clinical trials; however, the findings built on the ever-growing knowledge about the effects of glutamine and aspartate on prostate cancer and breast cancer development is for sure greatly relevant to dietary recommendations, along with providing guidance for cancer prevention as well as public health in general.
Availability of data and materials
All data analyzed in this study are included in the manuscript and supplementary materials.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492 PubMed PMID: 30207593Epub 2018/09/13.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551 PubMed PMID: 30620402 Epub 2019/01/09.
Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global burden of urologic cancers, 1990–2013. Eur Urol. 2017;71(3):437–46. https://doi.org/10.1016/j.eururo.2016.10.008 PubMedPMID:WOS:000396333700027.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. https://doi.org/10.3322/caac.21387 PubMedPMID:WOS:000393807800003.
Granja S, Pinheiro C, Reis RM, Martinho O, Baltazar F. Glucose addiction in cancer therapy: advances and drawbacks. Curr Drug Metab. 2015;16(3):221–42. https://doi.org/10.2174/1389200216666150602145145 PubMedPMID:WOS:000359604700005.
Luo M, Brooks M, Wicha MS. Asparagine and glutamine: co-conspirators fueling metastasis. Cell Metab. 2018;27(5):947–9. https://doi.org/10.1016/j.cmet.2018.04.012 PubMedPMID:WOS:000432438100002.
Alkan HF, Walter KE, Luengo A, Madreiter-Sokolowski CT, Stryeck S, Lau AN, et al. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab. 2018;28(5):706–20. https://doi.org/10.1016/j.cmet.2018.07.021 PubMedPMID:WOS:000449440000007.
Giunchi F, Fiorentino M, Loda M. The Metabolic landscape of prostate cancer. Eur Urol Oncol. 2019;2(1):28–36. https://doi.org/10.1016/j.euo.2018.06.010 PubMedPMID:WOS:000474608500004.
Kelly RS, Heiden MGV, Giovannucci E, Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis, and recurrence. Cancer Epidemiol Biomark Prev. 2016;25(6):887–906. https://doi.org/10.1158/1055-9965.Epi-15-1223 PubMedPMID:WOS:000377528100002.
Ni H, Lu L, Deng J, Fan W, Li T, Yao J. Effects of glutamate and aspartate on serum antioxidative enzyme, sex hormones, and genital inflammation in boars challenged with hydrogen peroxide. Mediators Inflamm. 2016;2016:4394695 PubMed PMID: MEDLINE:27777497.
Okoye CN, Ochiogu IS, Onah CE. The effects of monosodium L-glutamate administration on the reproduction and serum biochemistry of adult male rabbits. Vet Med. 2016;61(3):141–7. https://doi.org/10.17221/8765-vetmed PubMedPMID:WOS:000376027000005.
Nechuta SJ, Caan BJ, Chen WY, Lu W, Chen Z, Kwan ML, et al. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr. 2012;96(1):123–32. https://doi.org/10.3945/ajcn.112.035972 PubMedPMID:WOS:000305670100017.
Thomson CA, Rock CL, Thompson PA, Caan BJ, Cussler E, Flatt SW, et al. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the women’s healthy eating and living study. Breast Cancer Res Treat. 2011;125(2):519–27. https://doi.org/10.1007/s10549-010-1014-9 PubMedPMID:WOS:000285344000022.
Zhang M, Wang K, Chen L, Yin B, Song Y. Is phytoestrogen intake associated with decreased risk of prostate cancer? A systematic review of epidemiological studies based on 17,546 cases. Andrology. 2016;4(4):745–56. https://doi.org/10.1111/andr.12196 PubMedPMID:WOS:000383291000021.
Zhang Q, Feng H, Qluwakemi B, Wang J, Yao S, Cheng G, et al. Phytoestrogens and risk of prostate cancer: an updated meta-analysis of epidemiologic studies. Int J Food Sci Nutr. 2017;68(1):28–42. https://doi.org/10.1080/09637486.2016.1216525 PubMedPMID:WOS:000394030100004.
