Abstract
Background
Dependence on marine natural resources threatens the sustainability of Atlantic salmon aquaculture. In the present study, Atlantic salmon fed for 14 weeks with an experimental diet based on animal by-products and vegetable oil (ABP) exhibited reduced growth performance compared with others fed a fish meal/fish oil based experimental diet (MAR) and a plant protein/vegetable oil-based experimental diet (VEG). To characterize the molecular changes underlying the differences in growth performance, we conducted a 44 K microarray study of the liver transcriptome of the three dietary groups.
Results
The microarray experiment identified 122 differentially expressed features (Rank Products, PFP < 10%). Based on their associated Gene Ontology terms, 46 probes were classified as metabolic and growth-relevant genes, 25 as immune-related, and 12 as related to oxidation-reduction processes. The microarray results were validated by qPCR analysis of 29 microarray-identified transcripts. Diets significantly modulated the transcription of genes involved in carbohydrate metabolism (gck and pfkfb4), cell growth and proliferation (sgk2 and htra1), apoptosis (gadd45b), lipid metabolism (fabp3, idi1, sqs), and immunity (igd, mx, ifit5, and mhcI). Hierarchical clustering and linear correlation analyses were performed to find gene expression patterns among the qPCR-analyzed transcripts, and connections between them and muscle and liver lipid composition. Overall, our results indicate that changes in the liver transcriptome and tissue lipid composition were driven by cholesterol synthesis up-regulation by ABP and VEG diets, and the lower carbohydrate intake in the ABP group. Two of the microarray-identified genes (sgk2 and htra1) might be key to explaining glucose metabolism regulation and the dietary-modulation of the immune system in fish. To evaluate the potential of these genes as predictive biomarkers, we subjected the qPCR data to a stepwise discriminant analysis. Three sets of no more than four genes were found to be able to predict, with high accuracy (67–94%), salmon growth and fatty acid composition.
Conclusions
This study provides new findings on the impact of terrestrial animal and plant products on the nutrition and health of farmed Atlantic salmon, and a new method based on gene biomarkers for potentially predicting desired phenotypes, which could help formulate superior feeds for the Atlantic salmon aquaculture industry.
Similar content being viewed by others
Background
World aquaculture production continues to rely on the supply of fish meal (FM) and fish oil (FO) from overexploited fish stocks to feed farmed fish [1, 2]. Research on replacement of FM and FO with alternative ingredients has made notable progress since economic and ecological sustainability of aquafeeds became a growing concern for industry and consumers [2]. In recent years, traditional methods for evaluating feed performance like the monitoring of morphometric and biochemical parameters have been complemented by molecular techniques [3]. The nutrigenomic approach has facilitated the understanding of the physiological mechanisms behind impaired growth rates of fish fed diets low in marine ingredients [4]. However, the dietary modulation of some metabolic and immune pathways in fish is yet to be elucidated [4, 5], and high FM/FO replacement levels in feeds can be challenging for carnivorous fish growth performance [6], although significant advances have recently been made in this direction [7, 8].
Several different alternative ingredients to FM and FO have been tested on farmed fish [9]. Soy protein concentrate (SPC) has become an extensively used ingredient in commercial aquafeeds due to its high protein proportion and low contents of indigestible fibers and anti-nutrients, nutritional characteristics that contribute to better performance compared with plant meals [10,11,12]. Furthermore, Atlantic salmon (Salmo salar) have been found to grow well on diets with moderate replacement levels of FM by SPC [13]. Other plant ingredients with high protein content are wheat and corn glutens, which have proven to be nutritionally valuable when combined with oilseed protein sources [14, 15]. Rendered terrestrial animal products can also be used as an alternative protein source to FM [9, 16]. Meals from animal by-products often present adequate amino acid profiles compared with plant protein sources, higher levels of digestible phosphorus, and competitive prices and availability in the global market. However, use of these products is not well accepted by some consumers because of health concerns in the past (e.g., bovine spongiform encephalopathy) [9]. Nevertheless, the use of animal by-products in aquafeeds is widespread, and their quality has improved greatly since the 1980s with the refinement of the production methods [17]. Recent work on salmonids has shown that FM can be replaced at high levels by poultry by-products without affecting growth performance [18, 19]. However, in contrast to plant protein sources, there is a lack of studies on the effects of these feedstuffs on the fish transcriptome.
FO in aquafeeds can effectively be replaced at high levels by vegetable oils without significant detriment to fish growth performance [14, 20,21,22,23,24]. Despite the apparent positive results, lipid class and fatty acid profiles of vegetable oils may negatively impact the welfare of the fish and their quality as a commodity (nutritional value and flavor). Most vegetable oils are known to be devoid of omega-3 long-chain polyunsaturated fatty acids (ω3 LC-PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and to have high content of omega-6 fatty acids (ω6 FAs) [25], a combination known to be pro-inflammatory [26, 27]. Differences in diet ratios of ω6 and ω3 FAs, especially arachidonic acid (ARA)/EPA, have been found to modulate the inflammatory and immune responses of Atlantic salmon [28,29,30]. Indeed, among all farmed fish species, diet FA composition takes on particular importance for Atlantic salmon producers and consumers. Atlantic salmon is a highly valued product due to the elevated content of EPA (20:5ω3) and DHA (22:6ω3) in the flesh. Besides their anti-inflammatory properties, EPA and DHA are also essential for the proper functioning of important physiological processes in humans and fish [31,32,33]. Low FO levels in feeds can result in reduced levels of ω3 LC-PUFAs in muscle of Atlantic salmon, as salmonids have limited capacity for de novo synthesis of such metabolites [34,35,36]. This was shown recently by Sprague et al. [25] for Scottish farmed salmon: the EPA and DHA levels in salmon flesh in 2015 had decreased to almost half the values of 2006’s production. Several solutions have been proposed to break the interdependency of the use of more sustainable oil sources and the decline of the nutritional value of Atlantic salmon. The culture of transgenic fish [37, 38], and the use of transgenic plants [23] and new oil sources [35, 36, 39], have been studied. Regardless, for all of the potential paths to address the problem, a better understanding of lipid metabolism in Atlantic salmon (and fish in general) is needed.
In 2013, the main suppliers of aquafeeds for farmed Atlantic salmon (EWOS, Skretting, and BioMar) included 18% FM and 11% FO in their formulations [40]. According to FAO’s estimations [1], over the period 2016–2025 FM inclusion in salmon feeds will be around 15–10%. However, these estimations are subjected to multiple uncertainties such as adverse climatic conditions (i.e., climate change impacts), or changes in the economic and social environment [1]. Therefore, more ambitious replacement levels need to be explored. In the present study, we investigated the effects of almost completely replacing marine ingredients with terrestrial animal and plant alternatives on the liver transcriptome of Atlantic salmon. We correlated growth performance and lipid composition with the transcription of diet-responsive genes, which has allowed us to identify phenotype-predicting gene expression biomarkers. Taken together, our study not only provides new insights into the nutrition (and health) of Atlantic salmon under challenging dietary conditions, but also new tools for industry to assess the performance of future aquafeed candidates.
Methods
Experimental diets and animals
The present study was conducted using liver RNA samples from Atlantic salmon fed three experimental diets with different combinations of protein and oil sources for 14 weeks. The diets were designed by EWOS Innovation (now Cargill Innovation) and were as follows: a diet with a high proportion of FM and FO (MAR diet), a high animal by-product/high rapeseed oil diet (ABP diet), and a high plant protein/high rapeseed oil diet (VEG). Regardless of the source of the ingredients utilized, all feeds were formulated to be isonitrogenous and isoenergetic and to meet the nutritional requirements of salmonids [41]. The formulation of MAR, ABP, and VEG diets was published in previous investigations [28, 42]. However, since diet formulation is pertinent to the present study, it is provided herein (Table 1).
For the feeding trial, salmon smolts from Northern Harvest Sea Farms (Stephenville, NL, Canada) were transported to the Dr. Joe Brown Aquatic Research Building (JBARB, Ocean Sciences Centre, Memorial University of Newfoundland, Canada), and held in 3800-L tanks. For individual identification, the salmon were PIT (passive integrated transponder)-tagged. Once adapted to the holding conditions at JBARB (flow-through seawater system, 12-h light photoperiod, and water temperature of ~ 12 °C) and having reached the desired weight, the Atlantic salmon were randomly distributed in twenty-eight 620-L tanks, with 40 fish each. Fish were considered as adapted to holding conditions once accepting a feed ration of 1% wet body weight per day. After 2 weeks of acclimation to the 620-L tanks, salmon feeding switched from the commercial diet (Nutra Transfer NP, 3 mm, Skretting Canada, St. Andrews, NB, Canada) to the experimental diets. Salmon weight at the beginning of the trial was 179 ± 29 g (mean value ± standard deviation); no significant differences were found across tanks (one-way ANOVA). Four tanks were dedicated to each experimental diet, which were manually supplied to the fish to apparent satiation twice a day for 14 weeks. Apparent feed intake, and water temperature and oxygen levels, were recorded daily. Salmon weight was measured at the beginning and at the end of the feeding trial to monitor differences in growth performance.
Tissue collection and sample selection
At the end of the trial, salmon were starved for 24 h and then five individuals per tank were euthanized with an overdose of MS-222 (400 mg/L; Syndel Laboratories, Vancouver, BC, Canada) and dissected for tissue collection. After collection, liver samples of 50–100 mg were immediately flash-frozen in liquid nitrogen and stored at − 80 °C until processing for RNA extraction. Liver and muscle samples for the determination of lipid classes and fatty acid composition were collected, processed, and analyzed as described in Hixson et al. [36].
Biological variability can strongly determine fish performance on a given diet, and thus complicate the finding of common dietary-related transcriptomic patterns among specimens. For this reason, we decided only to utilize liver samples from salmon showing growth performances close to the tank average. Therefore, we selected samples from those fish with weight gains within one standard deviation below and above the mean value of the tank. Tank average and not diet average was chosen for sample selection so that inter-tank variability could be included in our statistical contrasts.
RNA extraction, DNase treatment, and column purification
Liver samples were homogenized in TRIzol Reagent (Invitrogen/Life Technologies, Burlington, Canada) and subjected to RNA extraction as described previously in Xue et al. [35] and Xu et al. [43]. Thirty micrograms of each total RNA sample were treated with 6.8 Kunitz units of DNaseI (RNase-Free DNase Set, QIAGEN) with the manufacturer’s buffer (1X final concentration) at room temperature for 10 min to degrade any residual genomic DNA. DNase-treated RNA samples were column-purified using the RNeasy Mini Kit (QIAGEN) following the manufacturer’s instructions. RNA integrity was verified by 1% agarose gel electrophoresis, and RNA purity was assessed by A260/280 and A260/230 NanoDrop UV spectrophotometry (NanoDrop, Wilmington, DE, USA) at all stages of the RNA preparation (i.e., after Trizol-extraction, after re-extraction, and after column-purification). Column-purified RNA samples had A260/280 ratios between 2.1 and 2.3 and A260/230 ratios between 1.9 and 2.4.
Microarray hybridization and data acquisition
Each of the three dietary groups (MAR, ABP, and VEG) contributed liver RNA samples of eight individual fish (two from each quadruplicate tank) to the microarray experiment (i.e., 24 fish in total). Differences among dietary groups in the liver transcriptome of fish were assessed by contrasting individual RNA samples against a common reference pool of equal quantities of RNA from all 24 fish. Twenty-four arrays were used for the experiment: one array for each individual/common reference RNA hybridization. The microarray experiment was performed as described in Xue et al. [35]. Briefly, anti-sense amplified RNA (aRNA) was in vitro transcribed from 1 μg of each experimental RNA or reference RNA pool using Ambion’s Amino Allyl MessageAmp™ II aRNA Amplification kit (Life Technologies), following the manufacturer’s instructions. The quality and quantity of aRNA were assessed using NanoDrop spectrophotometry and agarose gel electrophoresis. Twenty micrograms of aRNA were subsequently precipitated overnight following standard molecular biology procedures and re-suspended in coupling buffer. Individual aRNA samples were labeled with Cy5, whereas reference aRNA samples were labeled with Cy3 (GE HealthCare, Mississauga, ON), following the manufacturer’s instructions. The labeling efficiency was measured using the “microarray” function of the NanoDrop spectrophotometer. For each array, an equal quantity (825 ng) of an individual Cy5-labeled and a reference Cy3-labeled aRNA sample were fragmented and co-hybridized to a consortium for Genomic Research on All Salmonids Project (cGRASP)-designed Agilent 44 K salmonid oligonucleotide microarray (GEO accession number: GPL11299) [44], following manufacturer instructions (Agilent, Mississauga, ON). The arrays were hybridized at 65 °C for 17 h with 10 rpm rotation in an Agilent hybridization oven. The array slides were washed immediately after hybridization as per the manufacturer’s instructions.
