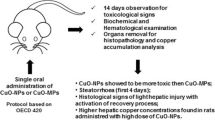
Abstract
Background
The widespread use of nano-copper as a feed additive in the absence of toxicological studies has potential risks to humans and animals. Toxicity studies on nano-copper in animals usually exposure from the respiratory tract, however, it is necessary to study the oral exposure toxicity of nano-copper to understand its risks as a feed additive.
Results
Currently the hepatotoxicity and mechanism of nano-copper after sub-choronic oral exposure at equivalent doses of 50, 100, and 200 mg/kg/day were evaluated in rats; micro-copper and Cu ions were used as controls. Nano-copper (200 mg/kg) increased serum alanine aminotransferase and aspartate aminotransferase significantly, further promoting hepatic oxidative stress, inflammation, and corresponding histopathological changes, and exhibited significant dose-dependent changes. Nano-copper also decreased the level of nuclear receptors, resulting in significant reductions in mRNA, protein, and activity of hepatic CYP450 enzymes. The molecular mechanisms responsible for these toxic effects involved the signaling pathway of NF-κB, MAPK, and STAT5.
Conclusions
Nano-copper caused strong hepatic toxicity by inducing oxidative stress and inflammation. The decreased drug metabolism enzymes lead to nano-copper–drug interaction that provoked the concerns on animal-derived food safety.
Similar content being viewed by others
Introduction
With the increasing resistance of bacteria to antibiotics, there have been increased concerns about the widespread use of veterinary antimicrobials for antibacterial and growth promotion [1]. The growing demand for meat products is an important reason to promote the use of antibiotics in farms; however, a dilemma persists in the selection and use of antibiotics to deal with animal infection [2]. Large-scale investigations have found that many resistant plasmids can drift between different bacteria, which increases the challenge of zoonosis for public health [3]. Therefore, we are in urgent need of new antibacterial substances that can be used to treat animal diseases with lower risks to animals and humans. Currently, due to the antibacterial and growth promoting effects on animals, metal nano-preparation has become a good choice as a feed additive [4, 5]. People have been eager to use nano-copper as a feed additive [6, 7]. Therefore, we think it is a suitable time to evaluate whether nano-copper is safe for animal with large-dose and long-term oral exposure, and the further impacts on humans will be discussed.
Copper (Cu) is one of the essential nutrients to maintain normal physiological and biochemical functions in animals and humans [8]. Copper nanoparticles (nano-copper) are used as antibacterial and antifouling agents in all kinds of industrial products [9]. The excessive application of nano-copper increased exposure to humans, animals, and the environment. We need more toxicity data to ensure the safe use of nano-copper in future, especially the application as the drug sources of antibacterial, anti-tumor, and feed additive [10,11,12].
Since the efficacy and toxicity of nano-copper are directly related to their particle size, we have evaluated the LD50 values of Cu ions, 30 nm, 50 nm, 80 nm, and 1 μm copper particles were 359.6, 1022, 1750, 2075, and > 5000 mg/kg, respectively [13]. Nanoparticles can break through a variety of biological barriers and widely disseminate and accumulate in multiple organs, especially the liver and kidneys, when exposed to respiratory and oral routes [14, 15]. When nano-copper entered into the blood circulation system, it mediated oxidative responses and activated a series of pathological events such as genotoxicity, inflammation, fibrosis, and carcinogenesis [16]. The liver, kidney, and spleen are the main toxic target organs of nano-copper particles [17]. The increased oxidative stress will activate related regulation pathways resulting in increased expression of proinflammatory cytokines [18, 19]. Therefore, the toxic effects of nano-copper on the liver may be more extensive than currently evaluated, and more toxicity studies need to be done to ensure their rational use.
The liver plays a crucial role in determining the toxicity of drugs due to its key role in the metabolism, transport, and clearance of foreign substances. Most of the xenobiotic substances metabolized in liver by cytochrome P450 enzymes (CYP450), and three CYP families, CYP1A, 2A, and 3A, which account for 77% of the reported xenobiotic biotransformation [20]. However, the metabolism function of liver can be affected by many factors, and the inhibition and induction of drug-metabolizing enzymes are important causes of drug–drug interactions [21,22,23]. Inflammation and oxidative stress have been shown to be one of the potential factors for the inhibition of CYP450 [24]. Therefore, it is important to study whether nano-copper in feed causes liver damage in animals, especially the impacts on drug metabolism enzymes.
In this work, we discussed the hepatoxicity and mechanism of nano-copper in rats after sub-chronic oral exposure, especially on hepatic CYP450 enzymes. The information may help us to predict the risks of nano-copper on human and animals when it’s used in medications or animal feed.
