Abstract
Delirium, an acute and fluctuating disturbance of consciousness and cognition, is a common manifestation of acute brain dysfunction in critically ill patients, occurring in up to 80% of the sickest intensive care unit (ICU) populations. Critically ill patients are subject to numerous risk factors for delirium. Some of these, such as exposure to sedative and analgesic medications, may be modified to reduce risk. Although dysfunction of other organ systems continues to receive more clinical attention, delirium is now recognized to be a significant contributor to morbidity and mortality in the ICU, and it is recommended that all ICU patients be monitored using a validated delirium assessment instrument. Patients with delirium have longer hospital stays and lower 6-month survival than do patients without delirium, and preliminary research suggests that delirium may be associated with cognitive impairment that persists months to years after discharge. Little evidence exists regarding the prevention and treatment of delirium in the ICU, but multicomponent interventions reduce the incidence of delirium in non-ICU studies. Strategies for the prevention and treatment of ICU delirium are the subjects of multiple ongoing investigations.
Similar content being viewed by others
Introduction
Recent advances in critical care medicine have improved survival in patients cared for in intensive care units (ICUs) worldwide, and in doing so they have revealed a major public health concern that previously had been under-appreciated. Critical care clinicians have historically been attuned to pulmonary, cardiac, and renal dysfunction as a source of morbidity and mortality in ICU patients but have underestimated the impact of brain dysfunction [1]. Delirium, a common manifestation of acute brain dysfunction in critically ill patients, is associated with poor short-term outcomes and may result in adverse sequelae years after ICU discharge [2–4], and an executive summary on preventable medical injuries commissioned by the American Association of Retired Persons identified delirium as one of six leading causes of injuries associated with hospitalization in patients over 65 years of age [5]. In this article we provide a general overview of the research to date regarding the epidemiology, diagnosis, and pathophysiology of ICU delirium, its association with health outcomes, and possible options for prevention and treatment.
Definition
Delirium is defined in the American Psychiatric Association's (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV [6] as a disturbance of consciousness and cognition that develops over a short period of time (hours to days) and fluctuates over time. Many different terms have been used to describe this syndrome of cognitive impairment in critically ill patients, including ICU psychosis, ICU syndrome, acute confusional state, encephalopathy, and acute brain failure [1, 7]. However, the critical care literature has recently conformed to the recommendations of the APA and other experts that the term 'delirium' be used uniformly to describe this syndrome of brain dysfunction.
Prevalence and subtypes
The prevalence of delirium reported in medical and surgical ICU cohort studies has varied from 20% to 80%, depending upon severity of illness observed and diagnostic methods used [3, 8–12]. Despite high prevalence rates in the ICU, delirium often goes unrecognized by clinicians [13] or its symptoms are incorrectly attributed to dementia, depression, or ICU syndrome (considered an expected, inconsequential complication of critical illness).
Delirium can be categorized into subtypes according to psychomotor behavior, and the high prevalence of hypoactive delirium in critically ill patients probably contributes to clinicians' lack of recognition of delirium. Hypoactive delirium is characterized by decreased responsiveness, withdrawal, and apathy, whereas hyperactive delirium is characterized by agitation, restlessness, and emotional lability [14]. Peterson and coworkers [15] examined delirium subtypes in a cohort of ventilated and nonventilated medical ICU patients, and they observed that purely hyperactive delirium was rare (1.6%). In contrast, 43.5% of patients had purely hypoactive delirium and 54.1% had mixed delirium. Among non-ICU patients, hyperactive delirium has been associated with a better prognosis than hypoactive delirium [16], but this relationship has not been evaluated thoroughly among ICU patients. Because it is clear, however, that delirium is associated with poor clinical outcomes in critically ill patients, routine monitoring using valid and reliable delirium diagnosis instruments is recommended in all ICUs so that the prognostic significance of delirium does not go unnoticed.