The microarray slides were scanned at 5 μm resolution with 90% laser power using a ScanArray Gx Plus scanner and ScanExpress v4.0 software (Perkin Elmer, Waltham, Massachusetts, USA), and the Cy3 and Cy5 channel photomultiplier tube (PMT) settings were adjusted to balance the fluorescence signal. The raw data contained in the TIFF images resulting from the scanning were extracted using Imagene 9.0 (BioDiscovery, El Segundo, California, USA). The removal of low-quality/flagged spots on the microarray, as well as the background signal correction, the log2-transformation and Loess-normalization of the data, were performed using R and the Bioconductor package mArray [45]. Features absent in more than 30% of the arrays were discarded, and the missing values were imputed using the EM_array method and the LSimpute package [35, 46, 47]. The final dataset used for statistical analyses consisted of 11,149 probes for all arrays (GEO accession number: GSE106604; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE106604).
Microarray data analysis
The differentially expressed genes among diets were determined using Rank Products (RP), a non-parametric statistical method that is less sensitive to high biological variability than Significance Analysis of Microarrays (SAM) [47,48,49,50]. RP analysis was conducted at a percentage of false-positives (PFP) threshold of 10%, using the Bioconductor package, RankProd [51]. The resulting gene lists were annotated using the contiguous sequences (contigs) employed to design the 60mer oligonucleotide probes of the array [44]. Annotation was carried out manually with BLASTx/BLASTn searches against the NCBI non-redundant (nr) amino acid and nucleotide sequence databases using an E-value threshold of 10− 5. The best BLASTx hits corresponding to putative Danio rerio or Homo sapiens orthologues were used to obtain gene ontology (GO) terms from the UniProt Knowledgebase (http://www.uniprot.org/).
qPCR analysis
We selected 29 genes of interest (GOIs) from the list of microarray-identified genes for real-time quantitative polymerase chain reaction (qPCR) analysis. Gene selection was based on the different metabolic pathways or biological processes represented among the diet-responsive identified genes. Three more GOIs not identified by the microarray analysis (i.e., acyl-coenzyme A oxidase 1, carnitine palmitoyltransferase 1, and ATP-citrate synthase) were added to the qPCR experiment. For the qPCR analysis, we also included seven additional RNA samples from the three dietary treatments (two from MAR group, two from ABP, and three from VEG; 31 samples in total), all of them satisfying the criterion for sample selection (see above in ‘Tissue collection and sample selection’ section).
For each of the selected GOIs, we BLASTn-searched for paralogues using published Atlantic salmon cDNA sequences in the non-redundant nucleotide (nt) sequence and the expressed sequence tags (EST) databases of NCBI. BLASTn-searches were performed between October and November, 2015, and thus limited to the sequences available during that period. Putative paralogue sequences were compiled and aligned using Vector NTI (Vector NTI Advance 11, Life Technologies) to determine identity between paralogues and find suitable regions where to design paralogue-specific qPCR primers (Additional file 1). Paralogue-specific primers were intended to amplify areas with at least 2 bp difference between sequences to ensure specificity. Primers not complying with this rule (i.e., 1 bp difference) were paired with primers targeting more heterogeneous areas of the cDNA sequence. All primers were designed using Primer 3 v.0.4.0 software [available at (http://bioinfo.ut.ee/primer3-0.4.0/)]. Each primer pair was quality tested using the 7500 Fast Real Time PCR system (Applied Biosystems/Life Technologies). Quality testing ensured that a single product was amplified (dissociation curve analysis) and that there was no primer-dimer present in the no-template control. Amplicons were electrophoretically separated on 2% agarose gels and compared with a 1 kb Plus DNA Ladder (Invitrogen/Life Technologies) to verify that the correct size fragment was being amplified. Amplification efficiencies [52] were determined using 5-point 1:3 dilution series starting with cDNA representing 10 ng of input total RNA. For primer quality testing, a reference RNA pool was prepared with an equal contribution of all specimens included in the qPCR study.
Transcript (mRNA) levels of the GOIs were normalized against two endogenous control genes. To select these endogenous controls, qPCR primer pairs were designed for six candidate normalizers and quality tested as described above. Two of the candidate normalizers genes were selected as they were among the most stable transcripts represented in our 11,149 microarray probe dataset: transmembrane protein 85, and ATPase H+ transporting V1 subunit E1. The other candidate normalizers were chosen based on Atlantic salmon literature [53] and previous studies of our group [35] (60S ribosomal protein L32, β-actin, and two paralogues of elongation factor 1-alpha). The fluorescence threshold cycle (CT) values of the 31 samples were measured for each of these genes using cDNA representing 5 ng of input total RNA and then analyzed using geNorm (qBASE plus, Biogazelle NV, Belgium) [54]. Using this software, 60S ribosomal protein L32 (rpl32; geNorm M = 0.255) and elongation factor 1-alpha 1 (eef1a1; geNorm M = 0.250) were determined to be the most stable.
First-strand cDNA templates for qPCR were synthesized in 20 μL reactions from 1 μg of DNaseI-treated, column-purified total RNA using random primers (250 ng; Invitrogen/Life Technologies) and M-MLV reverse transcriptase (200 U; Invitrogen/Life Technologies) with the manufacturer’s first strand buffer (1X final concentration), dNTPs (0.5 mM final concentration), and DTT (10 mM final concentration) at 37 °C for 50 min. Once primer quality testing was completed, the transcript levels of the selected GOIs and normalizer genes were qPCR-analyzed in technical triplicates using Power SYBR Green I dye chemistry in 384-well format on a ViiA 7 Real Time PCR system (Applied Biosystems/Life Technologies, Foster City, CA). In all qPCR analyses, a no-template control (in triplicate) was included in the plate. The qPCR amplifications were performed in 13 μL reactions using 1X Power SYBR Green PCR Master Mix (Applied Biosystems/Life Technologies), 50 nM of both the forward and reverse primers, and 4 μL of diluted cDNA (5 ng input total RNA). The qPCR program consisted of 1 cycle of 50 °C for 2 min, 1 cycle of 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min, with fluorescence detection at the end of each 60 °C step.
The relative quantity (RQ) of each transcript was determined using the ViiA 7 Software Relative Quantification Study Application (Version 1.2.3) (Applied Biosystems/Life Technologies), with normalization to both rpl32 and eef1a1 transcript levels, and with amplification efficiencies incorporated. For each GOI, the sample with the lowest normalized expression (mRNA) level was set as the calibrator sample (i.e., assigned an RQ value = 1).
Fold-change values calculated from microarray log2 ratios, and log2-transformed qPCR RQs, were analyzed for correlation via linear regression (see below). The formula used to calculate gene expression fold-changes between a dietary group (A) and another (B) was 2A-B [55, 56]. For down-regulated genes, fold-change values were inverted (− 1/fold-change). A significant correlation between both datasets was considered as proof of the validity of the microarray results.
Statistical analyses
Comparing mean values among dietary groups
GOI RQs, growth performance (i.e., weight gain and AFI) and tissue composition (i.e., lipid class and FA profiles) data for one-way ANOVA were first checked for outliers using Grubb’s test, and for homoscedasticity using Levene’s test. The ‘Tank’ effect was nested to ‘Diet’ factor in the one-way ANOVA. If variances were found not to be statistically equal among dietary groups (failure to comply with the homoscedasticity assumption), Games-Howell tests were used for pair-wise comparisons. If the homoscedasticity assumption was satisfied, the mean values of the three dietary treatments were compared using Tukey’s post hoc test. The accepted level of significance was p < 0.05. All the statistical analyses above were conducted using IBM SPSS Statistics (version 24.0.0; IBM Corp, Armonk, NY).
Linear regression analyses and hierarchical clustering
Differences in dietary lipid composition could lead to contrasted tissue lipid class and FA profiles, as well as changes in the liver transcriptome of the salmon. Therefore, we analyzed the relationships between all three datasets (qPCR results, and tissue lipid class and FA composition) for each individual fish by linear regression analysis. In this regression analysis, we included only GOIs showing significant differences among diets, sterols based upon our transcriptomic data, and those ω3 and ω6 FAs accounting for at least 0.5% of the total FAs in the tissue. Fish weight gain and other relevant tissue composition parameters (e.g., EPA/ARA ratios) were also included. To facilitate the discussion of the possible correlation patterns, GOIs and tissue composition parameters were grouped by hierarchical clustering. The methods and results of these tissue composition analyses are published elsewhere [42].
Differences in feed intake can have a significant effect on fish gene expression [57,58,59]. Therefore, we analyzed the possible correlation between the qPCR results and AFI values of each tank (as AFI cannot be calculated for each individual fish) by linear regression.
For linear regression analyses and hierarchical clustering, all datasets were log2-transformed to meet the normality assumption (checked by Kolmogorov-Smirnov test). The linear regression analyses were performed with IBM SPSS Statistics, whereas the complete linkage hierarchical clustering was carried out with PRIMER (Version 6.1.15, Ivybridge, UK) using Pearson correlation resemblance matrices. The significance of the regression was determined by an F-test (p < 0.05).
Stepwise discriminant analysis
In addition to the statistical analyses described above, we analyzed our qPCR results in search of potential phenotype-predictive biomarkers. A stepwise discriminant analysis was deemed to be the best method. Discriminant analysis linearly combines variables into predictive functions to classify experimental subjects between different levels of a categorical variable. Discriminant scores are calculated using the predictive functions and used to classify individuals based on cut-off values determined by maximum likelihood [60]. As categorical variables, we considered ‘Diet’ (MAR, ABP, and VEG), ‘Growth’ (weight gain; low, and high), and the sum of DHA and EPA levels in the muscle (‘EPA + DHA’; low, and high). The number of individual samples available did not allow the division of ‘Growth’ and ‘EPA + DHA’ values in more than two levels. In both cases, values above the mean across individuals were denoted as ‘high’, whereas those values below the mean were ‘low’. The number of predictive functions that a discriminant analysis can generate equals the number of levels of the categorical variable minus 1. We chose the stepwise method for the selection of the predictor variables (i.e., diet-responsive GOIs). Thus, GOIs were entered provided the predictive ability of the function (measured as Wilks’ lambda statistic) was significantly improved (minimum partial F-to-enter = 3.84 [61]), and removed if significantly reduced (maximum partial F-to-remove = 2.81). The accuracy of the predictive functions was assessed using a leave-one-out classification method. This method classifies each individual by the functions derived for all subjects except the one being classified [62]. In this way, we obtain a less biased estimate of the true predictive ability of the functions. Only log2-transformed RQ values of diet-responsive transcripts were included in the analysis. We used IBM SPSS Statistics to conduct the stepwise discriminant analyses.
Results
Diet effect on fish growth performance
The feeding trial concluded with salmon fed MAR diet showing significantly higher final weight and weight gain than ABP diet (Table 2). In contrast, based on final weight and weight gain, VEG diet performed similarly to MAR diet. However, the hepatosomatic index (HSI) was significantly higher in fish fed MAR diet than in fish fed VEG diet. Salmon fed ABP diet showed a trend towards lower apparent feed intake (AFI), but the differences among group mean values were not statistically significant (p = 0.069). Results in Table 2 were previously published [28, 42]. However, they are included herein as also pertinent to this study, as were used in the selection of the three dietary groups for the transcriptomic analysis.
Microarray profiling of fish liver transcriptome
One hundred and twenty-two differentially expressed probes were detected in the microarray analysis of the liver transcriptome of fish fed the three experimental diets (Additional file 2). Based on their associated Gene Ontology (GO) terms and the information available in the literature, 83 BLAST-identified and functionally characterized probes were classified as genes involved in metabolic pathways (e.g., carbohydrate metabolism, lipid metabolism) and biological processes (e.g., autophagy, antibacterial response) (Tables 3, 4, 5 and 6). The other 39 differentially expressed probes did not fall into any of these categories or encoded putative unknown or uncharacterized proteins and, therefore, were classified as miscellaneous and unknown (see Additional file 2).
Twenty-eight microarray probes putatively involved in nutrient metabolism were identified (Table 3). Among them, 7 probes were classified as involved in carbohydrate metabolism (e.g., glucokinase), 17 probes related to lipid metabolism (e.g., fatty acid-binding protein, heart; alias: fatty acid-binding protein 3), and 4 probes related to nucleotide metabolism (e.g., adenylosuccinate synthetase). Eighteen probes were classified as involved in cellular processes (Table 4): 5 related to protein synthesis and degradation (e.g., trypsin), 10 related to cell growth and proliferation (e.g., serine/threonine-protein kinase Sgk2), and 3 related to autophagy and apoptosis (e.g., serine/threonine-protein kinase ULK4). A list of 24 immune-related microarray probes was generated (Table 5), comprising 9 probes classified as antibacterial (e.g., leukocyte cell-derived chemotaxin 2), 2 as antiviral (e.g., interferon-induced GTP-binding protein Mx), and 13 as involved in other immune processes (e.g., major histocompatibility complex class I). Finally, the microarray experiment also identified 12 probes putatively related to oxidation-reduction processes, and therefore could serve as indicators of oxidative stress (Table 6).