Melville GW, Siegler JC, Marshall PWM. Three and six grams supplementation of d-aspartic acid in resistance trained men. J Int Soc Sports Nutr. 2015;12:15. https://doi.org/10.1186/s12970-015-0078-7 PubMed PMID: WOS:000352053600001.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65. https://doi.org/10.1002/gepi.21758 PubMedPMID:WOS:000325980600002.
Sekula P, Del Greco FM, Pattaro C, Koettgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–65. https://doi.org/10.1681/asn.2016010098 PubMedPMID:WOS:000386538300006.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. J Am Med Assoc. 2017;318(19):1925–6. https://doi.org/10.1001/jama.2017.17219 PubMedPMID:WOS:000415870300022.
Hartwig FP, Davies NM, Hemani G, Smith GD. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45(6):1717–26. https://doi.org/10.1093/ije/dyx028 PubMedPMID:WOS:000398261100003.
Long T, Hicks M, Yu H-C, Biggs WH, Kirkness EF, Menni C, et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 2017;49(4):568–78. https://doi.org/10.1038/ng.3809 PubMedPMID:WOS:000397603700020.
Zhang H, Ahearn TU, Lecarpentier J, Barnes D, Beesley J, Qi G, et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet. 2020;52(6):572–81. https://doi.org/10.1038/s41588-020-0609-2 PubMed PMID: 32424353 Epub 2020/05/20.
Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–36. https://doi.org/10.1038/s41588-018-0142-8 PubMed PMID: 29892016; PubMed Central PMCID: PMCPMC6568012 Epub 2018/06/13.
Bowden J, Del Greco FM, Minelli C, Smith GD, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-egger regression: the role of the I-2 statistic. Int J Epidemiol. 2016;45(6):1961–74. https://doi.org/10.1093/ije/dyw220 PubMedPMID:WOS:000398261100034.
Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica. 1997;65(3):557–86. https://doi.org/10.2307/2171753 PubMedPMID:WOS:A1997WV90300003.
Stearns FW. Anecdotal, historical and critical commentaries on genetics one hundred years of pleiotropy: a retrospective. Genetics. 2010;186(3):767–73. https://doi.org/10.1534/genetics.110.122549 PubMedPMID:WOS:000283996100001.
Golubnitschaja O, Debald M, Yeghiazaryan K, Kuhn W, Pesta M, Costigliola V, et al. Breast cancer epidemic in the early twenty-first century: evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumor Biology. 2016;37(10):12941–57. https://doi.org/10.1007/s13277-016-5168-x PubMedPMID:WOS:000387538700004.
Lambertini M, Santoro L, Del Mastro L, Nguyen B, Livraghi L, Ugolini D, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76. https://doi.org/10.1016/j.ctrv.2016.07.006 PubMedPMID:WOS:000383006100007.
Wilson KM, Giovannucci EL, Mucci LA. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J Androl. 2012;14(3):365–74. https://doi.org/10.1038/aja.2011.142 PubMedPMID:WOS:000303804500005.
Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32(20):3207–9. https://doi.org/10.1093/bioinformatics/btw373 PubMedPMID:WOS:000386020700023.
Burgess S, Zuber V, Gkatzionis A, Foley CN. Modal-based estimation via heterogeneity-penalized weighting: model averaging for consistent and efficient estimation in Mendelian randomization when a plurality of candidate instruments are valid. Int J Epidemiol. 2018;47(4):1242–54. https://doi.org/10.1093/ije/dyy080 PubMedPMID:WOS:000444559900033.
Larsson SC, Traylor M, Burgess S, Boncoraglio GB, Jern C, Michaelsson K, et al. Serum magnesium and calcium levels in relation to ischemic stroke Mendelian randomization study. Neurology. 2019;92(9):E944–50. https://doi.org/10.1212/wnl.0000000000007001 PubMedPMID:WOS:000465408500016.