Another important categorization scheme was recently studied in the critical care literature. Ouimet and colleagues [17] evaluated 600 ICU patients for symptoms of delirium and categorized them according to the number of symptoms present. Patients with no symptoms were considered to have 'no delirium', those with four or more symptoms to have 'clinical delirium', and those with one to three symptoms to have 'subsyndromal delirium' (because the full syndrome, according to DSM-IV criteria, was not present). This important study corroborated in the ICU setting a finding that had previously been reported from non-ICU studies; subsyndromal delirium represents an intermediate state that is different from both clinical delirium and a normal neurologic state.
Prognostic significance
Among medical ICU patients, delirium is associated with multiple complications and adverse outcomes, including self-extubation and removal of catheters [9], failed extubation [18], prolonged hospital stay [2], increased health care costs [19], and increased mortality [3, 20, 21]. Ely and coworkers [3] studied 275 mechanically ventilated medical ICU patients and determined that delirium was associated with a threefold increase in risk for 6-month mortality after adjusting for age, severity of illness, co-morbidities, coma, and exposure to psychoactive medications. The association between ICU delirium and increased mortality was subsequently confirmed in two other cohort studies [20, 21].
Delirium may be a predictor of long-term cognitive impairment in survivors of critical illness. Jackson and colleagues [4] reviewed nine prospective studies that included nearly 1,900 non-ICU patients who were hospitalized for medical and surgical treatments, and reported that delirium was associated with cognitive decline over 1 to 3 years after hospital discharge. The relationship between ICU delirium and long-term cognitive impairment is the subject of ongoing investigations, but preliminary data suggest that the association is significant. Jackson and coworkers [22] recently examined this association in 98 patients who were mechanically ventilated for acute respiratory failure in medical ICUs. Prolonged periods of ICU delirium were associated with an increased risk for long-term cognitive impairment at 3 months post-discharge after adjusting for covariates (P < 0.001).
Pathophysiology
The pathophysiology of delirium is poorly understood but multiple promising hypotheses are subjects of ongoing research. The majority of studies supporting the hypotheses reviewed here were conducted in non-ICU patients. Thus, significant research is needed to elucidate the complex interplay between the mechanisms of critical illness and ICU delirium.
Neurotransmitter imbalance
Delirium is theorized to be a neurobehavioral manifestation of imbalances in the synthesis, release, and inactivation of neurotransmitters that normally control cognitive function, behavior, and mood [23]. Derangements of multiple neurotransmitter systems have been implicated in the pathophysiology of delirium, with the greatest focus being given to dopamine and acetylcholine. These neurotransmitters work in opposition, with dopamine increasing and acetylcholine decreasing neuronal excitability. An imbalance in one or both of these neurotransmitters results in neuronal instability and unpredictable neurotransmission. Specifically, an excess of dopamine [24] or depletion of acetylcholine [25] have been associated with delirium. Other neurotransmitters are likely to play a role in the pathogenesis of delirium as well, including γ-aminobutyric acid (GABA), serotonin, endorphins, and glutamate [23].
Inflammation
Inflammation plays a significant role in the dysfunction of multiple organs caused by critical illness [26], and inflammatory abnormalities induced by endotoxin and cytokines probably contribute to the development of ICU delirium. Inflammatory mediators produced during critical illness (for example, tumor necrosis factor-α, interleukin-1, and other cytokines and chemokines) initiate a cascade of endothelial damage, thrombin formation, and microvascular compromise [27]. Studies in animal models have revealed that these inflammatory mediators cross the blood-brain barrier [28], increase vascular permeability in the brain [29], and result in changes on electroencephalography (EEG) that are consistent with those seen in septic patients with delirium [30]. Inflammation may incite brain dysfunction by decreasing cerebral blood flow via the formation of microaggregates of fibrin, platelets, neutrophils, and erythrocytes in the cerebral microvasculature; by constricting cerebral vasculature through activation of α1-adrenoceptors [31]; or by interfering with neurotransmitter synthesis and neurotransmission [32].