Overall, microarray features identified as genes putatively related to carbohydrate metabolism were down-regulated in salmon fed the non-marine based diets (i.e., ABP and VEG diets) compared with those fed MAR diet (Table 3). Of the genes related to lipid metabolism, those involved in the metabolism of fatty acids and triacylglycerols (e.g., diacylglycerol O-acyltransferase 2, acetyl-CoA carboxylase) showed down-regulation by the non-marine diets, contrary to those participating in the synthesis of cholesterol (e.g., squalene synthase), which showed up-regulation by the same diets (Table 3). A feature encoding putative heart-type fatty acid-binding protein (fabp3), an intracellular lipid transport protein, and putative apolipoprotein B-100, an extracellular lipid transport protein, showed contrasted dietary modulation. The first, fabp3, was significantly down-regulated by VEG diet compared with MAR and ABP diets, while apolipoprotein B-100 was down-regulated by ABP diet compared with MAR diet, thus resulting in an apparent higher transcription with VEG diet than with ABP diet. The microarray-identified genes related to nucleotide synthesis were mostly down-regulated by the non-marine diets.
According to the results of the microarray analysis, genes associated with proteolysis and protein synthesis (Table 4) were, in general, up-regulated by the non-marine diets. However, 60S ribosomal protein L7 transcript levels showed down-regulation by the non-marine diets, this down-regulation being statistically significant for ABP diet. Similarly, microarray-identified genes related to cell growth were largely down-regulated by non-marine diets. Three genes annotated with GO terms for cell proliferation showed opposed trends as two were up-regulated (i.e., protein regulator of cytokinesis 1-like isoform X1 and pre-mRNA-splicing factor syf2) while the other one was down-regulated (i.e., cbp/p300-interacting transactivator 2-like) by the non-marine diets. Among the genes putatively associated with apoptotic processes, serine/threonine-protein kinase ULK4 and growth arrest and DNA-damage-inducible protein GADD45 beta showed a trend towards up-regulation by ABP diet.
Regarding the immune-related genes (Table 5), five of those classified as antibacterial were down-regulated by non-marine diets. However, four features identified as immunoglobulins, also classified as antibacterial, were up-regulated by ABP diet. The two putative antiviral genes identified in the microarray experiment (i.e., mxb and ifit5) presented up-regulated transcript levels in fish fed VEG diet. The transcript levels of the genes putatively related to other immune pathways showed trends similar to either that of the antibacterial (e.g., alpha-1-acid glycoprotein 1, glucosidase 2), the immunoglobulins (e.g., dedicator of cytokinesis protein 11), or the antiviral genes (e.g., major histocompatibility complex class I). Of the four features annotated as major histocompatibility complex class I (mhcI), one showed an opposite trend, showing down-regulated transcript levels in the VEG group (probe ID C027R162). Given that the probe was designed based on a Salmo trutta cDNA sequence (best BLASTn hit is a Salmo trutta mhcI UBA allele; GenBank accession number AM262751) and the highly polymorphic nature of the mhcI genes, it is difficult to assert if observed expression discrepancies are due to differential paralogue regulation or to the specificity of the microarray probe.
The microarray features identified as transcripts involved in oxidation-reduction processes (Table 6) displayed contrasted expression patterns. Some transcripts encoding oxidizing enzymes showed up-regulation in salmon fed ABP diet compared with MAR or VEG diets (e.g., cytochrome oxidase subunit I, glycine dehydrogenase). By contrast, others were downregulated by ABP diet compared with MAR or VEG diets (e.g., glutathione S-transferase theta-2B). On the other hand, the expression levels of cytochrome P450 1A1 and other transcripts were significantly reduced by VEG diet compared with MAR diet.
qPCR validation
Fold-change values (FCs) calculated from microarray log2 ratios, and log2-transformed qPCR RQ ratios, showed a significant correlation (Fig. 1). The main deviation from linearity consisted of a group of dots corresponding to microarray-derived FCs between − 2 and − 1 and qPCR-derived FCs between 1 and 2. A closer look at these FCs revealed that most of them pertained to VEG vs MAR (six out of 12) and VEG vs ABP (five out of 12) comparisons. None of the genes was especially represented among those FCs. The sum of the potential divergences between microarray and qPCR results (see Discussion), and the inclusion of more biological replicates in the qPCR study, would likely be behind this deviation.
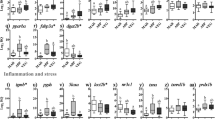
The qPCR results showed that feeding ABP diet resulted in significantly lower transcript levels of gck and pfkfb4 compared with MAR diet (Fig. 2A). In addition, the qPCR analysis showed a significant down-regulation of gck transcription by ABP diet compared with VEG diet. No significant differences among dietary treatments were found in the transcription of 6pgd and acac (Fig. 2A). Nevertheless, adssl1a transcription was significantly down-regulated by non-marine diets (Fig. 2A).
Results from the qPCR analysis of selected transcripts involved in carbohydrate (A: gck, pfkfb4, 6pgd), nucleotide (A: adssl1a), and lipid metabolism (A: acac, dgat2a, dgat2b; B: fabp3a, fabp3b, acox1, cpt1, acly, idi1, sqs). Columns and error bars represent mean relative quantity (RQ) values and S.E., respectively. Different letters above error bars represent significant differences between diets [one-way ANOVA, Tukey’s (homogeneity of variances among groups) or Games-Howell (variances not homogeneous across groups) post-hoc test, p < 0.05]. Gene abbreviations (below their corresponding columns) are according to UniProt terminology
The qPCR experiment did not find significant changes in dgat2a transcription among dietary groups (Fig. 2A). However, dgat2b showed a trend towards lower transcript levels in the liver of salmon fed non-marine diets (Fig. 2A; p = 0.09). The qPCR analysis also showed that ABP diet significantly up-regulated the transcript levels of fabp3a and fabp3b compared with MAR diet (Fig. 2B). Neither acox1, nor cpt1 were found to be significantly modulated by the diets (Fig. 2B). In contrast, the qPCR analysis found up-regulated acly, idi1 and sqs hepatic mRNA levels in salmon fed VEG diet compared with those fed MAR diet (Fig. 2B).
The qPCR analysis of sgk2a and sgk2b mRNA levels confirmed their down-regulation by ABP diet compared with MAR and VEG diets (Fig. 3). Both htra1 paralogues were down-regulated by VEG diet as compared with ABP (only htra1a) and MAR (both). Similarly, gadd45ba and gadd45bb transcript levels were down-regulated by VEG diet compared with ABP diet.
Results from the qPCR analysis of selected transcripts involved in cell growth and proliferation (sgk2a, sgk2b, htra1a, htra1b), and apoptosis (gadd45ba, gadd45bb). Columns and error bars represent mean relative quantity (RQ) values and S.E., respectively. Different letters above error bars represent significant differences between diets [one-way ANOVA, Tukey’s (homogeneity of variances among groups) or Games-Howell (variances not homogeneous across groups) post-hoc test, p < 0.05]. Gene abbreviations (below their corresponding columns) are according to UniProt terminology
According to the qPCR results, lect2a transcription was not responsive to diet (Fig. 4A). Although not statistically significant, lect2b mRNA levels seemed to be down-regulated by the non-marine diets (Fig. 4A; p = 0.14). Igma, igmb, and igd showed a similar trend towards up-regulation by ABP, which was only statistically significant for igd as compared with the results of MAR-fed salmon (Fig. 4A). The expression of the antiviral transcripts mxa, mxb, and ifit5 was significantly increased by VEG diet compared with ABP diet (Fig. 4B). VEG diet also up-regulated mhcI transcription as compared with salmon fed MAR diet (Fig. 4B). The transcripts representative of the oxidation-reduction processes in the qPCR experiment, mtco1, mtco2a, and mtco2b, did not show significant expression differences among dietary groups (Fig. 4B).
Results from the qPCR analysis of selected antibacterial (A: lect2a, lect2b, igma, igmb, igd), antiviral (B: mxa, mxb, ifit5), and other immune-related (A: mhcI) transcripts, as well as transcripts involved in oxidation-reduction processes (A: mtco1, mtco2a, mtco2b). Columns and error bars represent mean relative quantity (RQ) values and S.E., respectively. Different letters above error bars represent significant differences between diets [one-way ANOVA, Tukey’s (homogeneity of variances among groups) or Games-Howell (variances not homogenous across groups) post-hoc test, p < 0.05]. Gene abbreviations (below their corresponding columns) are according to UniProt terminology
For more details on DNA sequence identity between paralogues, and between genes and 60-mer microarray probes and their related contigs, see Additional file 2. For supporting information on paralogue-specific primer design, see Additional files 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12.
Correlations between gene expression and liver fatty acids
Hierarchical clustering segregated the qPCR-validated genes of interest (GOIs) into four groups (Fig. 5). Gadd45ba, gadd45bb, htra1a, and htra1b constituted the first cluster and were all repressed in fish fed diet VEG. The second cluster comprised all those genes related to lipid metabolism, and thus, they are referred to as ‘lipid metabolism-related’ genes. Genes directly or indirectly involved in the metabolism of carbohydrates were grouped in Cluster III and referred to as ‘carbohydrate metabolism-related’ genes. All immune-related genes were grouped in Cluster IV.
Matrix representing Pearson’s correlation coefficients between log2-transformed RQs of the qPCR-analyzed transcripts (rows) and the log2-transformed levels of different lipid composition parameters (columns) in the liver of salmon fed the experimental diets. Significant differences among diets (one-way ANOVA, p < 0.05) are indicated on the right of the transcript abbreviations and below the phenotypic variables. Transcriptomic and phenotypic data were arranged based on a hierarchical clustering performed using Pearson correlation resemblance matrices (PRIMER, Version 6.1.15, Ivybridge, UK). Dendrograms next to transcript abbreviations and above lipid parameters represent the results of the hierarchical clustering analyses. EPA: eicosapentaenoic acid (20:5ω3); ARA: arachidonic acid (20:4ω6); SFA: saturated fatty acids; PUFA: polyunsaturated fatty acid; DHA: docosahexaenoic acid (22:6ω3)
Liver FA levels and ratios were segregated into three major groups by hierarchical clustering (Fig. 5). The ω3 LC-PUFAs EPA (20:5ω3), 22:5ω3, and DHA (22:6ω3), together with EPA/ARA, the ω3/ω6 ratio, and the sum of saturated FAs (∑SFA) constituted Cluster I. All these parameters showed significantly higher values in the liver of salmon fed MAR diet. In contrast, omega-6 (ω6) LC-PUFAs (i.e., 20:2ω6, linoleic acid [18:2ω6], 20:3ω6, and ARA [20:4ω6]) and α-linolenic acid (18:3ω3) in Cluster II were present at lower levels in the liver of fish fed MAR diet, although differences were only statistically significant for 20:3ω6 (for MAR vs ABP and VEG) and ARA (for MAR vs ABP). Hepatic sterol levels and PUFA/SFA, and DHA/EPA ratios were grouped in Cluster III as values tended to be higher in salmon fed VEG diet, and lower in those fed MAR diet. This trend was statistically significant for hepatic PUFA/SFA and DHA/EPA ratios, but not for sterol levels.
The transcription of gadd45ba, gadd45bb, and htra1a showed negative correlation with 20:2ω6 (Fig. 5). Also, htra1a and htra1b were inversely proportional to liver 20:3ω6 levels. The hepatic mRNA levels of acly, idi1, sqs, and fabp3a correlated negatively with ω3/ω6. The transcription of acly, idi1, and sqs and the hepatic levels of EPA, 22:5ω3, and DHA were also negatively correlated. Separately, idi1, and sqs transcript levels correlated negatively with ∑SFA, and positively with DHA/EPA. Idi1, sqs, and fabp3a showed negative correlation with EPA/ARA and positive correlation with sterols. Among the carbohydrate metabolism-related genes, sgk2b showed transcript levels that correlated negatively with ∑SFA, 20:2ω6, 18:2ω6, 18:3ω3, and 20:3ω6, whereas those of sgk2a were inversely correlated with DHA. Gck transcript levels correlated negatively with hepatic ARA levels. Overall, the transcription of the immune-related genes (i.e., igd, mhcI, mxb, mxa, and ifit5) seemed to correlate negatively with FAs levels and ratios of Cluster I, and positively with those of Clusters II and III. However, the trends above were significant only in some cases. The mRNA levels of igd and mhcI were significantly positively correlated with ARA hepatic levels. The transcript levels of both mx paralogues showed significant positive correlation with PUFA/SFA, and those of mxb correlated negatively with liver ω3/ω6. The liver levels of ifit5 transcripts and 20:2ω6 were significantly and positively correlated.
Correlations between gene expression and muscle fatty acids
Three major groups could be identified after the hierarchical clustering of muscle FA levels and ratios (Fig. 6). Cluster I is comprised of PUFA/SFA and DHA/EPA ratios, both of them showing higher values in the muscle of salmon fed ABP diet compared with those fed MAR diet. All individual FAs included in the analysis and ∑SFA were grouped in Cluster II, and all showed a trend towards higher values with MAR diet than with ABP diet, a trend that was significant in all cases but for 18:2ω6 and ARA. Similarly, muscle ω3/ω6 and EPA/ARA ratios, and sterol levels in Cluster III were increased with MAR diet compared with VEG diet, and compared to ABP diet in the case of sterols and EPA/ARA.