He Y, Zhang H, Wang T, Han Z, Ni Q-b, Wang K, et al. Impact of serum calcium levels on alzheimer’s disease: a Mendelian randomization study. J Alzheimers Dis. 2020;76(2):713–24. https://doi.org/10.3233/jad-191249 PubMed PMID: WOS:000551104100026.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. https://doi.org/10.1007/s10654-017-0255-x PubMedPMID:WOS:000405184200004.
Bowden J, Smith GD, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. https://doi.org/10.1002/gepi.21965 PubMedPMID:WOS:000374542600005.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. https://doi.org/10.1002/gepi.21965 PubMed Central PMCID: PMCPMC4849733 Epub 2016/04/12.
Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat. 2020;48(3):1742–69. https://doi.org/10.1214/19-aos1866 PubMedPMID:WOS:000551644000022.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9. https://doi.org/10.1093/ije/dyx034 PubMedPMID:WOS:000417745100003.
Patel N, Itakura T, Jeong S, Liao C-P, Roy-Burman P, Zandi E, et al. Expression and functional role of orphan receptor GPR158 in Prostate cancer growth and progression. Plos One. 2015;10(2):e0117758. https://doi.org/10.1371/journal.pone.0117758 PubMed PMID: WOS:000350061500073.
Jasienska G, Bribiescas RG, Furberg A-S, Helle S, de la NunezMora A. Human reproduction and health: an evolutionary perspective. Lancet. 2017;390(10093):510–20. https://doi.org/10.1016/s0140-6736(17)30573-1 PubMed PMID: WOS:000406463400032.
Burgess S, Scott RA, Timpson NJ, Smith GD, Thompson SG, Epic-Interact C. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52. https://doi.org/10.1007/s10654-015-0011-z PubMedPMID:WOS:000358649900003.
Zhao JV, Kwok MK, Schooling CM. Effect of glutamate and aspartate on ischemic heart disease, blood pressure, and diabetes: a Mendelian randomization study. Am J Clin Nutr. 2019;109(4):1197–206. https://doi.org/10.1093/ajcn/nqy362 PubMedPMID:WOS:000478064700018.
Ye Won H, Soo Young K, Sun Ha J, Youn Nam K, Chung Mo N. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61(5):598–606 PubMed PMID: FSTA:2010-01-Jn0213.
Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer. 2005;117(4):667–9. https://doi.org/10.1002/ijc.21266 PubMedPMID:WOS:000232666500020.
Kim MK, Kim JH, Nam SJ, Ryu S, Kong G. Dietary intake of soy protein and tofu in association with breast cancer risk based on a case-control study. Nutr Cancer. 2008;60(5):568–76. https://doi.org/10.1080/01635580801966203 PubMedPMID:WOS:000259969800004.
Bos C, Metges CC, Gaudichon C, Petze KJ, Pueyo ME, Morens C, et al. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J Nutr. 2003;133(5):1308–15 PubMed PMID: WOS:000182727000014.
Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Smith GD. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63. https://doi.org/10.1002/sim.3034 PubMedPMID:WOS:000255210700001.
Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet. 2017;49(9):1385–91. https://doi.org/10.1038/ng.3913 PubMedPMID:WOS:000408672000017.
Kim MS, Patel KP, Teng AK, Berens AJ, Lachance J. Genetic disease risks can be misestimated across global populations. Genome Biol. 2018;19:179. https://doi.org/10.1186/s13059-018-1561-7 PubMed PMID: WOS:000450190600001.
Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Bruning U, et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36(16):2334–52. https://doi.org/10.15252/embj.201695518 PubMedPMID:WOS:000407786500005.
Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554(7692):378–81. https://doi.org/10.1038/nature25465 PubMedPMID:WOS:000424996300042.
Chen Y, Wang K, Liu T, Chen J, Lv W, Yang W, et al. Decreased glucose bioavailability and elevated aspartate metabolism in prostate cancer cells undergoing epithelial-mesenchymal transition. J Cell Physiol. 2020;235(7–8):5602–12. https://doi.org/10.1002/jcp.29490 PubMedPMID:WOS:000510766500001.