Impaired oxidative metabolism
Early hypotheses that remain relevant today attempted to explain delirium as a behavioral manifestation of a 'widespread reduction of cerebral oxidative metabolism resulting in an imbalance of neurotransmission' [33]. Engel and Romano [34] conducted classic experiments by evaluating delirious patients using EEG. These studies showed that delirium is associated with diffuse slowing on EEG, a finding that is believed to represent a reduction in brain metabolism. Thus, they hypothesized that delirium is the result of 'cerebral insufficiency' (a global failure of cerebral oxidative metabolism), a factor that is known to be important in the pathogenesis of multiple organ dysfunction in critical illness [35].
Availability of large neutral amino acids
Neurotransmitter levels and function can be affected by changes in the plasma concentrations of various amino acid precursors, and some investigators have proposed that altered availability of large neutral amino acids contributes to the development of delirium [32]. Amino acid entry into the brain is regulated by a sodium-independent large neutral amino acid transporter type 1 (LAT1) [36]. Tryptophan, an essential amino acid and precursor for serotonin, competes with several large neutral amino acids (for example, tyrosine, phenylalanine, valine, leucine, and isoleucine) for transport across the blood-brain barrier via LAT1 transporters. Phenylalanine also competes with large neutral amino acids for transport across the blood-brain barrier. Increased cerebral uptake of tryptophan and phenylalanine, compared with that of other large neutral amino acids, leads to elevated levels of dopamine and norepinephrine (noradrenaline), two neurotransmitters that have been implicated in the pathogenesis of delirium [33].
Risk factors
Risk factors for delirium can be divided into predisposing factors (host factors) and precipitating factors [37]. Although predisposing factors are present before ICU admission and are difficult to alter, precipitating factors occur during the course of critical illness. They may involve factors of the acute illness or be iatrogenic; these factors represent areas of risk that are potentially modifiable by preventive or therapeutic intervention.
Only a few studies have examined risk factors for delirium in the ICU, but numerous delirium risk factors have been identified in non-ICU patients. Table 1 highlights factors that have been identified in both ICU and non-ICU studies. In the largest study to date that examined risk factors for ICU delirium, Ouimet and coworkers [21] studied 820 general ICU patients and determined that hypertension, alcoholism, severity of illness, and exposure to sedatives and analgesics (when used to induce coma) increased the likelihood of delirium. Dubois and colleagues [9] similarly found hypertension to be a risk factor for delirium among 216 general ICU patients. In addition, smoking history, hyperbilirubinemia, morphine, and analgesia administered via an epidural route were associated with delirium. To date, however, no study has corroborated this association between analgesia administered via an epidural route and delirium.
In the only study to date to examine whether there is a genetic predisposition to ICU delirium in some patients, Ely and coworkers [38] evaluated the association between apolipoprotein E (APOE) genotype and duration of delirium among 53 mechanically ventilated medical ICU patients. Patients with the APOE4 polymorphism (a risk factor for Alzheimer's disease) were delirious for twice as long as those without the APOE4 polymorphism (median [interquartile range]: 4 [3 to 4.5] days versus 2 [1 to 4] days; P = 0.05). Larger studies are ongoing to confirm this association.
Other factors associated with delirium in the ICU include older age, baseline cognitive impairment, metabolic disturbances (for instance, derangements in sodium, calcium, and blood urea nitrogen), acute infection, respiratory disease, acidosis, anemia, and hypotension [10, 39, 40]. Some factors that are clearly associated with delirium in hospitalized patients without critical illness may carry similar risks among ICU patients, although studies to date have not yet proven associations with ICU delirium. These potential risk factors include hearing or vision impairment, depression, and immobilization resulting from placement of catheters or restraints [37, 41]. Critically ill patients are typically exposed to numerous factors that may precipitate delirium. In a study of 53 medical ICU patients, Ely and coworkers [2] identified an astounding average of 11 delirium risk factors per patient. Two delirium risk factors nearly universally experienced by ICU patients are exposure to sedative and analgesic medications and sleep deprivation. The risk associated with both of these factors is potentially modifiable, as is discussed in greater detail below.