Materials and methods
Test materials and their characterization
The tested copper particles were compared with copper ions (CuCl2·2H2O), which were manufactured by Aladdin Industrial Corporation. The size of copper particles was characterized with a scanning electron microscope (Phenom ProX, Nani Scientific Instruments LTD, Shanghai, China).
The nano- and micro-coppers were added to 1% HPMC to obtain a stock suspension of 10 g/L, which was shaken and sonicated in ice bath. The distribution of particle sizes in this suspension was characterized with dynamic light scattering performed with a Zeta sizer Nano ZS (Malvern Instruments, Malvern, UK) immediately after sonication.
Experiment design
The protocols for animal studies were reviewed and approved by the Animal Ethical Committee of Sichuan Agricultural University (#:20171120). 63 Specific pathogen-free (SPF) male Sprague–Dawley rats (aged 8 weeks; 100–120 g) were bought from Chengdu Dossy Biological Technology Co., Ltd (Chengdu, China) and housed under SPF conditions. The room temperature was kept between 20 and 24 °C, and humidity ranged from 40 to 70% with a 12 h light-12 h dark cycle, food and water were available ad libitum. Rats were divided as one control group and 6 test groups (n = 9). In addition to the control group (1% hydroxypropyl methylcellulose solution, HPMC), the experimental groups were treated with 1 μm copper (200 mg/kg), CuCl2·2H2O (100 mg/kg), and nano-copper 50 mg/kg, nano-copper 100 mg/kg, and nano-copper 200 mg/kg. 80 nm copper was usually used as a feed additive and these dosages were based on the LD50 and the amount was added to the animal feed (50–500 mg/kg diet in China). Since CuCl2·2H2O was the main copper source used as custom feed additive, it was chosen as one of the controls. The micro-copper was another control for comparing the sizes–cytotoxicity relationship.
All copper particles were dispersed in a 1% HPMC solution before use. The rats were exposure to different coppers for 28 days by continuous gavage. All rats were anesthetized at the end of the treatment, and blood samples were collected by cardiac puncture. After collecting blood, rats were euthanized, and the liver tissues were quickly removed and divided into four portions as the different lobes. Left lateral hepatic lobes were used to isolate liver microsomes by differential centrifugation [25], the left middle lobes were fixed in paraformaldehyde for histopathological analysis, the middle lobes were used to detect inflammatory factors, oxidative stress, and the right lobes were used for gene and protein expression detection. All liver tissues were stored in liquid nitrogen until analysis.
Measurements of cytokines and oxidative stress
After liver tissues were fully homogenized, the levels of interleukin (IL)-1β, IL-2, IL-4, IL-6, tumor necrosis factor-alpha (TNF-α), cyclooxygenase-2 (COX-2), monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory protein (MIP)-1α were determined by MAGPIX (R&D Systems) in accordance with the manufacturer’s instructions.
The levels of total antioxidant capacity (T-AOC: 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid(ABTS) oxidation), catalase (CAT: ammonium molybdate colorimetry), superoxide dismutase [SOD: (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazole monosodium salt (WST-8) catalytic reaction], glutathione peroxidase (GSH-Px: hydroperoxide catalytic reduction reaction), nitric oxide synthase (iNOS: ELISA kit), and the contents of malondialdehyde [MDA: thiobarbituric acid (TBA) color reaction] and nitric oxide (NO: griess reagent detection) were evaluated in accordance with the manufacturer’s instructions for the appropriate kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Gene expression analyze
RNA extractions and analysis of liver tissue gene expression were performed as described, with minor changes [26]. The quantity of extracted RNA was assessed with UV spectroscopy (NanoDrop 2000 UV–Vis Spectrophotometer, Thermo Scientific; Waltham, MA, USA). The following target genes were analyzed for their expression: constitutive androstane receptor (CAR), pregnane X receptor (PXR), aryl hydrocarbon receptor (AHR), CYP450 1A2, 2C11, 2D6, 2E1, and 3A2. GAPDH was used as the housekeeping gene for data normalization (the PCR primers were shown in Table 1.).
Measurement of protein expression
The protein expression of CYP450 1A2, 2C11, 2D6, 2E1, 3A2, CAR, PXR, and AhR in the liver microsomes of rats were estimated with Western immunoblot analyses [27, 28]. 30 µg of microsomal proteins were separated by SDS polyacrylamide gel electrophoresis (Bio-Rad Laboratories, Inc.). After incubation with a primary and secondary antibody, the protein bands were visualized with enhanced chemiluminescence, and intensity of the bands was quantified with ImageJ software.