Grabon W. Arginine as a crucial amino acid in carcinogenesis and tumor growth. Postepy Hig Med Dosw (Online). 2006;60:483–9 PubMed PMID: MEDLINE:17013367.
Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, et al. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des. 2008;14(11):1049–57. https://doi.org/10.2174/138161208784246199 PubMedPMID:WOS:000255683300002.
Pi D, Liu Y, Shi H, Li S, Odle J, Lin X, et al. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J Nutr Biochem. 2014;25(4):456–62. https://doi.org/10.1016/j.jnutbio.2013.12.006 PubMedPMID:WOS:000333513100011.
Kondoh T, Torii K. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male sprague-dawley rats. Physiol Behav. 2008;95(1–2):135–44. https://doi.org/10.1016/j.physbeh.2008.05.010 PubMedPMID:WOS:000259536100022.
Iwatsuki K, Torii K. Peripheral chemosensing system for tastants and nutrients. Curr Opin Endocrinol Diabetes Obes. 2012;19(1):19–25. https://doi.org/10.1097/MED.0b013e32834ec7f8 PubMedPMID:WOS:000298400600005.
Kong XF, Zhou XL, Feng ZM, Li FN, Li YJ, Tan BE, et al. Dietary supplementation with monosodium L-glutamate modifies lipid composition and gene expression related to lipid metabolism in growing pigs fed a normal- or high-fat diet. Livest Sci. 2015;180:247–52. https://doi.org/10.1016/j.livsci.2015.06.023 PubMedPMID:WOS:000362382200034.
Russell RR, Taegtmeyer H. Changes in citric-acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Investig. 1991;87(2):384–90. https://doi.org/10.1172/jci115008 PubMedPMID:WOS:A1991EW29500002.
Beger RD, Dunn W, Schmidt MA, Gross SS, Kirwan JA, Cascante M, et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective.” Metabolomics. 2016;12(9):149. https://doi.org/10.1007/s11306-016-1094-6 PubMed PMID: WOS:000384337700007.
Schooling CM, Yeung SL, Freeman G. Mendelian randomization estimates may be inflated. J Am Coll Cardiol. 2013;61(18):1931. https://doi.org/10.1016/j.jacc.2012.12.049 PubMedPMID:WOS:000318607400017.
Acknowledgements
The breast cancer genome-wide association analyses for BCAC and CIMBA were supported by Cancer Research UK (C1287/A10118, C1287/A16563, C1287/A10710, C12292/A20861, C12292/A11174, C1281/A12014, C5047/A8384, C5047/A15007, C5047/A10692, C8197/A16565), The National Institutes of Health (CA128978, X01HG007492- the DRIVE consortium), the PERSPECTIVE project supported by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research (grant GPH-129344) and the Ministère de l‘Économie, Science et Innovation du Québec through Genome Québec and the PSRSIIRI-701 grant, the Quebec Breast Cancer Foundation, the European Community’s Seventh Framework Programme under grant agreement n° 223175 (HEALTH-F2-2009-223175) (COGS), the European Union’s Horizon 2020 Research and Innovation Programme (634935 and 633784), the Post-Cancer GWAS initiative (U19 CA148537, CA148065 and CA148112 – the GAME-ON initiative), the Department of Defence (W81XWH-10-1-0341), the Canadian Institutes of Health Research (CIHR) for the CIHR Team in Familial Risks of Breast Cancer (CRN-87521), the Komen Foundation for the Cure, the Breast Cancer Research Foundation and the Ovarian Cancer Research Fund. All studies and funders are listed in Zhang H et al. (Nat Genet, 2020).
Funding
This work was supported by the China National 973 Project (2014CB542003), the China Natural Sciences Foundation Project (81372179) and the Zhejiang Provincial SciTech Commission Project (2014C03048-2) to Y. Luo.
