Abstract
Background
The efficacy and safety of platelet-rich plasma (PRP) augmentation for arthroscopic meniscal repair is controversial. This meta-analysis compared arthroscopic meniscal repair performed in isolation or augmented with PRP.
Methods
The present study was conducted according to PRISMA 2020 guidelines. Pubmed, Web of Science, Google Scholar and Embase were accessed in August 2021. All the clinical trials which compared arthroscopic meniscal repair performed in isolation or augmented with PRP were included.
Results
Eight hundred thirty-seven patients were included: 38% (318 of 837 patients) were women; the mean age of the patients was 35.6 (range, 20.8–64.3) years; the mean follow-up was 26.2 (range, 6–54) months. Similarity was found in analogue scale (VAS) (P = 0.5) and Lysholm (P = 0.9), and International Knee Documentation Committee (IKDC) scores (P = 0.9). Similarity was found in the rate of failure (P = 0.4) and rate of revision (P = 0.07).
Conclusion
The current published scientific evidence does not support PRP augmentation for arthroscopic meniscal repair.
Similar content being viewed by others
Introduction
Meniscal lesions are common, with an estimated prevalence of 12% in the adult population [1]. Arthroscopic meniscectomy has been widely performed to reduce pain deriving from these lesions and restore patients’ quality of life [2, 3]. Observational studies have demonstrated that meniscectomy is associated with early onset osteoarthritis [4,5,6,7,8,9]. Therefore, resection of the meniscal structures should be minimised or even avoided [2, 10, 11]. Patients with a meniscal tear but otherwise healthy meniscal tissue who wish to remain active may benefit from a meniscal repair [9, 12, 13]. Intra-meniscal injections of growth factors, including those present in platelets, could stimulate cell activity and favour meniscal healing [14,15,16]. The regenerative potential of platelet rich plasma (PRP) has been documented [15,16,17,18]. PRP is obtained by centrifugation of platelets extracted from peripheral venous blood [19, 20]. Given its regenerative properties (e.g. neoangiogenesis, proteins synthesis, cell proliferation and migration), PRP has been used in the conservative management of several knee ailments including osteoarthritis [21, 22] and meniscal lesions [21, 23, 24]. The efficacy of PRP augmentation has also been investigated in arthroscopic meniscal repair [15, 20, 25,26,27,28,29,30,31]. However, the results from these studies are controversial, and the actual benefit of PRP augmentation for arthroscopic meniscal repair is unclear. Therefore, a meta-analysis was conducted hypothesising that PRP augmentation in combination with arthroscopic meniscal repair would lead to greater patient-reported outcome measures (PROMs) and accelerate the healing process.
Material and methods
Eligibility criteria
All the clinical trials comparing arthroscopic isolated meniscal repair with meniscal repair augmented with PRP were accessed. According to the authors’ language capabilities, articles in English, German, Italian, French and Spanish were eligible. Only studies with evidence levels I–III, according to the Oxford Centre of Evidence-Based Medicine [32], were considered. Reviews, technical notes, comments, letters, editorials, protocols and guidelines were excluded. Biomechanical, computational, in vitro, animal and cadaveric studies were also not eligible. Only studies published in peer reviewed journals were eligible. Studies combining PRP with other procedures were not considered, nor were those augmenting arthroscopic meniscal repair with other compounds. Only studies reporting data with a minimum of 6 months follow-up were eligible. Studies evaluating experimental rehabilitation programs were not considered. Studies which performed mini-arthrotomy and/or those concerning meniscal repair in revision settings were not eligible. Only studies reporting quantitative data under the outcomes of interest were considered for inclusion.
Search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [33]. The PICOT framework was followed:
-
P (Population): meniscal tears;
-
I (Intervention): isolated arthroscopic meniscal repair;
-
C (Comparison): arthroscopic meniscal repair augmented with PRP;
-
O (Outcomes): PROMs, complications;
-
T (Timing): minimum 6 months follow-up.