Measurements of CYP450 1A2, 2C11, 2D6, 2E1, and 3A2 activity
The activities of CYP450 1A2, 2C11, 2D6, 2E1, and 3A2 were assessed [29,30,31]. Five specific probe substrates were incubated with liver microsomes. The concentrations of probe substrates were monitored by high performance liquid chromatography (HPLC). The decreasing amount of specific probe drugs were used to show the activity of microsomal enzymes (Fig. 1). The regression equations and lower limit of quantitation concentrations for the analytes are shown in Table 2.
Luminex analyze for key signal pathway proteins
Liver tissue homogenate was prepared with lysate and a protease inhibitor mixed solution at the ratio of 1:4 and lysed on ice for 15 min then centrifuged at 16000g at 4 °C for 15 min. Concentrations of signal pathway proteins were measured with a multiplexed particle-based flow cytometric assay [32, 33]. Luminex technology was used according to the manufacturer’s instructions to measure protein and phosphoproteins of CERB, NF-κB, P38, ERK1/2, Akt, p70s6k, STAT3, and STAT5 in rat livers (R&D Systems).
Statistical analysis
All statistical results are expressed as the mean ± standard deviation (n = 9), and all experimental groups were compared with the control group. Differences between mean values were analyzed with a one-way ANOVA, and values with superscripts are significantly different (p < 0.05 or 0.01) in the results.
Results
Physicochemical characterization of copper nanoparticles
The results of dynamic light scattering analysis (Fig. 2) showed that the spherical copper particles had mean sizes of 82.5 ± 33.4 nm (80 nm) and 987.4 ± 436.7 (1 μm). The polydispersity index (PDI) of nano- and micro- copper were 0.14 and 0.20, respectively, according to the laser particle size analyzer test. The size and shape of the copper particles were consistent with the data provided by the manufacturer.
Blood chemistry analyses
The serum biochemistry results of nano-copper, micro-copper, and Cu ions-treated rats are presented in Table 3. In the 1 μm copper-treated rats, the serum concentrations of aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin/globulin (A/G), and creatine kinase (CK) increased significantly, while globulin (GLB), total protein (TP), and creatinine (CREA) decreased significantly. In the Cu ions-treated rats, the serum levels of ALP, A/G, and triglyceride (TG) increased significantly, while GLB and TP decreased significantly. In the 200 mg/kg nano-copper-treated rats, the serum levels of AST increased significantly. The level of serum ALP increased significantly only in the 200 mg/kg group. In the nano-copper-treated rats, the serum level of ALB, GLB, TP, UREA, and CREA decreased significantly, while the level of A/G increased significantly. The serum level of total cholesterol (TC) decreased significantly in 200 mg/kg-treated rats, while the level of CK decreased in the 100 mg/kg- and 200 mg/kg-treated rats. The serum level of TG increased significantly in the 100 mg/kg-treated rats. Nano-copper caused strong liver injury and dysfunction by increasing the release of transaminase and decreasing the protein synthesis.
Histopathology
Histopathological results are illustrated in Fig. 3. The obvious liver sinus congestion can be found in Cu ions-treated rats. In the micro- and nano-copper-treated rats, the main changes were small vacuoles in hepatocytes and some inflammation infiltration, meanwhile obvious liver structural disorder and hepatocyte swelling were found in 200 mg/kg nano-copper-treated rats. Liver cells vacuolar degeneration and the appearance of inflammatory cells were the main pathological manifestations of liver damage caused by nano-copper. The pathological changes were dose-dependent in nano-copper-treated rats.
Cytokine analysis
Nano-copper induced the massive release of cytokines (Fig. 4). The levels of IL-1β, IL-2, TNF-α, MIP-1, and MCP-1 increased significantly in 200 mg/kg nano-copper-treated rats. The amount of IL-6 increased significantly in Cu ions and 200 mg/kg nano-copper-treated rats. The level of IFN-γ decreased significantly in the 100 mg/kg nano-copper-treated rats, while it increased significantly in the 1 μm micro-copper and 200 mg/kg nano-copper-treated groups. The concentrations of COX-2 decreased in 1 μm copper and Cu ions-groups rats but induced in rats treated with 200 mg/kg nano-copper. Only high dose of nano-copper caused significant increasing of different inflammation cytokines, which were different from the impacts of micro-copper and Cu ions.