Sedative and analgesic medications
Sedative and analgesic medications are routinely administered to patients receiving mechanical ventilation to reduce pain and anxiety, as recommended by the Society of Critical Care Medicine (SCCM) [42]. These medications, however, are not without detrimental effects. Continuous intravenous sedation, for example, is associated with prolonged mechanical ventilation as compared with sedation via intermittent boluses [43].
Multiple studies have demonstrated an association between delirium and exposure to sedative and analgesic medications. In a mixed surgical/medical ICU, Dubois and coworkers [9] determined that morphine was the strongest predictor of delirium in a multivariable model. Ouimet and colleagues [21] observed that sedative and analgesic medications used to induce coma were associated with delirium (delirium among patients with drug-induced coma: odds ratio [OR] = 3.2, 95% confidence interval [CI] = 1.5 to 6.8). Marcantonio and coworkers [44] reported that patients treated with benzo-diazepines were more likely to have postoperative delirium than were those not treated with benzodiazepines (OR = 2.7, 95% CI = 1.3 to 5.5). Meperidine also increased the likelihood of postoperative delirium (OR = 3.0, 95% CI = 1.3 to 6.8).
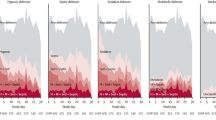
A recent study examined the temporal relationship between administration of sedatives and analgesics and ICU delirium. Pandharipande and colleagues [40] used Markov regression modeling to determine that lorazepam is an independent risk factor for daily transition to delirium (OR = 1.2, 95% CI = 1.2 to 1.4); specifically, patients treated with lorazepam on a given ICU day are more likely to be delirious on the following day than if lorazepam were not administered (Figure 1). Treatment with fentanyl, morphine, and propofol were not significantly associated with transition to delirium, but larger studies are ongoing to examine further the associations of these medications with delirium. Midazolam was shown to increase the likelihood of transition to delirium in another recently completed study of trauma and surgical ICU patients [11].
Lorazepam and the probability of transitioning to delirium. The probability of transitioning to delirium increased with the dose of lorazepam administered during the previous 24 hours. This incremental risk was large at low doses and plateaued at approximately 20 mg/day. Data from Pandharipande and coworkers [40].
Sedative agents that are GABA receptor sparing, such as opioids and dexmedetomidine (a novel α2-receptor agonist), may reduce the risk for delirium in ICU patients as compared with the risk attributable to benzodiazepines. Although studies have consistently identified lorazepam and midazolam as risk factors for delirium, the data regarding opioids are less consistent. For example, Ouimet and coworkers [21] noted that mean daily opioid doses were higher among ICU patients without delirium than among ICU patients with delirium. Similarly, in 541 hip fracture patients, Morrison and colleagues [45] determined that those treated liberally with opioid analgesics (>10 mg/day parenteral morphine sulfate equivalent) were less likely to develop delirium than patients who received less analgesia. Treatment with meperidine was an exception, because this drug increased the risk for delirium as compared with other opioids. These investigations point to the importance of judicious use of these psychoactive medications, with focus on adequate analgesia.
Sleep disturbances
Although the association between sleep disturbances and ICU delirium has not been studied, sleep deprivation impairs cognition [46] and the relationship between sleep during critical illness and delirium is a promising area of ongoing research. On average, ICU patients sleep only 2 hours per day [47], and less than 6% of their sleep is random eye movement sleep. Cooper and coworkers [48] studied 20 mechanically ventilated ICU patients using polysomnography and observed that every patient had severely disrupted sleep or none at all. Such disturbances may detrimentally affect protein synthesis, cellular and humoral immunity, and energy expenditure, and ultimately they may contribute to organ dysfunction such as delirium. Excessive noise and patient care activities account for only a minority of arousals in ICU patients, whereas metabolic derangements, mechanical ventilation, and exposure to sedative and analgesic medications probably play significant roles in disturbing sleep in the ICU [49]. Because sedative and analgesic medications may lead directly to delirium or may contribute to impaired cognition via disruption of sleep, and because traditional methods of sedation and analgesia are amenable to change, some investigators have prioritized studies seeking to understand the relationships between sedative and analgesic exposure, sleep disturbance, and brain dysfunction in critically ill patients.