Matrix representing Pearson’s correlation coefficients between log2-transformed RQs of hepatic transcripts (rows) and phenotypic features such as omega-3 and omega-6 FAs levels in muscle and weight gains (columns) of the salmon fed the experimental diets. Significant differences among diets (one-way ANOVA, p < 0.05) are indicated on the right of the transcript abbreviations and below the phenotypic variables. Transcriptomic and phenotypic data were arranged based on a hierarchical clustering performed using Pearson correlation resemblance matrices (PRIMER, Version 6.1.15, Ivybridge, UK). Dendrograms next to transcript abbreviations and above phenotypic parameters represent the results of the hierarchical clustering analysis. PUFA: polyunsaturated fatty acid; SFA: saturated fatty acids; DHA: docosahexaenoic acid (22:6ω3); EPA: eicosapentaenoic acid (20:5ω3); WG: weight gain; ARA: arachidonic acid (20:4ω6)
The expression of gadd45b and gadd45b correlated negatively with most FA parameters in Cluster II (all except 22:5ω3 and DHA for gadd45ba; Fig. 6). In contrast, htra1a and htra1b correlated positively with muscle EPA and DHA levels in Cluster II, as well as with muscle FA parameters in Cluster III. The transcription of the lipid metabolism-related genes (i.e., idi1, sqs, fabp3a, and fabp3b) in the liver of salmon correlated negatively with muscle parameters in Cluster III. Also, fabp3a transcript levels correlated negatively with many of the ω3 FAs (i.e., 20:4ω3, EPA, 22:5ω3, and DHA) and ∑SFA in Cluster II, and positively with DHA/EPA in Cluster I. Of the carbohydrate metabolism-related genes, the hepatic mRNA levels of adssl1a, gck, and sgk2a correlated positively with all muscle FA parameters in Cluster II, except for ARA, which showed significant correlations only with adssl1a and sgk2a. Regarding the connection between immune-related genes and muscle FA composition: hepatic igd transcript levels correlated negatively with muscle sterol and EPA/ARA; those of mhcI showed negative correlation with 20:4ω3 and 22:5ω3; and mxb transcription was inversely correlated with ω3/ω6. Interestingly, weight gain (WG) values correlated positively with several carbohydrate metabolism-related (i.e., sgk2b, pfkfb4, gck, sgk2a) and immune-related genes (i.e., mxa, mxb, ifit5), and negatively with the gadd45b paralogues.
Correlation between apparent feed intake, weight gain, and gene expression
As indicated before, differences among dietary groups in apparent feed intake (AFI) were not statistically significant (p = 0.069, Table 2). However, AFI showed to be highly and positively correlated with salmon weight gain (r = 0.94, p < 0.0001; Additional file 13). In addition, AFI was negatively correlated with the transcript levels of igd (r = − 0.71, p = 0.009) and fabp3a (r = − 0.65, p = 0.022). Conversely, tank AFI values correlated positively with WG (r = 0.94, p < 0.0001), and the levels of the carbohydrate metabolism-related transcripts sgk2b (r = 0.65, p = 0.022) and gck (r = 0.74, p = 0.006), as well as with those of the immune-related transcripts mxa (r = 0.59, p = 0.044) and ifit5 (r = 0.65, 0.022).
Gene biomarker identification by discriminant analysis
The stepwise discriminant analysis of the qPCR data generated two functions capable of classifying fish individuals among dietary groups with a 90% accuracy (cross-validated, Table 7). The diet-discriminating functions were constructed using expression data of sqs, gck, gadd45ba, sgk2b, and fabp3a. On the other hand, the analysis produced a function capable of discriminating salmon with high or low growth performance with a 94% accuracy. The growth-discriminating function included sgk2b, gck, mxa, and fabp3b. Another function to discriminate fish with high or low muscle EPA + DHA levels was obtained using fabp3a and igd transcript levels. This latter function was less accurate (67%) than the diet- and growth-discriminating functions, but had a significant discriminant ability (χ2, p < 0.05).
Discussion
Replacing FM and FO with terrestrial alternative ingredients affected the liver transcriptome of Atlantic salmon. Moreover, our findings indicate that feed formulation had an impact not only on liver transcripts related to nutrient metabolism [e.g., glucokinase (gck), squalene synthase (sqs)] but also on immune-related transcripts [e.g., interferon-induced protein with tetratricopeptide repeats 5 (ifit5)]. Changes in the liver transcriptome with replacement of FM by plant protein sources have been reported in previous studies on Atlantic salmon [63, 64] and other fish species [65]. FO replacement by vegetable oils had also been found to modulate gene expression in the liver of Atlantic salmon [23, 36, 66, 67]. As could be expected, the combination of both FM and FO replacement by plant products also results in an adaptation of the liver transcriptome of Atlantic salmon [35] and rainbow trout [68]. In the present study, the experimental diets proposed as potential substitutes to the conventional marine-based (i.e., ABP and VEG diets) were formulated with rapeseed oil as the main source of dietary lipids and different proportions of plant and animal by-product meals as the main source of dietary proteins. To date, little is known about the effect of replacing FM by animal by-products on the fish liver transcriptome. Liland et al. [69] analyzed through qPCR the effects of feeding Atlantic salmon feeds formulated with a mix of animal by-products on the transcription of a selection of metabolic and immune-related genes. They did not detect significant changes in the expression of any of the selected genes. To the best of our knowledge, ours is the first experiment conducted to explore the liver transcriptome of Atlantic salmon fed high levels of protein from animal by-products.
The transcript levels of some genes (e.g., 6pgd, acac, and igma) showed high variability among fish individuals of the same dietary treatment, which could be a major source of mismatch between microarray and qPCR fold-changes, as the sample size was increased for the qPCR analyses. Other genes (e.g., gck, sgk2a, and ifit5) were less affected by inter-individual variability or were more sensitive to dietary modulation, and showed better microarray-qPCR correlation. Technical limitations of the microarray platform could also explain discrepancies between qPCR and microarray results. As discussed by Booman et al. [45], one of these limitations could be the lower specificity of the microarray probe compared with that of the qPCR primers. This could be the case for those microarray features designed using cDNA sequences from other salmonid species, such as those targeting mtco1, and mtco2 transcripts. Another of the limitations listed by Booman et al. [45] is that the microarray probe and qPCR primers may target different regions of the mRNA, which occurs with most of the qPCR-analyzed genes in the present study (see Additional files 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12). On this point, it should be noted that paralogue-specific analyses restrict the suitable nucleotide regions for primer design (see Methods). Finally, the possibility of contig misassembly should be considered as a potential source of conflict between microarray and qPCR results [45].
Effects of terrestrial ingredients on liver lipid metabolism: promoted cholesterol biosynthesis
The transcription of genes involved in the metabolism of carbohydrates and lipids in the liver was modulated significantly, thus showing adaptation of the hepatic molecular machinery to the different nutritional conditions. Further, the nutritional composition of the feeds based on terrestrial products appeared to be metabolically challenging, especially for lipid metabolism. Numerous studies have shown cholesterol biosynthesis in fish is affected by dietary replacement of marine ingredients by vegetable alternatives [36, 67, 70,71,72,73,74]. In our study, the transcripts involved in the synthesis of cholesterol (i.e., idi1 and sqs) were significantly up-regulated by VEG diet. A moderate increase in their transcription was also observed in fish fed ABP diet, but it was only statistically significant in the microarray experiment. Up-regulation of idi1 transcription in the liver of Atlantic salmon fed vegetable oils was previously reported [70]. Cholesterol is the main sterol in animal fats and oils [75]. In contrast, in vegetable oils like the rapeseed oil of the non-marine diets ABP and VEG, sterols consist mainly of phytosterols, which are known to impede normal cholesterol absorption through the intestine of fish and mammals [75,76,77]. In response to this impaired absorption, the hepatic metabolism of Atlantic salmon promotes the biosynthesis of cholesterol [67, 78]. Higher hepatic idi1 and sqs transcript levels in salmon fed ABP and VEG diets would agree with the higher inclusion of rapeseed oil and thereby higher phytosterol levels in those diets. Other anti-nutrients present in plant meals (e.g., oligosaccharides and saponins) can reduce bile acid reabsorption by the intestine by inducing enteritis, increasing intestinal content viscosity, or by directly binding or trapping bile acids [11, 67, 79]. Decreased intestinal bile acid reabsorption drives an increased de novo synthesis in the liver, that requires cholesterol molecules as a precursor, thus increasing the metabolic demand for cholesterol [80]. No transcripts related to bile acid synthesis were detected in the present microarray experiment. In this regard, it should be noted that the vegetable protein sources chosen for our experimental diets were soy protein concentrate and glutens from wheat and corn, which contain low levels of anti-nutrients compared with other plant meals.
The up-regulation of cholesterol biosynthesis would contribute to maintaining cholesterol homeostasis in salmon fed non-marine diets. At the end of the trial, sterol concentrations in the liver of Atlantic salmon were similar among dietary treatments. Furthermore, liver sterol concentrations were positively correlated with idi1 and sqs mRNA levels. In contrast, the muscle of fish fed ABP and VEG diets had sterol levels ~ 4 times lower than those of fish fed MAR diet [42]. Consequently, muscle sterol levels and idi1 and sqs transcript levels were negatively correlated. As the liver is the main organ for the regulation of cholesterol homeostasis, with a faster turnover than skeletal muscle [77], it is not surprising that fish fed the non-marine diets could metabolically maintain liver but not muscle sterols levels. The question arises as to what pathway supplied the acetyl-CoA required for an up-regulated synthesis of cholesterol. One possible source could be the β-oxidation of fatty acids [81]. Up-regulated fabp3a and fabp3b transcription in the liver of fish fed ABP diet – also in fish fed VEG diet, although to a smaller extent – seems to support this hypothesis, as FABP3 transports fatty acids from cell membranes to mitochondria for β-oxidation [82]. In line with this, the microarray analysis identified the gene encoding adiponectin, a protein widely accepted to stimulate FA β-oxidation in mammals [83], as up-regulated by ABP and VEG diets. Moreover, idi1 and sqs transcript levels correlated negatively with the liver ∑SFA levels, which were reduced in salmon fed the non-marine diets, even though all diets presented similar SFA contents. However, and despite all this evidence, genes directly involved in mitochondrial (cpt1) and the peroxisomal (acox1) β-oxidation were not induced by the increased acetyl-CoA demand. On the other hand, acetyl-CoA can also be synthesized from citrate by ACLY [84], a cytosolic enzyme that showed to be up-regulated at the mRNA level by VEG diet. The precursor citrate derives from the mitochondrial TCA cycle, making of ACLY a connection between the catabolism of glucose, FAs, and amino acids, and the synthesis of cholesterol [84, 85]. In other words, the source of carbon skeletons for the increased cholesterol synthesis rates remains unclear, since acly dietary modulation differed from that of the glycolysis-related transcripts (i.e., gck and pfkfb4), and there is no clear trend among the transcripts involved in fatty acid β-oxidation. Contradictory findings on the effects of plant feedstuffs on the expression of transcripts related to β-oxidation can be found in the Atlantic salmon literature [35, 36, 70, 82, 83]. Also, the post-translational regulation of these pathways should be considered as suggested by previous research in mammals [85, 86]. In sum, further research is needed on the molecular mechanisms controlling acetyl-CoA availability for cholesterol synthesis in the liver of farmed Atlantic salmon.
Besides acetyl-CoA, SQS and other enzymes involved in the anabolism of cholesterol would also need NADPH as cofactors. Most of the NADPH in the cytosol is supplied by the oxidative branch of the pentose phosphate pathway (PPP) [87]. Glucose-6-phosphate dehydrogenase (G6PD) and 6PGD mediate in this pathway that converts glucose-6-phosphate into ribulose-5-phosphate, producing two molecules of NADPH. Although identified in the microarray experiment, the qPCR analyses could not detect significant differences in 6pgd transcription among diets. Moreover, all transcriptomic evidence leaned more towards a slight downregulation of 6pgd in fish fed ABP and VEG diets. Other NADPH-producing pathways such as malic enzyme could help cover the increased demand for NADPH in fish fed the non-marine diets [70], but none of them was detected in the microarray experiment. Instead, a more likely explanation could be found in the modulation of other metabolic-related genes. Our microarray and qPCR data suggest a decrease in the expression of genes related to the synthesis of triacylglycerols (e.g., dgat2b). Furthermore, fatty acid synthesis also appears to be down-regulated by non-marine diets, according to the microarray data (e.g., acac, fatty acid hydroxylase domain-containing protein 2, fatty acid synthase), although it could not be confirmed by the qPCR analysis of acac mRNA levels. Just as for cholesterol, synthesis of fatty acids and triacylglycerols requires NADPH. Therefore, increased cholesterol synthesis in fish fed ABP and VEG diets was possibly enabled by decreasing the synthesis of other lipid species.