Author information
Authors and Affiliations
Contributions
L.Z. and Y.L. conceived the study design and drafted the manuscript. Z.Y., J.L. and Y.S. participated in data extraction and data analysis, X.Z. and Z.Q. did the data checking and analysis. L.Z. and Y.L reviewed and edited the manuscript, and guided Y.L., Z.Y. and J.L. to design and carry out the experiments. All authors read and agreed to the published version of the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Forest plot of the causal effects of aspartate (3 independent SNPs, P value <5×10−8) on prostate and breast cancers. IVW, inverse-variance weighted. (a). The association of aspartate with prostate cancer. (b). The associationof aspartate with breast cancer.
Additional file 2: Fig. S2.
Causal associations between aspartate and prostate and breast cancers.
Additional file 3: Fig. S3.
Causal associations between glutamate and prostate and breast cancers.
Additional file 4: Fig. S4.
Forest plot of the causal effects of aspartate (13 independent SNPs, P value <5×10−6) on prostate and breast cancers. IVW, inverse-variance weighted. (a). The association of aspartate with prostate cancer. (b). The associationof aspartate with breast cancer.
Additional file 5: Fig. S5.
Forest plot of the causal effects of glutamate (5 independent SNPs, P value < 5×10−6) on prostate and breast cancers. IVW, inverse-variance weighted. (a). The association of glutamate with prostate cancer. (b). The association of glutamate with breast cancer.
Additional file 6: Fig. S6.
Leave-one-out sensitivity analysis for the effect of aspartate (3 independent SNPs with P value < 5×10−8) on prostate and breast cancers. IVW, inverse-variance weighted. (a). The association of aspartate with prostate cancer. (b). The association of aspartate with breast cancer.
Additional file 7: Fig. S7.
Leave-one-out sensitivity analysis for the effect of aspartate (13 independent SNPs with P value < 5×10−6) on prostate and breast cancers. IVW, inverse-variance weighted. (a). The association of aspartate with prostate cancer. (b). The association of aspartate with breast cancer.
Additional file 8: Fig. S8.
Leave-one-out sensitivity analysis for the effect of glutamate (5 independent SNPs with P value < 5×10−6) on prostate and breast cancers. IVW, inverse-variance weighted. (a). The association of glutamate with prostate cancer. (b). The association of glutamate with breast cancer.
Additional file 9: Fig. S9.
Single MR estimates from each of the genetic variants using MR-Egger method. The black scatter plots indicate single causal estimates from each of the genetic variants associated with serum aspartate or glutamate level on the x-axis and prostate cancer or breast cancer on the y-axis. The continuous line represents the causal effect of serum aspartate or glutamate level on prostate cancer or breast cancer. (a-b). The estimates between aspartate and cancers using 3 independent SNPs with P value < 5×10−8, (a). Prostate cancer; (b). Breast cancer. (c-d). The estimates between aspartate and cancers using 13 independent SNPs with P value < 5×10−6, (c). Prostate cancer; (d). Breast cancer. (e-f). The estimates between glutamate and cancers using 5 independent SNPs with P value < 5×10−6, (e). Prostate cancer; (f). Breast cancer.
Additional file 10: Table S1.
MR analysis using different methods for genetic associations between aspartate and prostate cancer1.
Additional file 11: Table S2.
MR analysis using different methods for genetic associations between aspartate and breast cancer1.
Additional file 12: Table S3.
MR analysis using different methods for genetic associations between glutamate and prostate cancer1.
Additional file 13: Table S4.
MR analysis using different methods for genetic associations between glutamate and breast cancer1.
Additional file 14: Table S5.
The characteristics of genome-wide association studies on the included outcomes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, Y., Yang, Z., Li, J. et al. Effects of glutamate and aspartate on prostate cancer and breast cancer: a Mendelian randomization study. BMC Genomics 23, 213 (2022). https://doi.org/10.1186/s12864-022-08442-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08442-7