Diagnosis of delirium in the intensive care unit
Delirium assessment instruments, such as the Intensive Care Delirium Screening Checklist (ICDSC) [8] and the Confusion Assessment Method for the ICU (CAM-ICU) [50, 51] allow nonpsychiatric physicians and other ICU personnel to diagnose delirium in ICU patients rapidly and reliably, even when the patient cannot speak because of endotracheal intubation. In fact, the SCCM [42] recommends that all ICU patients be routinely evaluated for delirium.
Bergeron and colleagues [8] validated the ICDSC in a study of 93 ICU patients who were evaluated by a consulting psychiatrist who served as the reference standard rater. Using the ICDSC, each patient is assigned a score from 0 to 8; a cut-off score of 4 has a sensitivity of 99% and specificity of 64% for identifying delirium. Thus, because of its very high sensitivity, the authors recommend that the ICDSC be used as a screening tool for ICU delirium.
When using the ICDSC, level of consciousness is first rated on a 5-point scale that ranges from unresponsive to exaggerated response (Table 2). Any patient who is not comatose or stuporous (A or B on the ICDSC scale) is then assessed for items on the checklist using information collected during the previous 24 hours. Including altered level of consciousness, the ICDSC consists of eight items (rated present or absent), and each patient is given a score from 0 to 8 (Table 2); 4 or greater is considered diagnostic of delirium.
The CAM-ICU, adapted from the Confusion Assessment Method [52], was designed for use in intubated patients and validated against a reference standard rater in mechanically ventilated ICU patients. Originally validated by Ely and coworkers [50, 53] in two cohorts of 38 and 111 medical ICU patients, the CAM-ICU has a high sensitivity (93% to 100%) and specificity (89% to 100%) for delirium as well as high inter-rater reliability (κ = 0.96, 95% CI = 0.92 to 0.99). Lin and colleagues [20] subsequently validated the CAM-ICU in a separate cohort of 102 mechanically ventilated medical ICU patients and reported similarly high sensitivity (91% to 95%), specificity (98%), and inter-rater reliability (κ = 0.91).
Using the CAM-ICU, delirium is diagnosed in two steps (Figure 2). Level of consciousness (level of arousal) is first assessed using a standardized sedation scale. One example, the Richmond Agitation-Sedation Scale (RASS) [54, 55], is a 10-point scale ranging from +4 to -5, with a RASS score of 0 denoting a calm and alert patient. By convention, RASS scores of -4 and -5 identify coma; a comatose patient cannot be assessed for delirium. All other patients, whether moderately sedated (RASS score -3) or more alert, should be evaluated for delirium. The CAM-ICU assesses patients for four features of delirium; three out of four features are required for a diagnosis of delirium (Figure 2).
The Confusion Assessment Method for the Intensive Care Unit. *Level of consciousness (depth of sedation) is assessed using the Richmond Agitation-Sedation Scale (RASS) [54,55]. †Content of consciousness is assessed (delirium is diagnosed) using the Confusion Assessment Method for the ICU (CAM-ICU) [50,53]. When three of four diagnostic features are present (either features 1, 2, and 3, or features 1, 2, and 4), delirium is diagnosed. Data from Ely and coworkers [50] and the CAM-ICU training manual [51].
Prevention and treatment
Multicomponent strategies
Interventions aimed at preventing delirium have not been adequately studied in critically ill patients (Table 3), and a higher rate of incident delirium (delirium present upon admission to the ICU or upon recovery from coma) in critically ill patients compared with that observed in hospitalized patients without critical illness may indicate that preventive strategies that are effective in non-ICU patients may be less likely to prevent delirium in the ICU. However, a review of studies of delirium prevention in non-ICU patients may inform ICU practitioners regarding appropriate management of high-risk patients treated in the ICU.