Oxidative stress analysis
Only nano-copper caused the oxidative stress in the liver of rats. The concentration of liver MDA increased in 200 mg/kg nano-copper-treated rats. The level of NO and iNOS increased significantly in a dose-dependent manner in nano-copper-treated rats. Similarly, the concentrations of CAT decreased in 50 mg/kg nano-copper-treated rats but increased significantly in 200 mg/kg nano-copper-treated rats. Nano-copper caused dose-dependent increase of oxidative stress in liver, but there were no changes on the expression of SOD and GSH-Px (Fig. 5).
mRNA expression of nuclear receptors and CYP enzymes
The different sources and dose of copper have different impact on the expression of CYP genes and receptors, but high dose of nano-copper shown strong inhibition effect on it. The level of CYP450 1A2 increased in 1 μm copper-treated rats, but it inhibited significantly by Cu ions and 100 mg/kg or 200 mg/kg of nano-copper (Fig. 6a). The level of CYP450 2C11 (Fig. 6b) significantly increased by 50 mg/kg and 100 mg/kg nano-copper, but it decreased with 200 mg/kg nano-copper. The level of CYP450 2D6 (Fig. 6c) decreased significantly with 100 mg/kg and 200 mg/kg nano-copper. The level of CYP450 2E1 (Fig. 6d) significantly increased with 1 μm Cu ions and 50 mg/kg nano-copper. The level of CYP450 3A1 (Fig. 6e) significantly decreased by 1 μm, 100, and 200 mg/kg nano-copper. The levels of PXR (Fig. 6f) and CAR (Fig. 6g) decreased in a dose-dependent manner by nano-copper, but the level of CAR increased by Cu ions. The level of rats AHR (Fig. 6h) decreased in a dose-dependent manner by nano-copper and decreased by 1 μm micro-copper and Cu ions.
Protein expression of nuclear receptors and CYP enzymes
The protein expression changes consistent with the changes of mRNA (Figs. 7 and 8), the inhibition mainly caused by 100 and 200 mg/kg nano-copper. The relative protein level of CYP450 1A2 increased significantly in rats treated with 1 μm copper, but decreased significantly in 100 mg/kg and 200 mg/kg nano-copper-treated rats. The level of CYP450 2C11 significantly increased with Cu ions, 50 and 100 mg/kg nano-copper, but significantly decreased in 200 mg/kg nano-coper. The level of CYP450 2D6 decreased significantly with 100 and 200 mg/kg nano-copper. The level of CYP450 2E1 increased significantly in 1 μm copper, Cu ions, 50 and 100 mg/kg nano-copper-treated rats. The level of CYP450 3A1 decreased significantly in rats treated with 1 μm copper, and decreased in a dose-dependent manner in nano-copper-treated rats. High dose of nano-copper strongly inhibited the expression of nuclear receptors, which were different to the impact of micro-copper and Cu ions.
CYP450 enzyme activities
Decrease in enzymatic activity was accompanied by the reduced genes and proteins expression (Fig. 9). CYP450 1A2 activity decreased significantly in rats treated with Cu ions, 100 mg/kg, and 200 mg/kg nano-copper. The activity of CYP450 2C11 decreased significantly in rats treated with 200 mg/kg nano-copper. The activities of CYP450 2D6 and CYP450 2E1 decreased in 100 mg/kg and 200 mg/kg nano-copper-treated rats. CYP450 3A1 decreased significantly in rats treated with 200 mg/kg nano-copper. Only high concentration of nano-copper inhibited all of the enzymes, and micro-copper and Cu ions did not have impacted the enzyme activities in liver.
Signaling pathway analysis
NF-κB, STAT, and MAPK were the main pathways activated by nano-copper during the process of treatment (Fig. 10). The level of NF-κB was induced by 100 and 200 mg/kg nano-copper, but p-NF-κB just increased significantly in 200 mg/kg nano-copper-treated rats. The total and phosphorylated levels of JNK, p38 and ERK1/2 were induced in 100 and 200 mg/kg nano-copper-treated rats. The signal pathway of STAT5 also activated by nano-copper, but the level of STAT3 just increased in total protein. The signals of Akt, p70S6K, CERB did not change during the process of treatment.
Discussion
The high dose of nano-copper caused obvious liver damage in rat, because the level of AST and ALP were increased significantly, which are used as common indicators of liver injury [34]. Liver is the main organ for protein synthesis; the contents of ALB, GLB, and TP decreased significantly after nano-copper causing liver dysfunction. Direct proof of liver damage was shown in the histopathological results, due to the dose-dependent degree of hepatocyte degeneration [35]. The increased CREA also indicated that kidney injury was caused by nano-copper [36].
Nano-copper can break different biological barriers and enter the body’s circulation [37,38,39], then accumulate in liver, and interact with biomacromolecules. Oxidative stress is the main cause of organ cytotoxicity caused by nano-copper [40]. Oxidative stress is also related to inflammation with the release of many inflammatory cytokines [41,42,43]. The levels of the inflammatory cytokines IL-1β, TNF-α, IL-6, and MIP-1 increased significantly, especially at the highest doses of the nano-copper-treated rats. Also, the markers of oxidative stress (MDA, NO, iNOS, and CAT) increased significantly. We found that Cu ions also caused inflammation in the rat liver, so we speculate that the liver inflammation and oxidative stress were caused by nano-copper and Cu ions together when part of the nano-copper was ionized in stomach acid [44, 45]. Oxidative stress and inflammatory response caused by nano-copper will further aggravate liver dysfunction [46].