In August 2021, the following databases were accessed: Pubmed, Web of Science, Google Scholar and Embase. No time constraints were used for the search. The following keywords were used in combination: meniscal, menisci, augmentation, PRP, repair, combined, isolated, knee, arthroscopy, platelet-rich plasma, meniscopathy, damage, injury, tear, patient reported outcome measures, PROMs, Lysholm, IKDC, failure, complications, pain, revision, visual analogue scale.
Selection and data collection
Two authors (F.M.;F.C.) independently performed the database search. All the resulting titles were screened and, if suitable, the abstracts were accessed. The full-text of the abstracts which matched the topic were subsequently accessed. A cross reference of the bibliography of the full-text articles was also accomplished to identify additional articles. Disagreements were debated and solved by a third author (N.M.*).
Data items
Two authors (**;**) independently performed data extraction. Study generalities (author and year, journal, study design, length of the follow-up) were collected. Patient demographic at baseline was retrieved: number of procedures, mean age, percentage of women, visual analogue scale (VAS), and time elapsed between the injury and arthroscopy. The following data were extracted at last follow-up: International Knee Documentation Committee (IKDC) [34], Lysholm score [35], VAS, rates of failure and revision. Failure was defined as the recurrence of meniscal symptoms or the request by the patient to repeat arthroscopy [20, 25].
Study risk of bias assessment
The risk of bias was assessed using Review Manager 5.3 software (The Nordic Cochrane Collaboration, Copenhagen). The risk of bias was evaluated based on the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions [36]. Two reviewers (**;**) evaluated the risk of bias of the extracted studies. The following endpoints were evaluated: selection, detection, performance, attrition, reporting and other bias. To assess the overall risk of publication bias, the funnel plot of the most commonly reported outcome was performed. The funnel plot charted the standard error (SE) of the log odds ratio (Log OR) versus its OR. The degree of asymmetry of the plot is directly proportional to the degree of bias. To assess the risk of bias of each included studies, a risk of bias graph was created.
Synthesis methods
The statistical analyses were performed by the main author (**) using Review Manager 5.3 software (The Nordic Cochrane Collaboration, Copenhagen). For descriptive statistics, mean difference and standard deviation were used. A t-test was performed to assess baseline comparability, with values of P > 0.1 considered satisfactory. For continuous data, the inverse variance method with mean difference (MD) effect measure was used. For binary data, the Mantel–Haenszel method with odds ratio (OR) effect measure was used. The confidence interval (CI) was set at 0.95 in all the comparisons. Heterogeneity was assessed using \(\chi\)2 and Higgins-I2 tests. If \(\chi\)2 > 0.05, no statistically significant heterogeneity was found. If \(\chi\)2 < 0.05 and Higgins-I2 > 60%, high heterogeneity was found. A fixed model effect was used as default. In case of high heterogeneity, a random model was used. Overall values of P < 0.05 were considered statistically significant.
Results
Study selection
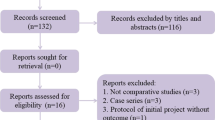
The literature search resulted in 1435 articles. Of these, 407 articles were duplicates. A further 1013 articles were not eligible as they did not match the following inclusion criteria: study design (N = 298), non-comparative studies (N = 109), not matching the topic of the study (N = 582), combining multiple or experimental procedures (N = 13), short follow-up and/or limited study size (N = 3) and uncertain data (N = 8). A further seven studies were excluded as they did not report quantitative data under the outcomes of interest. Finally, eight studies were included for formal analysis. The literature search results are shown in Fig. 1.
Methodological quality assessment
The limited number of randomized clinical trials (three out of nine studies) increased the risk of selection bias, which was low to moderate. The selection criteria were often biased, and the general heath measures were rarely reported. Given the lack of blinding in most studies, the risk of detection bias was moderate. Attrition and reporting biases were both low to moderate. The risk of other potential biases was moderate. In conclusion, the overall risk of bias among the included studies was moderate (Fig. 2).
Risk of publication bias
To evaluate the risk of publication bias, the funnel plot of the most commonly reported outcome (rate of revision) was performed. The plot evidenced good symmetry, with most of the referral points included within the pyramidal shapes. In conclusion, the risk of publications bias was low (Fig. 3).