Effect of feed intake and dietary carbohydrate levels on liver metabolism
Atlantic salmon fed ABP showed down-regulated hepatic glycolysis as indicated by lower transcript levels of gck and pfkfb4. GCK catalyzes the phosphorylation of glucose to glucose-6-phosphate in the liver of fish and other vertebrates [88]. Similar to mammals, the mRNA and activity levels of glucokinase in the liver of fish respond to the proportion of carbohydrates in the diet [89]. ABP diet had a lower proportion of raw wheat (Table 1) – the main source of carbohydrates in our experimental diets – than MAR diet. However, so did VEG diet and salmon under this dietary treatment also showed up-regulated gck transcription compared with ABP diet. Instead, the significant positive correlation between gck transcript levels and AFI suggests that feed intake could have been the main driver in the gck transcriptional regulation. Glucokinase gene expression and activity were previously found to adapt to feed ration size in gilthead seabream [58]. In mammals and fish [89], the bifunctional enzyme PFKFB controls the cytosolic concentration of fructose 2, 6-bisphosphate, a metabolite that allosterically regulates the activity of the enzymes phosphofructokinase-1 (glycolysis) and fructose-1, 6-biphosphatase (gluconeogenesis). The transcription of pfkfb has also been reported to increase with carbohydrate levels in the diet and feed ration [59]. The fact that pfkfb4 transcript levels did not correlate with AFI values and were a bit lower, although not significantly, with VEG diet could be due to an interaction of both factors: dietary carbohydrate levels, and feed intake.
GCK activity supplies the oxidative branch of the PPP with glucose-6-phosphate molecules, the oxidation of which generates NADPH for anabolic processes. 6PGD mediates in the pathway and was microarray-detected in our study as down-regulated by VEG diet. However, the qPCR analysis could only verify that 6pgd transcription was not up-regulated in response to the higher NADPH demand for cholesterol synthesis in salmon fed ABP and VEG diets. Thus, we infer that lower carbohydrate intake due to lower AFI (Table 2) or dietary carbohydrate proportion might have restricted the PPP. As discussed before, a limited production by PPP could have driven liver cells to divert NADPH from other anabolic pathways towards cholesterol synthesis. The influence of feed intake and dietary carbohydrates on hepatic cholesterol synthesis in Atlantic salmon warrants further investigation. Further downstream of the pathway, in the non-oxidative branch of the PPP, ribulose-5-phosphate originating from the oxidation of glucose-6-phosphate is metabolized into ribose-5-phosphate, which serves as a substrate for de novo synthesis of nucleotides [87]. In our study, nucleotide synthesis in the liver of salmon was likely down-regulated by ABP and VEG diets, as suggested by the microarray experiment, and confirmed by the qPCR analysis of adssl1a transcripts. Mammals possess two ADSS isozymes: a basic form (ADSS1) involved in the purine nucleotide cycle, and an acidic form (ADSS2) that catalyzes the de novo synthesis of adenosine monophosphate [90, 91]. ADSS proteins and genes have not been molecularly characterized in fish yet, although adssl1 has been related to cell apoptosis in zebrafish [92], and Atlantic salmon adssl2 was found to be down-regulated by dietary glucosinolate supplementation [93]. From a nutritional perspective, it is worth investigating the role of ADSS1 in fish glucose metabolism, as recent findings suggest a regulatory function in insulin secretion by mammalian β cells [94]. Interestingly, transcript levels of adssl1a correlated well with other hierarchically clustered transcripts (the ‘carbohydrate metabolism-related’ genes, especially pfkfb4). Taken together, differences in carbohydrate intake among dietary treatments seemed to have affected not only liver glucose metabolism but also other pathways that branch off from its intermediaries.
Based on their transcription patterns, two genes encoding SGK2a and SGK2b were also grouped within the ‘carbohydrate metabolism-related’ genes cluster in Figs. 5 and 6. SGKs are a family of serine/threonine kinases implicated in the regulation of ion transport, proliferation, apoptosis, and differentiation of mammalian cells [95], and therefore sgk2a and sgk2b were initially classified as related to “cell growth and proliferation” (Table 4). Three SGK proteins have been identified in mammals, and SGK2 was found to be the predominant form in the liver [96]. SGKs have been poorly studied in fish, and the little information available is limited to SGK1 [97, 98]. In the human hepatoma cell line HepG2, Gotoh and Negishi [99] found SGK2 dephosphorylation to contribute to the activation of drug-induced gluconeogenesis. Interestingly, these authors’ findings in mouse liver implied the existence of a different pathway by which SGK2 dephosphorylation would inactivate gluconeogenesis. Therefore, the molecular function of SGK2 may vary among species. Based on our correlation analyses, sgk2a and sgk2b transcripts responded to dietary conditions in a similar fashion to the transcripts involved in the metabolism of carbohydrates. Moreover, the transcription of sgk2b correlated significantly with AFI. Carnivorous fish such as Atlantic salmon have a limited ability to regulate hepatic gluconeogenesis in response to carbohydrate intake, which has been proposed as a possible explanation for fish glucose intolerance [100]. After considering all the above, future studies should functionally characterize SGK2 and investigate its significance in carbohydrate metabolism in the liver of fish.
Our analyses showed a correlation between the expression of the ‘carbohydrate metabolism-related’ transcripts and certain tissue FA levels. This strengthens the idea that the interaction between lipid and carbohydrate metabolisms through NADPH availability could have influenced the FA composition of salmon tissues, especially the muscle. Feed intake would have also contributed to the regulation of hepatic lipid metabolism, as indicated by the significant correlation between fabp3a transcript levels and AFI values. All our experimental diets were formulated by EWOS Innovation (now Cargill Innovation) to meet salmonid requirements of essential nutrients [41]. Therefore, salmon growth can only be attributed to the balance between metabolic resources provided by diet and the metabolic cost of maintaining existing tissues. In fact, weight gain was found to be positively correlated with AFI values. Considering the latter, it is not surprising that the mRNA levels of sgk2a, sgk2b, gck, and pfkfb4 correlated positively with salmon weight gain.
Dietary modulation of apoptosis, immunity, and inflammation
The liver of fish fed VEG diet showed decreased transcription of htra1a and htra1b. Tacchi et al. [63] previously reported hepatic htra1 transcription to be affected by feeding Atlantic salmon a diet with low FM/ high plant protein levels. In mammals, serine protease HTRA1 mediates in multiple biological processes by antagonizing IGF-binding proteins and proteins of the TGF-beta family [101]. Tacchi et al. [63] hypothesized that htra1 up-regulation by plant proteins could be a compensatory response upon the concurrent induction of apoptotic transcripts involved in the TGF-beta pathway. In contrast to Tacchi et al. [63], htra1a and htra1b transcription was repressed by replacing dietary FM with plant proteins in the present study. However, the interaction between htra1 and apoptosis may be inferred from the down-regulation of gadd45b paralogues (qPCR validated) and serine/threonine-protein kinase ULK4 (microarray identified) by VEG diet. In mammals, GADD45B and ULK4 were related to the regulation of apoptosis through p38 MAPK signaling pathways [102, 103], pathways in cross-talk with that of TGF-beta [104]. Piscine HTRA1 would require further investigation before drawing conclusions on its significance in salmon physiology. However, should HTRA1 in fish regulate the TGF-beta signaling pathway, then it may explain the promoted transcription of some immune transcripts in the liver of fish fed VEG diet (i.e., mxa, mxb, ifit5, and mhcI). Evidence in fish suggests that TGF-beta plays immunomodulatory roles similar to those observed in mammals [105]. Interestingly, recombinant human TGF-β1 was found to induce mhcI in grass carp [106]; and in the liver transcriptome of Atlantic salmon fed a plant-based diet, the induction of the transcript encoding the hypothesized TGF-β1-antagonist htra1 coincided with a repression of mhcI transcription [63].
Both qPCR and microarray agreed in that VEG diet up-regulated the basal expression of antiviral transcripts (i.e., mxa, mxb, and ifit5) and mhcI in the liver of salmon. In a study conducted subsequent to the present feeding trial, VEG diet was found to boost the expression of antiviral transcripts in the head kidney of Atlantic salmon 24 h after an intraperitoneal injection of the viral mimic polyriboinosinic polyribocytidylic acid (pIC) [28]. In mammals, the liver acts as a defense barrier against pathogens after they pass through the digestive tract [107]. Leukocytes in the liver of rainbow trout have been reported to represent 15–29% of non-hepatocytes [108]. In contrast, the head kidney of fish equates to the red bone marrow of higher vertebrates as a major hematopoietic and lymphoid organ [109]. Considering all the above, VEG diet appeared to fortify the constitutive immune defenses of the liver, and provide the metabolic conditions for the head kidney to mount a stronger immune response against immune challenges.
In the liver of salmon, the extent of the transcription of the antiviral genes and/or mhcI correlated positively with the proportion of some ω6 FAs and the PUFA/SFA ratio in the tissue. Omega-6 FAs are known to be pro-inflammatory as they are metabolized into more bioactive eicosanoids than ω3 FAs [24]. SFAs have also been proven to be pro-inflammatory in in vitro and in vivo mammalian models [110, 111]. In this regard, although neither of the lect2 paralogues showed a significant response to diet, the microarray analysis detected C-C motif chemokine 13-like (ccl13) transcripts as more highly expressed in fish fed VEG diet than in those fed ABP diet. In mammals, CCL13 (alias monocyte chemoattractant protein 4) attracts monocytes, T cells and immature dendritic cells [112]. In fish, ccl13 has been found to be highly responsive to pIC in cod macrophages [113], and to Piscirickettsia salmonis in the liver and head kidney of Atlantic salmon injected with the pathogen [114]. Likewise, VEG diet was found to up-regulate ccl19b transcription in head kidney of PBS-injected salmon compared with ABP diet [28]. Nevertheless, as VEG diet repressed the expression of head kidney transcripts involved in the synthesis of eicosanoids, the diet was not deemed pro-inflammatory [28], which would agree with its lower proportion in ARA compared with MAR and ABP diets. Therefore, the concurrence of up-regulated ccl13 and immune-related transcripts in the liver of salmon fed VEG diet may suggest an increased homing of leukocytes not related to diet-induced inflammation.
In contrast with VEG diet, ABP diet was previously taken as pro-inflammatory since it up-regulated the expression of transcripts related to the synthesis of leukotrienes in the head kidney of Atlantic salmon [28]. Furthermore, lower EPA/ARA ratios of ABP would have promoted the synthesis of ARA-derived eicosanoids, which possess stronger pro-inflammatory activity than those synthesized from EPA [26, 115, 116]. No evidence of changes in the hepatic eicosanoid metabolism was found in the present microarray experiment. However, Atlantic salmon fed ABP diet showed a trend towards higher transcription of igm and igd in the liver. IgM+ B cells have been found to dominate rainbow trout peritoneal inflammatory response to bacteria and viruses [117, 118]. On the other hand, IgD function in salmonids is yet to be investigated. Nevertheless, research on IgD in vertebrates is gaining interest as evidence accumulates regarding its activity enhancing basophil-mediated inflammatory responses [119, 120]. Attending to diet formulation, ABP’s pro-inflammatory properties would be most likely related to its higher content in animal by-products, which contributed to the lower EPA/ARA ratio in the diet. In line with this, igd mRNA levels correlated positively with hepatic ARA levels and negatively with muscle EPA/ARA ratios. In addition, ABP-promoted gadd45b transcription (compared to VEG diet) could also be considered as further evidence of the pro-inflammatory nature of the diet. Gadd45b has been reported to play a role in modulating inflammation in mice [121] and be induced upon infection by Piscirickettsia salmonis [114], and infectious salmon anemia virus [122] in the head kidney and liver, respectively, of Atlantic salmon. Considering that liver is central to regulating systemic nutrient homeostasis [123], and may act as barrier defense against pathogens [107], we can conclude that there is a need for a better understanding of the metabolic and immune implications of chronic inflammation in the liver of Atlantic salmon.
Identification of predictive gene biomarkers
The search for economically and environmentally superior sustainable feeds for the Atlantic salmon aquaculture industry has contributed to the development of new tools to analyze the performance of the fish under varying nutritional conditions. In the present study, we used qPCR data of hepatic diet-responsive transcripts to identify gene biomarkers capable of predicting desired salmon phenotypes. We report two discriminant functions based on the transcript levels of five genes (sqs, fabp3a, gck, sgk2b, gadd45ba) capable of classifying individual fish by dietary treatment with a 90% accuracy. These biomarkers cover the differences between MAR diet and the non-marine diets (sqs, fabp3a), and those between ABP diet and the other two (gck, sgk2b, gadd45ba). Another discriminant function could correctly classify 94% of the individuals by weight gain (two categories: low and high) using only four genes: gck, sgk2b, fabp3b, mxa. Understandably, all four genes were characteristically down-regulated by ABP diet in comparison with MAR and VEG diets. A third discriminant function with fabp3a and igd as predictors allowed the prediction of EPA + DHA levels in the muscle of Atlantic salmon with a 67% accuracy. As explained above, hepatic fabp3a mRNA levels showed a negative correlation with muscle EPA and DHA concentrations. What is not so evident is the connection between the transcription of igd and that of genes related to lipid metabolism. To our knowledge, no molecular mechanisms co-regulating the transcription of igd and lipid-metabolism genes have been reported to date. Nevertheless, as discussed above, igd transcript levels showed a negative correlation with muscle EPA/ARA ratios, which could explain its ability to predict EPA + DHA levels in muscle.