The CYP450 subfamily plays an important role in the metabolism of most kinds of drugs [47]. Oxidative stress and inflammation are important factors that affect the expression of CYP450 in the liver [48,49,50,51]. Many phase-I enzymes are also regulated by specifically transcription factors, such as the AhR, CAR, and PXR [52, 53]. In this work, mRNA expression and protein expression of PXR, CAR, and AHR were significantly suppressed by nano-copper administration. According to reported studies [52, 54], decreased PXR mainly affects the expression of CYP450 2C and 3A subtypes, while decreased CAR and AHR mainly affect the expression of CYP450 1A subtype. In this study, the mRNA, protein, and activity of CYP450 1A2 and 2D6 decreased significantly in medium and high doses of nano-copper; the mRNA and protein expression of CYP450 3A1 decreased significantly, but the activity only decreased in the 200 mg/kg dose. CYP450 2C11 and 2E1 were induced by low-dose but inhibited by high dose of nano-copper, which may be due to the increased levels of TNF-α and IL-6 [55].
The regulation of CYP450 s also related to key transcription and protein kinases, such as NF-κB and MAPKs [56, 57]. The signaling pathways also cross-talk with oxidative, inflammatory, and nuclear receptors to affect the expression of CYP450 enzymes [58]. In our results, nano-copper mainly affected NF-κB, MAPK, and STAT5, which significantly increased the phosphorylated proteins and the ratio of phosphorylated and total protein. The level of p-38, JNK, and ERK increased significantly, which was found to function upstream of the IκB-α/NF-κB signaling pathway [58]. NF-κB is an important transcription factor that regulates the expression of CYP450 [59]. The JAK/STAT signaling pathway is involved in the expression of various cytokines [60, 61], which indirectly regulates the expression of CYP450 enzymes. The regulation of CYP450 is a complex process, and the effects of nano-copper on CYP450 enzyme involve the activation of several regulatory signaling pathways, the mechanism of which deserves further study.
Conclusions
Our study has indicated that sub-chronic exposure to nano-copper caused obvious liver injury and decreased the expression and activity of drug metabolism enzymes in rats. These changes are accompanied by the appearance of oxidative stress, inflammatory reactions, and key regulation pathways activated in the liver of rats. To date, most of the literature only focused on the regular toxicity of nano-copper; our research focused on the potential risks of nano-copper–drug interactions, but more studies are still needed to explain the detail mechanism.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CYP450:
-
cytochrome P450 enzyme
- CAR:
-
constitutive receptor
- PXR:
-
pregnane X receptor
- PPAR:
-
peroxisome proliferator activated receptor
- IL:
-
interleukin
- CAR:
-
constitutive androstane receptor
- AHR:
-
aryl hydrocarbon receptor
- TNF-α:
-
tumor necrosis factor-alpha
- MCP-1:
-
monocyte chemotactic protein-1
- (MIP)-1α:
-
macrophage inflammatory protein-1α
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- ALP:
-
alkaline phosphatase
- A/G:
-
albumin/globulin
- CK:
-
creatine kinase
- GLB:
-
globulin
- TP:
-
total protein
- CREA:
-
creatinine
- TG:
-
triglyceride
- ALB:
-
albumin
- TC:
-
cholesterol
References
Walther B, Tedin K, Lübke-Becker A (2017) Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Vet Microbiol 200:71–78. https://doi.org/10.1016/j.vetmic.2016.05.017
Carmo LP, Schüpbach-Regula G, Müntener C, Chevance A, Moulin G, Magouras I (2017) Approaches for quantifying antimicrobial consumption per animal species based on national sales data: a Swiss example, 2006 to 2013. Eurosurveillance. https://doi.org/10.2807/1560-7917.ES.2017.22.6.30458
Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. https://doi.org/10.1016/S1473-3099(15)00424-7
Swain PS, Rao SB, Rajendran D, Dominic G, Selvaraju S (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim Nutr 2:134–141. https://doi.org/10.1016/j.aninu.2016.06.003
Gangadoo S, Stanley D, Hughes RJ, Moore RJ, Chapman J (2016) Nanoparticles in feed: progress and prospects in poultry research. Trends Food Sci Technol 58:115–126. https://doi.org/10.1016/j.tifs.2016.10.013