Study characteristics and patient demographic
A total of 837 patients were included: 38% (318 of 837 patients) were women; the mean age of the patients was 35.6 (range, 20.8–64.3) years; the mean follow-up was 26.2 (range, 6–54) months. Good comparability was found between the two groups in terms of mean age, percentage of women, time elapsed between injury and arthroscopy, and VAS (P > 0.1). Concerning the centrifugation procedure, a median rate of 1500 rpm for the first centrifugation, followed by a second centrifugation at 3400 rpm was found, with a mean extracted venepuncture volume of 94.8 ml. The mean platelet concentration after preparation was significantly greater than that of blood. The demographics of the included studies are presented in Table 1.
Synthesis of results
Similarity was found in VAS (P = 0.5), Lysholm score (P = 0.99) and IKDC (P = 0.9) (Fig. 4).
Similarity was found the rate of failure (P = 0.4) and rate of revision (P = 0.07) (Fig. 5).
Discussion
According to the main findings of the present meta-analysis, the current level I of evidence does not support PRP augmentation for arthroscopic meniscal repair. At approximately 2 years follow-up, PRP augmentation demonstrated similar VAS, Lysholm, and IKDC scores compared with isolated arthroscopic meniscal repair. Moreover, no differences were detected in failure and revision rates. Following its introduction in the 1950s, PRP application has been employed for regenerative medicine purposes, and extended to musculoskeletal disorders since the 1980s [19, 37, 38]. Given their limited vascularisation and metabolic activity, the menisci of the knee exhibit poor regenerative capacity [39,40,41]. Growth factor injections may potentially activate the meniscal cells and stimulate regeneration [42, 43]. Platelets exhibit a high concentration of growth factors and mediators, such as transforming growth factor (TGF)-β, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1) and basic fibroblast growth factor (b-FGF), which enhance chemotaxis, angiogenesis, cell proliferation and extracellular matrix formation [42]. Therefore, injections of PRP are believed to accelerate healing and improve regeneration [43,44,45,46,47,48]. The benefits of PRP for cartilage regeneration have been recognised [49, 69, 70]. PRP reduces catabolism and increases the anabolic activity of hyaline cartilage [23]. Meniscal catabolic activity is crucial for osteoarthritis progression in the knee [50,51,52]. In vitro studies demonstrated the antinociceptive and proliferative properties of PRP, which increased extracellular matrix production and meniscal tissue regeneration [50, 53]. Furthermore, PRP improved meniscal cell viability in animal studies, increasing meniscal compressive strength through the overexpression of proteoglycans [54,55,56]. The current evidence of PRP augmentation for arthroscopic meniscal repair is controversial. Four of the included studies [25, 26, 30, 31] reported no statistically significant differences in pain assessment using VAS scores. Current evidence on the effects of PRP on pain is controversial [29, 56,57,58]. In the present investigation, 63% (five out of eight) studies reported no significant differences in IKDC scores in the PRP-augmented groups. The IKDC score was also compared in recent systematic reviews, with similar results [29, 59].
A recent systematic review evaluating six studies (309 patients) reached similar conclusions [29]. However, other investigations, evidenced controversial results. In a systematic review including five studies (82 procedures), PRP enhanced meniscal repair and resulted in a lower failure rate, but the evidence was not compelling enough to support the use of PRP in meniscal repair [57]. Another systematic review of five studies (274 procedures) concluded that PRP augmentation during arthroscopic meniscal repair is related to better outcomes and leads to significantly lower failure rates (from 26.7% to 50%) [59]. Similar findings were evidenced in a meta-analysis of six studies (111 procedures), in which PRP augmentation did reduce the risk of failure (from 25.7% to 9.9%) [60]. In 5323 procedures (83 studies), PRP resulted in better outcomes following meniscectomy [61]. Wang et al. [58], in a meta-analysis of 293 patients (six studies), found that PRP injection can improve the efficacy of arthroscopic meniscal repair, reducing the failure rate and severity of pain. This diversity may be explained by the heterogeneous criteria, dosage and procedures included, which led to variable results.