In the present study, we propose the stepwise discriminant analysis as a promising method for predicting the performance of farmed Atlantic salmon based on the expression of biomarker genes. This method could also be used to predict other relevant diet effects for Atlantic salmon aquaculture, such as diet-derived immune resistance against pathogens. In fact, stepwise discriminant analysis has previously been utilized and proposed for prognosis prediction of cancer and tuberculosis patients [124, 125]. Notwithstanding the accuracy of the present post hoc predictions, for these functions to be used to classify cases in a predictive manner, they will need to be cross-validated with results from other feeding trials with Atlantic salmon. Ideally, new and more robust functions could be generated by using trancriptomic data compiled from multiple studies. In this way, the influence of differential genetic backgrounds and holding conditions (e.g., water quality, photoperiod, feeding regime) could be accounted for in the predictive function. Also, the applicability of these functions will depend on the diet formulations tested in the studies from which the gene expression data is collected. For example, the growth-predicting biomarkers identified here were associated with the inclusion of animal by-products and reduced feed intake. Well-established microarray platforms like the one used here are adequate for such analyses as the assays should be standardized across studies. Furthermore, a more inexpensive alternative could be the use of multiplex qPCR panels composed by highly predictive biomarker genes selected by stepwise discriminant analysis. This opens an opportunity for the development of routine assays for the screening of different fish performance parameters (e.g., growth, stress, immune status) by the Atlantic salmon farming industry. Lastly, the stepwise discriminant analysis may become a powerful tool for the formulation of superior feeds for Atlantic salmon should the phenotype-predictive functions be applied to transcriptomic data from early time points of the feeding trials (e.g., two to 3 weeks after the beginning of the trial).
Conclusions
We present new findings on the transcriptomic changes in the liver of Atlantic salmon fed diets with high replacement of FM and FO by terrestrial alternatives. Based on our results, the hepatic metabolic machinery of Atlantic salmon adapted to the nutritional challenges of such dietary formulations. The need for increased cholesterol biosynthesis in ABP and VEG groups seemed to be the main driver of the modulation of the hepatic metabolism and the changes in the tissue fatty acid composition. On the other hand, the poorer growth performance of fish fed ABP diet seemed to be related to lower feed intake, which was found to be correlated with the mRNA levels of the ‘carbohydrate metabolism-related’ transcripts analyzed (i.e., gck and sgk2b). We provide new evidence on the expression of htra1a and htra1b transcripts being modulated by the proportion of plant products in the diet of salmon. Concurrently, the plant-based formulation of VEG diet promoted higher mRNA levels of antiviral transcripts (i.e., mxa, mxb, and ifit5) and mhcI, as opposed to the trend observed with htra1a and htra1b. Therefore, we hypothesize a possible interaction between htra1 paralogues and antiviral immunity in the liver of Atlantic salmon. We found no indication of a pro-inflammatory effect by VEG diet, which agrees with our previous findings in the head kidney of Atlantic salmon fed these experimental diets [26]. In contrast, ABP diet up-regulated igd transcription, which correlated positively with a higher abundance of ARA in the liver, thus suggesting a pro-inflammatory effect of this diet. Using stepwise discriminant analysis, we identified gene biomarkers capable to accurately predict desired phenotypes within this study such as growth and muscle EPA + DHA levels. This study could lay the foundation for future research on the relevance of cholesterol and certain genes (i.e., sgk2 and htra1 paralogues) in the nutrition and immune system of Atlantic salmon and other fish species. From an industry perspective, on the other hand, we propose a molecular approach to analyze diet performance that could help manufacturers reduce the proportion of ingredients from overexploited fisheries in salmon aquafeeds.
Abbreviations
- 6pgd :
-
6-phosphogluconate dehydrogenase
- acac :
-
Acetyl-CoA carboxylase
- acly :
-
ATP-citrate synthase
- acox1 :
-
Acyl-coenzyme A oxidase 1
- adssl1a :
-
Adenylosuccinate synthetase isozyme 1 C-A
- AFI:
-
Apparent feed intake
- ARA:
-
Arachidonic acid
- ccl13 :
-
C-C motif chemokine 13-like
- cpt1 :
-
Carnitine palmitoyltransferase 1
- dgat2 :
-
Diacylglycerol O-acyltransferase 2
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- fabp3a :
-
Fatty acid-binding protein 3
- gadd45b :
-
Growth arrest and DNA-damage-inducible protein GADD45 beta
- gck :
-
Glucokinase
- GOI:
-
Gene of interest
- htra1 :
-
Serine protease HTRA1
- idi1 :
-
Isopentenyl-diphosphate delta-isomerase 1
- ifit5 :
-
Interferon-induced protein with tetratricopeptide repeats 5
- igd :
-
Immunoglobulin delta heavy chain
- igm :
-
Immunoglobulin mu heavy chain
- LC-PUFA:
-
Long-chain polyunsaturated fatty acids
- lect2 :
-
Leukocyte cell-derived chemotaxin 2
- mhcI :
-
MHC class I antigen
- mtco1 :
-
Cytochrome c oxidase subunit 1
- mtco2 :
-
Cytochrome c oxidase subunit 2
- mx :
-
Interferon-induced GTP-binding protein Mx
- pfkfb4 :
-
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4-like
- PFP:
-
Percentage of false-positives
- SFA:
-
Saturated fatty acids
- sgk2 :
-
Serine/threonine-protein kinase Sgk2
- sqs :
-
Squalene synthase
- WG:
-
Weight gain
- ω3:
-
Omega-3
- ω6:
-
Omega-6
References
FAO. The state of world fisheries and aquaculture 2016: contributing to food security and nutrition for all. Rome: Food and Agriculture Organization of the United Nations; 2016.
Hixson SM. Fish nutrition and current issues in aquaculture: the balance in providing safe and nutritious seafood, in an environmentally sustainable manner. J Aquac Res Dev. 2014;5(3):1.
Jobling M. Fish nutrition research: past, present and future. Aquacult Int. 2016;24(3):767–86.
Leaver MJ, Bautista JM, Björnsson BT, Jönsson E, Krey G, Tocher DR, Torstensen BE. Towards fish lipid nutrigenomics: current state and prospects for fin-fish aquaculture. Rev Fish Sci. 2008;16(sup1):73–94.
Martin SAM, Król E. Nutrigenomics and immune function in fish: new insights from omics technologies. Dev Comp Immunol. 2017;75:86–98.
Collins SA, Øverland M, Skrede A, Drew MD. Effect of plant protein sources on growth rate in salmonids: meta-analysis of dietary inclusion of soybean, pea and canola/rapeseed meals and protein concentrates. Aquaculture. 2013;400:85–100.
Torrecillas S, Robaina L, Caballero MJ, Montero D, Calandra G, Mompel D, Karalazos V, Kaushik S, Izquierdo MS. Combined replacement of fishmeal and fish oil in European sea bass (Dicentrarchus labrax): production performance, tissue composition and liver morphology. Aquaculture. 2017;474:101–12.
Simó-Mirabet P, Felip A, Estensoro I, Martos-Sitcha JA, de las Heras V, Calduch-Giner J, Puyalto M, Karalazos V, Sitjà-Bobadilla A, Pérez-Sánchez J. Impact of low fish meal and fish oil diets on the performance, sex steroid profile and male-female sex reversal of gilthead sea bream (Sparus aurata) over a three-year production cycle. Aquaculture. 2018;490:64–74.
Klinger D, Naylor R. Searching for solutions in aquaculture: charting a sustainable course. Annu Rev Environ Resour. 2012;37(1):247–76.
Kaushik SJ, Cravedi JP, Lalles JP, Sumpter J, Fauconneau B, Laroche M. Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture. 1995;133(3):257–74.
Francis G, Makkar HPS, Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 2001;199(3–4):197–227.
Hardy RW. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac Res. 2010;41(5):770–6.
Metochis C, Crampton VO, Ruohonen K, Bell JG, Adams A, Thompson KD. The effects of increasing dietary levels of amino acid-supplemented soy protein concentrate and constant dietary supplementation of phosphorus on growth, composition and immune responses of juvenile Atlantic salmon (Salmo salar L.). Fish Physiol Biochem. 2016;42(3):807-29.
Dias J, Conceição LEC, Ribeiro AR, Borges P, Valente LMP, Dinis MT. Practical diet with low fish-derived protein is able to sustain growth performance in gilthead seabream (Sparus aurata) during the grow-out phase. Aquaculture. 2009;293(3):255–62.
Kaushik SJ, Covès D, Dutto G, Blanc D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture. 2004;230(1):391–404.
Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, Farrell AP, Forster I, Gatlin DM, Goldburg RJ, Hua K, et al. Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci U S A. 2009;106(36):15103–10.
Bureau DP. Rendered products in fish aquaculture feeds. In: Meeker DL, Hamilton C, editors. Essential rendering: all about the animal by-product industry. Arlington: National Renderers Association; 2006. p. 179–94.
Sealey WM, Hardy RW, Barrows FT, Pan Q, Stone DAJ. Evaluation of 100% fish meal substitution with chicken concentrate, protein poultry by-product blend, and chicken and egg concentrate on growth and disease resistance of juvenile rainbow trout, Oncorhynchus mykiss. J World Aquacult Soc. 2011;42(1):46–55.
Hatlen B, Jakobsen JV, Crampton V, Alm M, Langmyhr E, Espe M, Hevrøy EM, Torstensen BE, Liland N, Waagbø R. Growth, feed utilization and endocrine responses in Atlantic salmon (Salmo salar) fed diets added poultry by-product meal and blood meal in combination with poultry oil. Aquac Nutr. 2015;21(5):714–25.
Bransden MP, Carter CG, Nichols PD. Replacement of fish oil with sunflower oil in feeds for Atlantic salmon (Salmo salar L.): effect on growth performance, tissue fatty acid composition and disease resistance. Comp Biochem Phys B. 2003;135(4):611–25.
Thanuthong T, Francis DS, Senadheera SD, Jones PL, Turchini GM. Fish oil replacement in rainbow trout diets and total dietary PUFA content: I Effects on feed efficiency, fat deposition and the efficiency of a finishing strategy. Aquaculture. 2011;320(1):82–90.
Sun S, Ye J, Chen J, Wang Y, Chen L. Effect of dietary fish oil replacement by rapeseed oil on the growth, fatty acid composition and serum non-specific immunity response of fingerling black carp, Mylopharyngodon piceus. Aquac Nutr. 2011;17(4):441–50.
Betancor MB, Sprague M, Sayanova O, Usher S, Campbell PJ, Napier JA, Caballero MJ, Tocher DR. Evaluation of a high-EPA oil from transgenic Camelina sativa in feeds for Atlantic salmon (Salmo salar L.): effects on tissue fatty acid composition, histology and gene expression. Aquaculture. 2015;444:1–12.
Bell JG, McEvoy J, Tocher DR, McGhee F, Campbell PJ, Sargent JR. Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J Nutr. 2001;131(5):1535–43.
Sprague M, Dick JR, Tocher DR. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci Rep. 2016;6:21892.
Calder PC. N-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013;72(3):326–36.
Schmitz G, Ecker J. The opposing effects of n−3 and n−6 fatty acids. Prog Lipid Res. 2008;47(2):147–55.
Caballero-Solares A, Hall JR, Xue X, Eslamloo K, Taylor RG, Parrish CC, Rise ML. The dietary replacement of marine ingredients by terrestrial animal and plant alternatives modulates the antiviral immune response of Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2017;64:24–38.
Seierstad SL, Haugland Ø, Larsen S, Waagbø R, Evensen Ø. Pro-inflammatory cytokine expression and respiratory burst activity following replacement of fish oil with rapeseed oil in the feed for Atlantic salmon (Salmo salar L.). Aquaculture. 2009;289(3–4):212–8.
Eslamloo K, Xue X, Hall JR, Smith NC, Caballero-Solares A, Parrish CC, Taylor RG, Rise ML. Transcriptome profiling of antiviral immune and dietary fatty acid dependent responses of Atlantic salmon macrophage-like cells. BMC Genomics. 2017;18(1):706.
Narayan B, Miyashita K, Hosakawa M. Physiological effects of eicosapentaenoic ecid (EPA) and docosahexaenoic acid (DHA)—a review. Food Rev Int. 2006;22(3):291–307.