In a landmark trial, Inouye and coworkers [56] evaluated 852 hospitalized older patients who were nonrandomly assigned to usual care or management with a multicomponent intervention consisting of multiple protocols designed to minimize the effects of delirium risk factors. These protocols directed repeated reorientation of the patient and provision of cognitively stimulating activities; a nonpharmacologic sleep protocol; early mobilization activities and range of motion exercises; timely removal of catheters and physical restraints; use of eyeglasses, magnifying lenses, and hearing aids; and early correction of dehydration. The intervention significantly reduced the incidence of delirium (15.0% in the usual care group versus 9.9% in the intervention group; matched OR = 0.60, 95% CI = 0.39 to 0.92). These results were confirmed in randomized trials studying the efficacy of multicomponent delirium prevention programs in postoperative patients [57] and geriatric inpatients [58].
Although the strategies emphasized in these studies (for instance, frequent reorientation, restoration of sleep/wake cycles, timely removal of catheters, early mobilization, and minimization of unnecessary noise/stimuli) are recommended for ICU patients, such strategies have not been studied in the ICU, and the nature and number of delirium risk factors in ICU patients are different from those observed among other inpatients. Preventive interventions, therefore, must address those risk factors that are most common in the ICU, including electrolyte abnormalities, infection, and exposure to sedative and analgesic medications. These drugs play a vital role in maintaining patient comfort, and adequate pain control is likely to reduce the risk for delirium [45], but practitioners must strive to avoid over-sedation by administering the drugs only as necessary. In fact, sedation protocols that use validated sedation scales [59], emphasizing intermittent boluses rather than continuous infusions [43], and promoting daily interruption of sedatives and analgesics [60] significantly improve multiple clinical outcomes. The effect of these methods of sedation on delirium is the subject of ongoing investigations.
Pharmacologic strategies
After the ICU team has used the multicomponent prevention strategies described above to minimize risk factors and after acute, life-threatening complications of critical illness that may lead to delirium (for example, hypoxia, hypercapnia, hypoglycemia, and shock) have been sought out and addressed, pharmacologic therapy for the prevention and treatment of delirium should be considered (Table 3). When using such medications, clinicians should be aware that any drug intended to improve cognition may have adverse psychoactive effects, paradoxically exacerbating delirium or causing excessive sedation in some patients. Also, evidence proving the efficacy of pharmacologic strategies for delirium is lacking. All psychoactive drugs should therefore be used judiciously in critically ill patients, in the smallest effective dose for the shortest time necessary.
Although no placebo-controlled clinical trials have been conducted to evaluate its efficacy in ICU patients, haloperidol is recommended as the drug of choice for the treatment of ICU delirium by the SCCM [42] and the APA [61]. A typical antipsychotic, haloperidol blocks D2 dopamine receptors, resulting in amelioration of hallucinations, delusions, and unstructured thought patterns. The optimal dose and regimen have not been defined in clinical trials, but the SCCM guidelines suggest that patients with hyperactive delirium should be treated with 2 mg intravenously, followed by repeated doses (doubling the previous dose) every 15 to 20 minutes while agitation persists. Once the agitation subsides, scheduled doses (every 4 to 6 hours) may be continued for a few days, followed by tapered doses for several days. Common doses for ICU patients range from 4 to 20 mg/day, but higher doses are frequently used for the treatment of acute agitation. Also, haloperidol has been evaluated as a prophylactic therapy for the prevention of postoperative delirium in elderly hip surgery patients. Kalisvaart and coworkers [62] conducted a randomized, double-blind, placebo-controlled trial in 430 elderly hip surgery patients and determined that 1.5 mg/day haloperidol significantly reduced the severity and duration of delirium. The incidence of delirium was not altered by haloperidol compared with placebo. A similar trial is currently ongoing in ICU patients.