This meta-analysis has several limitations. The limited number of studies included and the relatively small size in the cohorts in the various investigations do not allow reliable conclusions to be inferred. The retrospective design of most of the included studies represents another important limitation. Moreover, between-study heterogeneity with regard to the length of follow-up was evident. Post-operative rehabilitation was seldom described, and the length of the follow-up was limited in most of the included studies. The description of surgical technique was not adequately reported in some studies, which represents a further limitation. Heterogeneity in PRP preparation and processing protocols was evident, as were between-study differences with regard to the initial whole blood volume and centrifugation rate and duration [62,63,64,65]. Battaglia et al. [66] used 150 ml of venous blood followed by centrifugation at 1800 rpm for 15 min and a further centrifugation at 3500 rpm for 10 min, resulting in 20 ml of PRP (four units of 5 ml each). Dallari et al. [63] collected 150 ml of peripheral blood and performed two centrifugations.The first centrifugation to separate erythrocytes from platelets was performed at 1480 rpm for 6 min, the second to concentrate them was performed at 3400 rpm for 15 min. They further added 1 ml of calcium chloride to activate platelets [63]. Doria et al. [64] performed two centrifugations, lasting 6 and 15 min, without adding calcium chloride [64]. Calcium chloride (CaCl2) is an exogenous coagulation factors which aims to clot the PRP [67, 68]. However, its use is still debated, and consensus has not been reached. Further investigations to validate and standardise PRP preparation procedures are required. Between-study heterogeneity with regard to the timing of the injection was also detected. Some authors performed PRP injections during meniscal repair [25, 27, 28, 30]. To achieve closer contact between PRP and the meniscal lesion, Dai et al. [25] performed the injections after the meniscal suture but before those sutures were fastened. Kaminski et al. [30] performed PRP injections during the meniscal repair in an arthroscopically guided fashion. Duif et al. [26] performed PRP injections after the arthroscopic procedure with a sterile syringe through the anterolateral portal. In contrast, Kemmochi et al. [20] injected the PRP before the arthroscopic procedure. These differences between protocols may produce marked clinical differences and, given the limited quantitative data available, further subgroup analyses were not possible.
Conclusion
The current evidence does not support PRP augmentation when performing arthroscopic meniscal repair. At approximately 2 years follow-up, PRP augmentation demonstrated similar VAS, Lysholm, and IKDC scores compared with isolated arthroscopic meniscal repair. Moreover, no differences were detected in failure and revision rates.
Availability of data and materials
The data underlying this article are available in the article.
Abbreviations
- PRP:
-
Platelet rich plasma
- PROMs:
-
Patient-reported outcome measures
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- VAS:
-
Analogue scale
- IKDC:
-
International Knee Documentation Committee
- MD:
-
Mean difference
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Logerstedt DS, Snyder-Mackler L, Ritter RC, Axe MJ, Orthopedic Section of the American Physical Therapy A (2010) Knee pain and mobility impairments: meniscal and articular cartilage lesions. J Orthop Sports Phys Ther 40(6):A1–A35
LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF (2015) Meniscal root tears: a classification system based on tear morphology. Am J Sports Med 43(2):363–369
Shelbourne KD, Gray T (2000) Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med 28(4):446–52
Beaufils P, Becker R, Kopf S, Englund M, Verdonk R, Ollivier M et al (2017) Surgical management of degenerative meniscus lesions: the 2016 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc 25(2):335–346