Lorente-Cebrián S, Costa AGV, Navas-Carretero S, Zabala M, Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem. 2013;69(3):633–51.
Glencross BD. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev Aquacult. 2009;1(2):71–124.
Morais S, Monroig O, Zheng X, Leaver MJ, Tocher DR. Highly unsaturated fatty acid synthesis in Atlantic salmon: characterization of ELOVL5- and ELOVL2-like elongases. Mar Biotechnol. 2009;11(5):627–39.
Xue X, Hixson SM, Hori TS, Booman M, Parrish CC, Anderson DM, Rise ML. Atlantic salmon (Salmo salar) liver transcriptome response to diets containing Camelina sativa products. Comp Biochem Phys D. 2015;14:1–15.
Hixson SM, Parrish CC, Xue X, Wells JS, Collins SA, Anderson DM, Rise ML. Growth performance, tissue composition, and gene expression responses in Atlantic salmon (Salmo salar) fed varying levels of different lipid sources. Aquaculture. 2017;467:76–88.
Alimuddin KV, Satoh S, Takeuchi T, Yoshizaki G. Cloning and over-expression of a masu salmon (Oncorhynchus masou) fatty acid elongase-like gene in zebrafish. Aquaculture. 2008;282(1):13–8.
Kabeya N, Takeuchi Y, Yamamoto Y, Yazawa R, Haga Y, Satoh S, Yoshizaki G. Modification of the n-3 HUFA biosynthetic pathway by transgenesis in a marine teleost, nibe croaker. J Biotechnol. 2014;172:46–54.
Hixson SM, Parrish CC, Anderson DM. Full substitution of fish oil with camelina (Camelina sativa) oil, with partial substitution of fish meal with camelina meal, in diets for farmed Atlantic salmon (Salmo salar) and its effect on tissue lipids and sensory quality. Food Chem. 2014;157:51–61.
Ytrestøyl T, Aas TS, Åsgård T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture. 2015;448:365–74.
Jobling M. National Research Council (NRC): nutrient requirements of fish and shrimp. Aquacult Int. 2012;20(3):601–2.
Beheshti Foroutani M, Parrish CC, Wells J, Taylor RG, Rise ML, Shahidi F. Minimizing marine ingredients in diets of farmed Atlantic salmon (Salmo salar): effects on growth performance and muscle lipid and fatty acid composition. PLoS One. 2018;13(9):e0198538.
Xu Q, Feng CY, Hori TS, Plouffe DA, Buchanan JT, Rise ML. Family-specific differences in growth rate and hepatic gene expression in juvenile triploid growth hormone (GH) transgenic Atlantic salmon (Salmo salar). Comp Biochem Phys D. 2013;8(4):317–33.
Jantzen SG, Sanderson DS, von Schalburg KR, Yasuike M, Marass F, Koop BF. A 44K microarray dataset of the changing transcriptome in developing Atlantic salmon (Salmo salar L.). BMC Res Notes. 2011;4(1):88.
Booman M, Borza T, Feng CY, Hori TS, Higgins B, Culf A, Léger D, Chute IC, Belkaid A, Rise M, et al. Development and experimental validation of a 20K Atlantic cod (Gadus morhua) oligonucleotide microarray based on a collection of over 150,000 ESTs. Mar Biotechnol. 2011;13(4):733–50.
Bø TH, Dysvik B, Jonassen I. LSimpute: accurate estimation of missing values in microarray data with least squares methods. Nucleic Acids Res. 2004;32(3):e34.
Brown TD, Hori TS, Xue X, Ye CL, Anderson DM, Rise ML. Functional genomic analysis of the impact of camelina (Camelina sativa) meal on Atlantic salmon (Salmo salar) distal intestine gene expression and physiology. Mar Biotechnol. 2016;18(3):418–35.
Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573(1–3):83–92.
Jeffery IB, Higgins DG, Culhane AC. Comparison and evaluation of methods for generating differentially expressed gene lists from microarray data. BMC Bioinformatics. 2006;7(1):359.
Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98(9):5116–21.
Hong F, Breitling R, McEntee CW, Wittner BS, Nemhauser JL, Chory J. RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics. 2006;22(22):2825–7.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29(9):e45.
Olsvik PA, Lie KK, Jordal AEO, Nilsen TO, Hordvik I. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Biol. 2005;6(1):21.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–12.
Cui X, Churchill GA. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 2003;4(4):210.
Hori TS, Gamperl AK, Booman M, Nash GW, Rise ML. A moderate increase in ambient temperature modulates the Atlantic cod (Gadus morhua) spleen transcriptome response to intraperitoneal viral mimic injection. BMC Genomics. 2012;13(1):431.
Salas-Leiton E, Anguis V, Martín-Antonio B, Crespo D, Planas JV, Infante C, Cañavate JP, Manchado M. Effects of stocking density and feed ration on growth and gene expression in the Senegalese sole (Solea senegalensis): potential effects on the immune response. Fish Shellfish Immunol. 2010;28(2):296–302.
Caseras A, Metón I, Fernández F, Baanante IV. Glucokinase gene expression is nutritionally regulated in liver of gilthead sea bream (Sparus aurata). BBA-Gene Struct Expr. 2000;1493(1–2):135–41.
Metón I, Caseras A, Fernández F, Baanante IV. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene expression is regulated by diet composition and ration size in liver of gilthead sea bream, Sparus aurata. BBA-Gene Struct Expr. 2000;1491(1–3):220–8.
Lachenbruch PA, Goldstein M. Discriminant analysis. Biometrics. 1979;35(1):69–85.
Dixon WJ, Brown MB. BMDP statistical software. Berkeley: University of California Press; 1985.
Huberty CJ. Applied discriminant analysis. New York: Wiley; 1994.
Tacchi L, Secombes CJ, Bickerdike R, Adler MA, Venegas C, Takle H, Martin SAM. Transcriptomic and physiological responses to fishmeal substitution with plant proteins in formulated feed in farmed Atlantic salmon (Salmo salar). BMC Genomics. 2012;13(1):363.
Skugor S, Grisdale-Helland B, Refstie S, Afanasyev S, Vielma J, Krasnov A. Gene expression responses to restricted feeding and extracted soybean meal in Atlantic salmon (Salmo salar L.). Aquac Nutr. 2011;17(5):505–17.
Geay F, Ferraresso S, Zambonino-Infante JL, Bargelloni L, Quentel C, Vandeputte M, Kaushik S, Cahu CL, Mazurais D. Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-sibfamilies showing different growth rates with the plant-based diet. BMC Genomics. 2011;12(1):522.
Morais S, Pratoomyot J, Taggart JB, Bron JE, Guy DR, Bell JG, Tocher DR. Genotype-specific responses in Atlantic salmon (Salmo salar) subject to dietary fish oil replacement by vegetable oil: a liver transcriptomic analysis. BMC Genomics. 2011;12(1):1–17.
Kortner TM, Gu J, Krogdahl Å, Bakke AM. Transcriptional regulation of cholesterol and bile acid metabolism after dietary soyabean meal treatment in Atlantic salmon (Salmo salar L.). Brit J Nutr. 2012;109(4):593–604.
Panserat S, Hortopan GA, Plagnes-Juan E, Kolditz C, Lansard M, Skiba-Cassy S, Esquerré D, Geurden I, Médale F, Kaushik S, et al. Differential gene expression after total replacement of dietary fish meal and fish oil by plant products in rainbow trout (Oncorhynchus mykiss) liver. Aquaculture. 2009;294(1–2):123–31.
Liland NS, Hatlen B, Takle H, Venegas C, Espe M, Torstensen BE, Waagbø R. Including processed poultry and porcine by-products in diets high in plant ingredients reduced liver TAG in Atlantic salmon, Salmo salar L. Aquac Nutr. 2015;21(5):655–69.
Leaver MJ, Villeneuve LA, Obach A, Jensen L, Bron JE, Tocher DR, Taggart JB. Functional genomics reveals increases in cholesterol biosynthetic genes and highly unsaturated fatty acid biosynthesis after dietary substitution of fish oil with vegetable oils in Atlantic salmon (Salmo salar). BMC Genomics. 2008;9(1):299.
Morais S, Pratoomyot J, Torstensen BE, Taggart JB, Guy DR, Bell JG, Tocher DR. Diet × genotype interactions in hepatic cholesterol and lipoprotein metabolism in Atlantic salmon (Salmo salar) in response to replacement of dietary fish oil with vegetable oil. Brit J Nutr. 2011;106(10):1457–69.
Norambuena F, Lewis M, Hamid NKA, Hermon K, Donald JA, Turchini GM. Fish oil replacement in current aquaculture feed: is cholesterol a hidden treasure for fish nutrition? PLoS One. 2013;8(12):e81705.
Liland NS, Espe M, Rosenlund G, Waagbø R, Hjelle JI, Lie Ø, Fontanillas R, Torstensen BE. High levels of dietary phytosterols affect lipid metabolism and increase liver and plasma TAG in Atlantic salmon (Salmo salar L.). Brit J Nutr. 2013;110(11):1958–67.
Limtipsuntorn U, Haga Y, Kondo H, Hirono I, Satoh S. Microarray analysis of hepatic gene expression in juvenile Japanese flounder Paralichthys olivaceus fed diets supplemented with fish or vegetable oils. Mar Biotechnol. 2014;16(1):88–102.
Turchini GM, Torstensen BE, Ng W-K. Fish oil replacement in finfish nutrition. Rev Aquacult. 2009;1(1):10–57.
Krogdahl Å, Penn M, Thorsen J, Refstie S, Bakke AM. Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquac Res. 2010;41(3):333–44.
van der Wulp MYM, Verkade HJ, Groen AK. Regulation of cholesterol homeostasis. Mol Cell Endocrinol. 2013;368(1–2):1–16.
Kortner TM, Björkhem I, Krasnov A, Timmerhaus G, Krogdahl Å. Dietary cholesterol supplementation to a plant-based diet suppresses the complete pathway of cholesterol synthesis and induces bile acid production in Atlantic salmon (Salmo salar L.). Brit J Nutr. 2014;111(12):2089–103.
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, et al. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res. 2007;38(6):551–79.
Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65(16):2461–83.
Houten SM, Wanders RJA. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33(5):469–77.
Haunerland NH, Spener F. Fatty acid-binding proteins – insights from genetic manipulations. Prog Lipid Res. 2004;43(4):328–49.
Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6(1):13–21.
Chypre M, Zaidi N, Smans K. ATP-citrate lyase: a mini-review. Biochem Biophys Res Commun. 2012;422(1):1–4.
Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr Opin Cell Biol. 2015;33:125–31.
O’Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol. 2013;366(2):135–51.
Wamelink MMC, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J Inherit Metab Dis. 2008;31(6):703–17.
Wilson RP. Utilization of dietary carbohydrate by fish. Aquaculture. 1994;124(1):67–80.
Enes P, Panserat S, Kaushik S, Oliva-Teles A. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem. 2009;35(3):519–39.
Li X, Zhu Z, Mo D, Wang H, Yang S, Zhao S, Li K. Comparative molecular characterization of ADSS1 and ADSS2 genes in pig (Sus scrofa). Comp Biochem Phys B. 2007;147(2):271–7.
Stayton MM, Rudolph FB, Fromm HJ. Regulation, genetics, and properties of adenylosuccinate synthetase: a review. Curr Top Cell Regul. 1983;22:103–41.
Kwon S, Ki SM, Park SE, Kim M-J, Hyung B, Lee NK, Shim S, Choi B-O, Na DL, Lee JE, et al. Anti-apoptotic effects of human Wharton's jelly-derived mesenchymal stem cells on skeletal muscle cells mediated via secretion of XCL1. Mol Ther. 2016;24(9):1550–60.
Skugor S, Holm HJ, Bjelland AK, Pino J, Evensen Ø, Krasnov A, Wadsworth S. Nutrigenomic effects of glucosinolates on liver, muscle and distal kidney in parasite-free and salmon louse infected Atlantic salmon. Parasit Vectors. 2016;9(1):639.
Gooding JR, Jensen MV, Dai X, Wenner BR, Lu D, Arumugam R, Ferdaoussi M, MacDonald PE, Newgard CB. Adenylosuccinate is an insulin secretagogue derived from glucose-induced purine metabolism. Cell Rep. 2015;13(1):157–67.
Leong MLL, Maiyar AC, Kim B, O'Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278(8):5871–82.
Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344(Pt 1):189–97.
Shaw JR, Sato D, VanderHeide J, LaCasse T, Stanton CR, Lankowski A, Stanton SE, Chapline C, Coutermarsh B, Barnaby R, et al. The role of SGK and CFTR in acute adaptation to seawater in Fundulus heteroclitus. Cell Physiol Biochem. 2008;22(1–4):069–78.
Burgon J, Robertson AL, Sadiku P, Wang X, Hooper-Greenhill E, Prince LR, Walker P, Hoggett EE, Ward JR, Farrow SN, et al. Serum and glucocorticoid–regulated kinase 1 regulates neutrophil clearance during inflammation resolution. J Immunol. 2014;192(4):1796–805.