Chang Z, Zhang H, Dong H, Mehmood K, Ijaz M, Ahmad H, Naeem M, Wu Q, Nabi F, Zhu H (2018) Effect of CuSO4 and nano copper on serum antioxidant capacity in Weaned piglets. J Biol Regul Homeost Agents 32:219–224
Chang Z, Zhang H, Mehmood K, Luo M, Zhao Y, Nabi WuX, Tian X, Liu X, Zhou D (2018) Effect of nano copper on visceral organs and the contents of trace elements in weanling pigs. Toxin Rev. https://doi.org/10.1080/15569543.2017.1421559
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67:952S–959S. https://doi.org/10.1093/ajcn/67.5.952S
Borkow G, Gabbay J (2005) Copper as a biocidal tool. Curr Med Chem 12:2163–2175. https://doi.org/10.2174/0929867054637617
Villanueva ME, Diez AMadR, González JA, Pérez CJ, Orrego M, Piehl L, Teves S, Copello GJ (2016) Antimicrobial activity of starch hydrogel incorporated with copper nanoparticles. ACS Appl Mater Interfaces 8:16280–16288. https://doi.org/10.1021/acsami.6b02955
Azizi M, Ghourchian H, Yazdian F, Dashtestani F, AlizadehZeinabad H (2017) Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB 231. PLoS ONE 12:e0188639. https://doi.org/10.1371/journal.pone.0188639
Mroczek-Sosnowska N, Łukasiewicz M, Wnuk A, Sawosz E, Niemiec J, Skot A, Jaworski S, Chwalibog A (2016) In ovo administration of copper nanoparticles and copper sulfate positively influences chicken performance. J Sci Food Agric 96:3058–3062. https://doi.org/10.1002/jsfa.7477
Tang H, Xu M, Zhou X, Zhang Y, Zhao L, Ye G, Shi F, Lv C, Li Y (2018) Acute toxicity and biodistribution of different sized copper nano-particles in rats after oral administration. Mater Sci Eng C 93:649–663. https://doi.org/10.1016/j.msec.2018.08.032
Lee I-C, Ko J-W, Park S-H, Shin N-R, Shin I-S, Moon C, Kim J-H, Kim H-C, Kim J-C (2016) Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Particle Fibre Toxicol 13:56. https://doi.org/10.1186/s12989-016-0169-x
Braakhuis HM, Gosens I, Krystek P, Boere JA, Cassee FR, Fokkens PH, Post JA, Van Loveren H, Park MV (2014) Particle size dependent deposition and pulmonary inflammation after short-term inhalation of silver nanoparticles. Particle Fibre Toxicol 11:49. https://doi.org/10.1186/s12989-014-0049-1
Zhang H, Chang Z, Mehmood K, Abbas RZ, Nabi F, Rehman MU, Wu X, Tian X, Yuan X, Li Z (2018) Nano copper induces apoptosis in PK-15 cells via a mitochondria-mediated pathway. Biol Trace Elem Res 181:62–70. https://doi.org/10.1007/s12011-017-1024-0
Cholewińska E, Ognik K, Fotschki B, Zduńczyk Z, Juśkiewicz J (2018) Comparison of the effect of dietary copper nanoparticles and one copper (II) salt on the copper biodistribution and gastrointestinal and hepatic morphology and function in a rat model. PLoS ONE 13:e0197083. https://doi.org/10.1371/journal.pone.0197083
Shi M, Kwon HS, Peng Z, Elder A, Yang H (2012) Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 6:2157–2164. https://doi.org/10.1021/nn300445d
Worthington KL, Adamcakova-Dodd A, Wongrakpanich A, Mudunkotuwa IA, Mapuskar KA, Joshi VB, Guymon CA, Spitz DR, Grassian VH, Thorne PS (2013) Chitosan coating of copper nanoparticles reduces in vitro toxicity and increases inflammation in the lung. Nanotechnology 24:395101. https://doi.org/10.1088/0957-4484/24/39/395101
Poloyac SM (2017) Drug and fatty acid cytochrome P450 metabolism in critical care. In: Drug metabolism in diseases. Academic Press, Boston, pp 115–138. https://doi.org/10.1016/B978-0-12-802949-7.00005-5
Wang J, Rahman MF, Duhart HM, Newport GD, Patterson TA, Murdock RC, Hussain SM, Schlager JJ, Ali SF (2009) Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 30:926–933. https://doi.org/10.1016/j.neuro.2009.09.005
Ekhart C, Matic M, Kant A, van Puijenbroek E, Schaik RV (2017) CYP450 genotype and aggressive behavior on selective serotonin reuptake inhibitors. Pharmacogenomics 18:613–620. https://doi.org/10.2217/pgs-2017-0197
Baer-Dubowska W, Szaefer H (2013) Modulation of carcinogen-metabolizing cytochromes P450 by phytochemicals in humans. Expert Opin Drug Metab Toxicol 9:927–941. https://doi.org/10.1517/17425255.2013.795219
Morgan ET (2017) Regulation of drug-metabolizing enzymes and drug metabolism by inflammatory responses. In: Drug metabolism in diseases. Academic Press, Boston, pp 21–58. https://doi.org/10.1016/B978-0-12-802949-7.00002-X