Abram SGF, Hopewell S, Monk AP, Bayliss LE, Beard DJ, Price AJ (2020) Arthroscopic partial meniscectomy for meniscal tears of the knee: a systematic review and meta-analysis. Br J Sports Med 54(11):652–663
Feeley BT, Lau BC (2018) Biomechanics and clinical outcomes of partial meniscectomy. J Am Acad Orthop Surg 26(24):853–863
Krych AJ, Hevesi M, Leland DP, Stuart MJ (2020) Meniscal root injuries. J Am Acad Orthop Surg 28(12):491–499
Longo UG, Ciuffreda M, Candela V, Rizzello G, D’Andrea V, Mannering N et al (2019) Knee osteoarthritis after arthroscopic partial meniscectomy: prevalence and progression of radiographic changes after 5 to 12 years compared with contralateral knee. J Knee Surg 32(5):407–413
Faucett SC, Geisler BP, Chahla J, Krych AJ, Kurzweil PR, Garner AM et al (2019) Meniscus root repair vs meniscectomy or nonoperative management to prevent knee osteoarthritis after medial meniscus root tears: clinical and economic effectiveness. Am J Sports Med 47(3):762–769
Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, Rodeo SA (2015) The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin Anat 28(2):269–287
Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K (2014) Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med 42(5):1049–1057
O’Donnell K, Freedman KB, Tjoumakaris FP (2017) Rehabilitation protocols after isolated meniscal repair: a systematic review. Am J Sports Med 45(7):1687–1697
Mueller BT, Moulton SG, O’Brien L, LaPrade RF (2016) Rehabilitation following meniscal root repair: a clinical commentary. J Orthop Sports Phys Ther 46(2):104–113
Andia I, Sanchez M, Maffulli N (2010) Tendon healing and platelet-rich plasma therapies. Expert Opin Biol Ther 10(10):1415–1426
Pujol N, Salle De Chou E, Boisrenoult P, Beaufils P (2015) Platelet-rich plasma for open meniscal repair in young patients: any benefit? Knee Surg Sports Traumatol Arthrosc 23(1):51–8
Oryan A, Alidadi S, Moshiri A (2016) Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther 16(2):213–232
Andia I, Maffulli N (2020) Dose-dependent effects of platelet-rich plasma powder on chondrocytes in vitro: letter to the editor. Am J Sports Med 48(14):NP59
Andia I, Sanchez M, Maffulli N (2012) Joint pathology and platelet-rich plasma therapies. Expert Opin Biol Ther 12(1):7–22
Wu PI, Diaz R, Borg-Stein J (2016) Platelet-rich plasma. Phys Med Rehabil Clin N Am 27(4):825–853
Kemmochi M, Sasaki S, Takahashi M, Nishimura T, Aizawa C, Kikuchi J (2018) The use of platelet-rich fibrin with platelet-rich plasma support meniscal repair surgery. J Orthop 15(2):711–720
Nourissat G, Mainard D, Kelberine F, French AS (2013) Current concept for the use of PRP in arthroscopic surgery. Orthop Traumatol Surg Res 99(8 Suppl):S407–S410
Bennell KL, Hunter DJ, Paterson KL (2017) Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep 19(5):24
Braun HJ, Wasterlain AS, Dragoo JL (2013) The use of PRP in ligament and meniscal healing. Sports Med Arthrosc Rev 21(4):206–212
Scotti C, Hirschmann MT, Antinolfi P, Martin I, Peretti GM (2013) Meniscus repair and regeneration: review on current methods and research potential. Eur Cell Mater 26:150–170
Dai WL, Zhang H, Lin ZM, Shi ZJ, Wang J (2019) Efficacy of platelet-rich plasma in arthroscopic repair for discoid lateral meniscus tears. BMC Musculoskelet Disord 20(1):113
Duif C, Vogel T, Topcuoglu F, Spyrou G, von Schulze PC, Lahner M (2015) Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial. Arch Orthop Trauma Surg 135(7):971–977
Everhart JS, Cavendish PA, Eikenberry A, Magnussen RA, Kaeding CC, Flanigan DC (2019) Platelet-rich plasma reduces failure risk for isolated meniscal repairs but provides no benefit for meniscal repairs with anterior cruciate ligament reconstruction. Am J Sports Med 47(8):1789–1796
Griffin JW, Hadeed MM, Werner BC, Diduch DR, Carson EW, Miller MD (2015) Platelet-rich plasma in meniscal repair: does augmentation improve surgical outcomes? Clin Orthop Relat Res 473(5):1665–1672
Belk JW, Kraeutler MJ, Thon SG, Littlefield CP, Smith JH, McCarty EC (2020) Augmentation of meniscal repair with platelet-rich plasma: a systematic review of comparative studies. Orthop J Sports Med 8(6):2325967120926145