Gotoh S, Negishi M. Statin-activated nuclear receptor PXR promotes SGK2 dephosphorylation by scaffolding PP2C to induce hepatic gluconeogenesis. Sci Rep. 2015;5:14076.
Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B. 2012;182(8):1015–45.
Oka C, Tsujimoto R, Kajikawa M, Koshiba-Takeuchi K, Ina J, Yano M, Tsuchiya A, Ueta Y, Soma A, Kanda H, et al. HtrA1 serine protease inhibits signaling mediated by Tgfβ family proteins. Development. 2004;131(5):1041–53.
Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Liebermann DA, Böttinger EP, Roberts AB. Transforming growth factor-β-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003;278(44):43001–7.
Lang B, Zhang L, Jiang G, Hu L, Lan W, Zhao L, Hunter I, Pruski M, Song N-N, Huang Y, et al. Control of cortex development by ULK4, a rare risk gene for mental disorders including schizophrenia. Sci Rep. 2016;6:31126.
Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18.
Wang T, Secombes CJ. The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol. 2013;35(6):1703–18.
Yang M, Zhou H. Grass carp transforming growth factor-β1 (TGF-β1): molecular cloning, tissue distribution and immunobiological activity in teleost peripheral blood lymphocytes. Mol Immunol. 2008;45(6):1792–8.
Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27(1):147–63.
Möller A-M, Korytář T, Köllner B, Schmidt-Posthaus H, Segner H. The teleostean liver as an immunological organ: intrahepatic immune cells (IHICs) in healthy and benzo[a]pyrene challenged rainbow trout (Oncorhynchus mykiss). Dev Comp Immunol. 2014;46(2):518–29.
Whyte SK. The innate immune response of finfish – a review of current knowledge. Fish Shellfish Immunol. 2007;23(6):1127–51.
Harvey KA, Walker CL, Pavlina TM, Xu Z, Zaloga GP, Siddiqui RA. Long-chain saturated fatty acids induce pro-inflammatory responses and impact endothelial cell growth. Clin Nutr. 2010;29(4):492–500.
Mozaffarian D. Trans fatty acids – effects on systemic inflammation and endothelial function. Atherosclerosis Supp. 2006;7(2):29–32.
Mendez-Enriquez E, García-Zepeda EA. The multiple faces of CCL13 in immunity and inflammation. Inflammopharmacology. 2013;21(6):397–406.
Eslamloo K, Xue X, Booman M, Smith NC, Rise ML. Transcriptome profiling of the antiviral immune response in Atlantic cod macrophages. Dev Comp Immunol. 2016;63:187–205.
Tacchi L, Bron JE, Taggart JB, Secombes CJ, Bickerdike R, Adler MA, Takle H, Martin SAM. Multiple tissue transcriptomic responses to Piscirickettsia salmonis in Atlantic salmon (Salmo salar). Physiol Genomics. 2011;43(21):1241–54.
Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n−6 essentiality and the balance between n−6 and n−3. Lipids. 2003;38(4):323–41.
Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68(5):280–9.
Castro R, Abós B, González L, Granja AG, Tafalla C. Expansion and differentiation of IgM+ B cells in the rainbow trout peritoneal cavity in response to different antigens. Dev Comp Immunol. 2017;70:119–27.
Korytář T, Jaros J, Verleih M, Rebl A, Kotterba G, Kühn C, Goldammer T, Köllner B. Novel insights into the peritoneal inflammation of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013;35(4):1192–9.
Ye J, Kaattari IM, Ma C, Kaattari S. The teleost humoral immune response. Fish Shellfish Immunol. 2013;35(6):1719–28.
Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm E-S, Santini PA, Rath P, Chiu A, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10(8):889–98.
Gupta SK, Gupta M, Hoffman B, Liebermann DA. Hematopoietic cells from gadd45a-deficient and gadd45b-deficient mice exhibit impaired stress responses to acute stimulation with cytokines, myeloablation and inflammation. Oncogene. 2006;25:5537.
Jørgensen SM, Afanasyev S, Krasnov A. Gene expression analyses in Atlantic salmon challenged with infectious salmon anemia virus reveal differences between individuals with early, intermediate and late mortality. BMC Genomics. 2008;9(1):1–16.
Gribble FM. Metabolism: a higher power for insulin. Nature. 2005;434(7036):965–6.
Komatsu N, Matsueda S, Tashiro K, Ioji T, Shichijo S, Noguchi M, Yamada A, Doi A, Suekane S, Moriya F, et al. Gene expression profiles in peripheral blood as a biomarker in cancer patients receiving peptide vaccination. Cancer. 2011;118(12):3208–21.
Brahmbhatt S, Black GF, Carroll NM, Beyers N, Salker F, Kidd M, Lukey PT, Duncan K, Van Helden P, Walzl G. Immune markers measured before treatment predict outcome of intensive phase tuberculosis therapy. Clin Exp Immunol. 2006;146(2):243–52.
Acknowledgements
The authors would like to thank the Dr. Joe Brown Aquatic Research Building (JBARB) staff for their assistance with fish husbandry and sampling. We are also grateful to Jeanette Wells for providing the tissue lipid composition and to Cara Kirkpatrick (Genome Atlantic, Halifax, Canada) for all the help as the Program Manager for this project.
Funding
This study was conducted within the Biomarker Platform for Commercial Aquaculture Feed Development project, a Genomic Applications Partnership Program (GAPP #6604), funded by the Government of Canada through Genome Canada and Genome Atlantic, and Cargill Innovation (formerly EWOS Innovation).
Availability of data and materials
The datasets supporting the conclusions of this study are included within the article (and its additional files). The list of the differentially expressed microarray features with more detailed information about the BLASTx/n gene identification results can be found in the supplementary files. The alignments of the Atlantic salmon cDNA sequences from NCBI’s databases [i.e., redundant nucleotide (nr/nt) sequence and the expressed sequence tags (EST)] are also included in a supplementary file. The whole microarray dataset of 11,149 probes is available in NCBI’s Gene Expression Omnibus database (GEO Accession: GSE106604; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE106604).
Author information
Authors and Affiliations
Contributions
ACS, XX, and MBF participated in the tissue sampling and growth monitoring throughout the feeding trial. ACS took the lead role in the experimental design, RNA preparations, the conduction of the microarray and qPCR experiments, the analysis and interpretation of the results, and the writing of the manuscript. XX helped with the microarray and qPCR analysis and data interpretation. CCP led the team in charge of the tissue lipid analyses, and helped with the data interpretation. MBF performed the tissue lipid composition analyses and determined the apparent feed intake in each tank. RGT lead the team that designed and elaborated the experimental diets, and provided comments regarding the experimental design and data analysis. MLR supervised and provided comments on all the steps of the experimentation, results interpretation, and the preparation of the present manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures involving fish handling, treatment, euthanasia, and dissection were performed in accordance with the guidelines of the Canadian Council of Animal Care (approved Memorial University Institutional Animal Care Protocol 14–71-MR).
Consent for publication
Not applicable.
Competing interests
RGT is employed by Cargill Innovation, one of the funders of this study. The experimental diets used in the present study were formulated and manufactured by Cargill Innovation (formerly EWOS Innovation). The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Primers used for qPCR analyses. (DOCX 21 kb)
Additional file 2:
Table S2. Complete list of microarray probes identified as diet-responsive. The results from the gene identification analysis, functional annotation, and microarray-based fold changes between diets are presented. For the qPCR-analyzed genes, data regarding the cDNA sequences utilized for primer design (e.g., the similarity between those corresponding to different paralogues) and qPCR-based fold changes between diets are also included. AA: amino acid; bp: base pair; RQ: relative quantity. (XLS 85 kb)
Additional file 3:
Figure S1. Alignment of nucleotide sequences corresponding to adssl1a and adssl1b. Conserved nucleotides in the aligned sequences are highlighted in blue. Adssl1a and adssl1b sequences share 93% identity over 597 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide regions covered by probes C107R157 and C098R022 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) are indicated within boxes. The forward qPCR primer for adssl1a is in bold and single underlined, whereas the reverse qPCR primer is in bold and double underlined. The qPCR primers designed for adssl1b are not included in the figure as none of them passed our quality tests. (DOCX 28 kb)
Additional file 4:
Figure S2. Alignment of nucleotide sequences corresponding to dgat2a and dgat2b. Nucleotides conserved in all sequences are highlighted in yellow, and in blue for those nucleotides shared by three sequences from different paralogues, or by two but the nucleotide is deleted in the third (or the sequence is not long enough); finally, non-conserved nucleotides between paralogues are indicated by highlighting the nucleotides of one of them in green. “Rc” after the GenBank accession numbers stands for reverse complement. Dgat2a and dgat2b sequences share 83% identity over 2170 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide regions covered by probes C103R066 and C134R089 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) are indicated within boxes. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 38 kb)
Additional file 5:
Figure S3. Alignment of nucleotide sequences corresponding to fabp3a and fabp3b. Conserved nucleotides in all the aligned sequences are highlighted in yellow. Fabp3a and fabp3b sequences share 92% identity over 400 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide region covered by the probe C086R144 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) is indicated within boxes. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 22 kb)
Additional file 6:
Figure S4. Alignment of nucleotide sequences corresponding to sgk2a and sgk2b. Conserved nucleotides in all the aligned sequences are highlighted in yellow. Sgk2a and sgk2b sequences share 92% identity over 1390 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide regions covered by the probes C050R117 and C168R030 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) is indicated within boxes. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 33 kb)
Additional file 7:
Figure S5. Alignment of nucleotide sequences corresponding to htra1a and htra1b. Conserved nucleotides in all three aligned sequences are highlighted in yellow, those conserved in two are highlighted in blue. The cDNA sequence named as “htra1b_assembly” was assembled using the Salmo salar EST sequences DW576053, DW556574, DW539580, EG831192, and EG831191. Htra1a and htra1b sequences share 91% identity over 1117 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide regions covered by the probes C265R134, C231R170 and C170R142 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) is indicated within boxes. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 37 kb)
Additional file 8:
Figure S6. Alignment of nucleotide sequences corresponding to gadd45ba and gadd45bb. Conserved nucleotides in all the aligned sequences are highlighted in yellow. “Rc” after the GenBank accession numbers stands for reverse complement. Gadd45ba and gadd45bb sequences share 78% identity over 767 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide regions covered by the probe C100R113 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) is indicated within a box. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 20 kb)
Additional file 9:
Figure S7. Alignment of nucleotide sequences corresponding to lect2a and lect2b. Conserved nucleotides in all the aligned sequences are highlighted in yellow. Lect2a and lect2b sequences share 88% identity over 531 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide regions covered by the probes C134R121, C164R142 and C159R112 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) is indicated within boxes. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 29 kb)
Additional file 10:
Figure S8. Alignment of nucleotide sequences corresponding to igma and igmb. Conserved nucleotides in all the aligned sequences are highlighted in yellow. Igma and igmb sequences share 96% identity over 1548 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide regions covered by the probes C134R121, C164R142 and C159R112 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) is indicated within boxes. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 32 kb)
Additional file 11:
Figure S9. Alignment of nucleotide sequences corresponding to mxa and mxb. Conserved nucleotides in both aligned sequences are highlighted in yellow. Mxa and mxb sequences share 89% identity over 1955 aligned nucleotides. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). The nucleotide region covered by the probe C236R043 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299) is indicated within a box. Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 23 kb)
Additional file 12:
Figure S10. Alignment of nucleotide sequences corresponding to two mtco2 paralogues and the probe C060R108 from the Agilent 44 K salmonid microarray (GEO accession number: GPL11299). Conserved nucleotides in all three aligned sequences are highlighted in yellow, those conserved in two are highlighted in blue. Mtco2a and mtco2b sequences share 96% identity over 547 aligned nucleotides, and 83 and 85% identity compared with the 60mer microarray probe, respectively. The alignment and percentage identity calculation were performed using AlignX (Vector NTI Advance 11). Forward qPCR primers are in bold and single underlined, whereas reverse qPCR primers are in bold and double underlined. (DOCX 13 kb)
Additional file 13:
Figure S11. Scatter plots showing linear correlations between log2 apparent feed intake (AFI) and: A) log2 weight gain (WG); B) log2 gck RQs; C) log2 sgk2b RQs; D) log2 mxa RQs; E) log2 ifit5 RQs; F) log2 fabp3a RQs; G) log2 igd RQs. The analyses were performed using the tank mean values of each variable as AFI could only be calculated by tank. Regression lines and 95% confidence intervals are represented by solid and dashed lines, respectively. (TIF 95 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Caballero-Solares, A., Xue, X., Parrish, C.C. et al. Changes in the liver transcriptome of farmed Atlantic salmon (Salmo salar) fed experimental diets based on terrestrial alternatives to fish meal and fish oil. BMC Genomics 19, 796 (2018). https://doi.org/10.1186/s12864-018-5188-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-018-5188-6