Goossens J, De Bock L, Osselaere A, Verbrugghe E, Devreese M, Boussery K, Van Bocxlaer J, De Backer P, Croubels S (2013) The mycotoxin T-2 inhibits hepatic cytochrome P4503A activity in pigs. Food Chem Toxicol 57:54–56. https://doi.org/10.1016/j.fct.2013.03.009
Xu S, Wu Q, Zhang B, Li H, Xu Y, Du Y, Wei L, Liu J (2016) Comparison of mercury sulfides with mercury chloride and methylmercury on hepatic P450, phase-2 and transporter gene expression in mice. J Trace Elem Med Biol 37:37–43. https://doi.org/10.1016/j.jtemb.2016.06.006
Daniel W, Haduch A, Syrek M, Boksa J (2006) Direct and indirect interactions between antidepressant drugs and CYP2C6 in the rat liver during long-term treatment. Eur Neuropsychopharmacol 16:580–587. https://doi.org/10.1016/j.euroneuro.2006.01.004
Haduch A, Wójcikowski J, Daniel W (2006) The effect of tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs) and newer antidepressant drugs on the activity and level of rat CYP3A. Eur Neuropsychopharmacol 16:178–186. https://doi.org/10.1016/j.euroneuro.2005.08.004
Zhu W-J, Zhang Z-W, Wang X-S, Xu S-W, Li M, Li S (2014) Effects of avermectin on microsomal cytochrome P450 enzymes in the liver and kidneys of pigeons. Environ Toxicol Pharmacol 38:562–569. https://doi.org/10.1016/j.etap.2014.08.011
Peng Y, Wu H, Zhang X, Zhang F, Qi H, Zhong Y, Wang Y, Sang H, Wang G, Sun J (2015) A comprehensive assay for nine major cytochrome P450 enzymes activities with 16 probe reactions on human liver microsomes by a single LC/MS/MS run to support reliable in vitro inhibitory drug–drug interaction evaluation. Xenobiotica 45:961–977. https://doi.org/10.3109/00498254.2015.1036954
Rao MN, Biju B, Ansar A, Mujeeb S, Ramesh M, Srinivas N (2003) ‘Open access’ generic method for continuous determination of major human CYP450 probe substrates/metabolites and its application in drug metabolism studies. Xenobiotica 33:1233–1245. https://doi.org/10.1080/00498250310001636877
Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J (2006) The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 26:4752–4762. https://doi.org/10.1523/JNEUROSCI.0099-06.2006
Chantratita N, Tandhavanant S, Myers ND, Seal S, Arayawichanont A, Kliangsa-ad A, Hittle LE, Ernst RK, Emond MJ, Wurfel MM (2013) Survey of innate immune responses to Burkholderia pseudomallei in human blood identifies a central role for lipopolysaccharide. PLoS ONE 8:e81617. https://doi.org/10.1371/journal.pone.0081617
Manna P, Ghosh M, Ghosh J, Das J, Sil PC (2012) Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: role of IκBα/NF-κB, MAPKs and mitochondrial signal. Nanotoxicology 6:1–21. https://doi.org/10.3109/17435390.2011.552124
Chen Z, Meng H, Yuan H, Xing G, Chen C, Zhao F, Wang Y, Zhang C, Zhao Y (2007) Identification of target organs of copper nanoparticles with ICP-MS technique. J Radioanal Nuclear Chem 272:599–603. https://doi.org/10.1007/s10967-007-0631-1
Xu M, Tang H, Zhou X, Chen H, Dong Q, Zhang Y, Ye G, Shi F, Lv C, Jing B (2018) Effects and mechanisms of sub-chronic exposure to copper nanoparticles on renal cytochrome P450 enzymes in rats. Environ Toxicol Pharmacol 63:135–146. https://doi.org/10.1016/j.etap.2018.08.004
Prabhu BM, Ali SF, Murdock RC, Hussain SM, Srivatsan M (2010) Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology 4:150–160. https://doi.org/10.3109/17435390903337693
Ingle AP, Duran N, Rai M (2014) Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol 98:1001–1009. https://doi.org/10.1007/s00253-013-5422-8
Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y (2011) Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 7:1322–1337. https://doi.org/10.1002/smll.201100001
Xu P, Xu J, Liu S, Yang Z (2012) Nano copper induced apoptosis in podocytes via increasing oxidative stress. J Hazard Mater 241:279–286. https://doi.org/10.1016/j.jhazmat.2012.09.041
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int. https://doi.org/10.1155/2013/942916
Sarkar A, Das J, Manna P, Sil PC (2011) Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology 290:208–217. https://doi.org/10.1016/j.tox.2011.09.086
Zhang H, Wu X, Mehmood K, Chang Z, Li K, Jiang X, Nabi F, Ijaz M, Rehman MU, Javed MT (2017) Intestinal epithelial cell injury induced by copper containing nanoparticles in piglets. Environ Toxicol Pharmacol 56:151–156. https://doi.org/10.1016/j.etap.2017.09.010