Kaminski R, Kulinski K, Kozar-Kaminska K, Wielgus M, Langner M, Wasko MK et al (2018) A prospective, randomized, double-blind, parallel-group, placebo-controlled study evaluating meniscal healing, clinical outcomes, and safety in patients undergoing meniscal repair of unstable, complete vertical meniscal tears (bucket handle) augmented with platelet-rich plasma. Biomed Res Int 2018:9315815
Kaminski R, Maksymowicz-Wleklik M, Kulinski K, Kozar-Kaminska K, Dabrowska-Thing A, Pomianowski S (2019) Short-term outcomes of percutaneous trephination with a platelet rich plasma intrameniscal injection for the repair of degenerative meniscal lesions. A prospective, randomized, double-blind, parallel-group, placebo-controlled study. Int J Mol Sci. https://doi.org/10.3390/ijms20040856
Howick J CI, Glasziou P, Greenhalgh T, Carl Heneghan, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M (2011) The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine. https://www.cebmnet/indexaspx?o=5653
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P et al (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29(5):600–613
Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD (1998) Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am 80(8):1132–1145
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP et al (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev 10:ED000142
Tayapongsak P, O’Brien DA, Monteiro CB, Arceo-Diaz LY (1994) Autologous fibrin adhesive in mandibular reconstruction with particulate cancellous bone and marrow. J Oral Maxillofac Surg 52(2):161–165 (discussion 6)
Marx RE (2001) Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 10(4):225–228
Arnoczky SP, Warren RF (1983) The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med 11(3):131–41
Kobayashi K, Fujimoto E, Deie M, Sumen Y, Ikuta Y, Ochi M (2004) Regional differences in the healing potential of the meniscus-an organ culture model to eliminate the influence of microvasculature and the synovium. Knee 11(4):271–278
Mesiha M, Zurakowski D, Soriano J, Nielson JH, Zarins B, Murray MM (2007) Pathologic characteristics of the torn human meniscus. Am J Sports Med 35(1):103–112
Goncalves NJN, Frantz N, de Oliveira RM (2020) Platelet-rich plasma (PRP) therapy: an approach in reproductive medicine based on successful animal models. Anim Reprod 16(1):93–98
Wasterlain AS, Braun HJ, Harris AH, Kim HJ, Dragoo JL (2013) The systemic effects of platelet-rich plasma injection. Am J Sports Med 41(1):186–193
Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA (2012) Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy 28(3):429–439
Weber AE, Bolia IK, Trasolini NA (2021) Biological strategies for osteoarthritis: from early diagnosis to treatment. Int Orthop 45(2):335–344
Centeno CJ, Pastoriza SM (2020) Past, current and future interventional orthobiologics techniques and how they relate to regenerative rehabilitation: a clinical commentary. Int J Sports Phys Ther 15(2):301–325
Mazzocca AD, McCarthy MB, Chowaniec DM, Dugdale EM, Hansen D, Cote MP et al (2012) The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med 40(8):1742–1749
Harris SE, Bonewald LF, Harris MA, Sabatini M, Dallas S, Feng JQ et al (1994) Effects of transforming growth factor beta on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res 9(6):855–863
Chellini F, Tani A, Zecchi-Orlandini S, Sassoli C (2019) Influence of platelet-rich and platelet-poor plasma on endogenous mechanisms of skeletal muscle repair/regeneration. Int J Mol Sci. https://doi.org/10.3390/ijms20030683
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. https://doi.org/10.3390/ijms21207794
Laudy AB, Bakker EW, Rekers M, Moen MH (2015) Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med 49(10):657–672
Nie LY, Zhao K, Ruan J, Xue J (2021) Effectiveness of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled clinical trials. Orthop J Sports Med 9(3):2325967120973284
Aghajanova L, Houshdaran S, Balayan S, Manvelyan E, Irwin JC, Huddleston HG et al (2018) In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J Assist Reprod Genet 35(5):757–770
Cook JL, Kuroki K, Stoker AM, Monibi FA, Roller BL (2017) Meniscal biology in health and disease. Connect Tissue Res 58(3–4):225–237
Ishida K, Kuroda R, Miwa M, Tabata Y, Hokugo A, Kawamoto T et al (2007) The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng 13(5):1103–1112
Popescu MB, Carp M, Tevanov I, Nahoi CA, Stratila MA, Haram OM et al (2020) Isolated meniscus tears in adolescent patients treated with platelet-rich plasma intra-articular injections: 3-month clinical outcome. Biomed Res Int 2020:8282460
Haunschild ED, Huddleston HP, Chahla J, Gilat R, Cole BJ, Yanke AB (2020) Platelet-rich plasma augmentation in meniscal repair surgery: a systematic review of comparative studies. Arthroscopy 36(6):1765–1774
Wang Y, Yao C, Yang Z, Guo W (2020) Clinical efficacy of platelet-rich plasma as adjuvant therapy in patients undergoing arthroscopic repair of meniscal injury. J Int Med Res 48(9):300060520955059
Sochacki KR, Safran MR, Abrams GD, Donahue J, Chu C, Sherman SL (2020) Platelet-rich plasma augmentation for isolated arthroscopic meniscal repairs leads to significantly lower failure rates: a systematic review of comparative studies. Orthop J Sports Med 8(11):2325967120964534
Zaffagnini S, Poggi A, Reale D, Andriolo L, Flanigan DC, Filardo G (2021) Biologic augmentation reduces the failure rate of meniscal repair: a systematic review and meta-analysis. Orthop J Sports Med 9(2):2325967120981627
Trams E, Kulinski K, Kozar-Kaminska K, Pomianowski S, Kaminski R (2020) The clinical use of platelet-rich plasma in knee disorders and surgery-a systematic review and meta-analysis. Life (Basel). https://doi.org/10.3390/life10060094
Garcia FL, Williams BT, Polce EM, Heller DB, Aman ZS, Nwachukwu BU et al (2020) Preparation methods and clinical outcomes of platelet-rich plasma for intra-articular hip disorders: a systematic review and meta-analysis of randomized clinical trials. Orthop J Sports Med 8(10):2325967120960414
Dallari D, Stagni C, Rani N, Sabbioni G, Pelotti P, Torricelli P et al (2016) Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis: a randomized controlled study. Am J Sports Med 44(3):664–671
Doria C, Mosele GR, Caggiari G, Puddu L, Ciurlia E (2017) Treatment of early hip osteoarthritis: ultrasound-guided platelet rich plasma versus hyaluronic acid injections in a randomized clinical trial. Joints 5(3):152–155
Redmond JM, Gupta A, Stake CE, Hammarstedt JE, Finch NA, Domb BG (2015) Clinical results of hip arthroscopy for labral tears: a comparison between intraoperative platelet-rich plasma and bupivacaine injection. Arthroscopy 31(3):445–453
Battaglia M, Guaraldi F, Vannini F, Rossi G, Timoncini A, Buda R et al (2013) Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics 36(12):e1501–e1508
Anitua E (1999) Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 14(4):529–535
Toyoda T, Isobe K, Tsujino T, Koyata Y, Ohyagi F, Watanabe T et al (2018) Direct activation of platelets by addition of CaCl2 leads coagulation of platelet-rich plasma. Int J Implant Dent 4(1):23
Alessio-Mazzola M, Felli L, Trentini R, Formica M, Capello AG, Lovisolo S et al (2021) Efficacy of autologous platelet-rich plasma injections for grade 3 symptomatic degenerative meniscal lesions: a 1-year follow-up prospective study. Sports Health. https://doi.org/10.1177/19417381211011074
Andia I, Maffulli N (2017) Biological therapies in regenerative sports medicine. Sports Med 47(5):807–828
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL. No external source of funding was used.
Author information
Authors and Affiliations
Contributions
F.M.: conceptualization, statistical analysis, writing, revision; F.C.: writing; L.C.: supervision; F.O.: revision; F.H.: supervision; N.M.: revision, writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Patient consent
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Migliorini, F., Cuozzo, F., Cipollaro, L. et al. Platelet-rich plasma (PRP) augmentation does not result in more favourable outcomes in arthroscopic meniscal repair: a meta-analysis. J Orthop Traumatol 23, 8 (2022). https://doi.org/10.1186/s10195-022-00630-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10195-022-00630-1