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4:707–716. https://doi.org/10.1016/j.actbio.2007.11.006
Lee H-J, Song JY, Kim BS (2013) Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity. J Chem Technol Biotechnol 88:1971–1977. https://doi.org/10.1002/jctb.4052
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163. https://doi.org/10.1016/S0300-483X(03)00159-8
Renton KW (2001) Alteration of drug biotransformation and elimination during infection and inflammation. Pharmacol Ther 92:147–163. https://doi.org/10.1016/S0163-7258(01)00165-6
Morgan E (2009) Impact of infectious and inflammatory disease on cytochrome P450–mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther 85:434–438. https://doi.org/10.1038/clpt.2008.302
Robertson G, Leclercq I, Farrell GC (2001) II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol 281:G1135–G1139. https://doi.org/10.1152/ajpgi.2001.281.5.G1135
Aitken AE, Morgan ET (2007) Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos 35:1687–1693. https://doi.org/10.1124/dmd.107.015511
Sunman JA, Hawke RL, LeCluyse EL, Kashuba AD (2004) Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab Dispos 32:359–363. https://doi.org/10.1124/dmd.32.3.359
Wang Y-M, Ong SS, Chai SC, Chen T (2012) Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol 8:803–817. https://doi.org/10.1517/17425255.2012.685237
Kodama S, Koike C, Negishi M, Yamamoto Y (2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24:7931–7940. https://doi.org/10.1128/MCB.24.18.7931-7940.2004
Aleksunes LM, Klaassen CD (2012) Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab Dispos 40:1366–1379. https://doi.org/10.1124/dmd.112.045112
Morgan ET (2001) Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab Dispos 29:207–212
Mallick P, Taneja G, Moorthy B, Ghose R (2017) Regulation of drug-metabolizing enzymes in infectious and inflammatory disease: implications for biologics–small molecule drug interactions. Expert Opin Drug Metab Toxicol 13:605–616. https://doi.org/10.1080/17425255.2017.1292251
Rushmore TH, Tony Kong A (2002) Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab 3:481–490. https://doi.org/10.2174/1389200023337171
Gerbal-Chaloin S, Iankova I, Maurel P, Daujat-Chavanieu M (2013) Nuclear receptors in the cross-talk of drug metabolism and inflammation. Drug Metab Rev 45:122–144. https://doi.org/10.3109/03602532.2012.756011
Li W, Qiu X, Jiang H, Zhi Y, Fu J, Liu J (2015) Ulinastatin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of AMPK/NF-κB pathway. Int Immunopharmacol 29:560–567. https://doi.org/10.1016/j.intimp.2015.09.028
Zordoky BN, El-Kadi AO (2009) Role of NF-κB in the regulation of cytochrome P450 enzymes. Curr Drug Metab 10:164–178. https://doi.org/10.2174/138920009787522151
Febvre-James M, Bruyère A, Le Vée M, Fardel O (2018) The JAK1/2 inhibitor ruxolitinib reverses interleukin-6-mediated suppression of drug-detoxifying proteins in cultured human hepatocytes. Drug Metab Dispos 46:131–140. https://doi.org/10.1124/dmd.117.078048
Acknowledgements
The authors thank Bo Jing for technical assistance and help in the lab, and Ms. Salazar Monique S for kind help to edit the language for us.
Funding
This work was supported by the Guizhou Provincial Science and Technology Fund Project, China (No: J[2019]1313).
Author information
Authors and Affiliations
Contributions
HT, MX and JL are the co-first authors, they performed the exposure experiment, the histological examination of the liver, the biochemical analyses and the statistical analyses. JL and LZ supervised the histological examinations and designed the study. GY supervised the biochemical analyses. FS did the data analysis. Cl, HC and YW performed the activity analyses. YL supervised all the analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments were conducted in strict accordance with Guiding Principles for the Experimental Animal Ethics Committee and were approved by the Animal Ethical Committee of Sichuan Agricultural University (#:20171120).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tang, H., Xu, M., Luo, J. et al. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ Sci Eur 31, 30 (2019). https://doi.org/10.1186/s12302-019-0214-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-019-0214-0