Atypical antipsychotics (for instance, risperidone, ziprasidone, quetiapine, and olanzapine) may also be helpful in the treatment of delirium, but only preliminary data exist supporting their use in the ICU. These medications target dopamine receptors as well as receptors for other neurotransmitters, including serotonin, acetylcholine, and nor-epinephrine (noradrenaline). Skrobik and coworkers [63] compared olanzapine with haloperidol in a nonrandomized trial that enrolled 73 medical and surgical ICU patients; they reported that resolution of delirium symptoms was similar in both treatment groups, but more side effects were observed in patients treated with haloperidol. Numerous studies have compared different antipsychotics with each other for the treatment of delirium in non-ICU patients, but all are limited by the absence of a placebo-treated control group as well as by other methodologic limitations [64]. Well designed, randomized, placebo-controlled trials are needed to inform critical care clinicians regarding the efficacy and safety of both typical and atypical antipsychotics in the prevention and treatment of ICU delirium.
Patients treated with haloperidol or other antipsychotics should be monitored for adverse effects, including hypotension, dystonia, extrapyramidal effects, laryngeal spasm, malignant hyperthermia, glucose and lipid dysregulation, and anticholinergic effects such as dry mouth, constipation, and urinary retention. Torsades de pointes is a life-threatening arrhythmia that may occur in patients treated with antipsychotics, and these medications should be avoided in patients with prolonged QT intervals.
Recently, investigators observed an association between antipsychotic use (typical or atypical) and increased mortality in elderly patients [65, 66]. The patients studied were not critically ill, and no study of ICU patients has demonstrated an increased risk for death due to antipsychotic use. In fact, Milbrandt and coworkers [67] studied 989 mechanically ventilated ICU patients and observed that haloperidol was associated with significantly lower hospital mortality.
Although they remain the drugs of choice for the treatment of delirium tremens (and other withdrawal syndromes), benzodiazepines are not recommended for the management of delirium because these medications are themselves risk factors for delirium [21, 40, 44]. Dexmedetomidine, a novel α2-receptor agonist that does not act on GABA receptors, may prove to be an alternative sedative agent that is less likely to cause delirium. In a preliminary report of an unblinded, randomized trial conducted in postoperative cardiac surgical patients, Maldonado and colleagues [68] described a significant reduction in the incidence of delirium associated with dexmedetomidine; 8% of patients sedated with dexmedetomidine at sternal closure developed delirium as compared with 50% of patients sedated with propofol or midazolam. Similarly, in a recently completed double-blind, randomized controlled trial [69] it was determined that ICU patients sedated with dexmedetomidine spent fewer days in coma and more days neurologically normal (without coma or delirium) than did those sedated with lorazepam. These pilot studies suggest that larger trials are warranted to evaluate the efficacy and safety of sedation with dexmedetomidine as well as clonidine, a less selective α2-receptor agonist, in ICU patients.
Conclusion
Delirium is a common manifestation of acute brain dysfunction in critically ill patients that is associated with poor short-term outcomes and may result in adverse sequelae years after ICU discharge. The strategies described here for the prevention, diagnosis, and treatment of ICU delirium are subjects of multiple ongoing investigations. Every ICU clinician should be aware of these strategies, institute routine monitoring for delirium in the ICU, seek to reduce the impact of risk factors for delirium when possible, and use treatment options when necessary.
Abbreviations
- APA:
-
American Psychiatric Association
- APOE:
-
apolipoprotein E
- CAM-ICU:
-
Confusion Assessment Method for the ICU
- CI:
-
confidence interval
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- EEG:
-
electroencephalography
- GABA:
-
γ-aminobutyric acid
- ICDSC:
-
Intensive Care Delirium Screening Checklist
- ICU:
-
intensive care unit
- LAT1:
-
large neutral amino acid transporter type 1
- OR:
-
odds ratio
- RASS:
-
Richmond Agitation-Sedation Scale
- SCCM:
-
Society of Critical Care Medicine.
References
McGuire BE, Basten CJ, Ryan CJ, Gallagher J: Intensive care unit syndrome: a dangerous misnomer. Arch Intern Med. 2000, 160: 906-909. 10.1001/archinte.160.7.906.
Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK: The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001, 27: 1892-1900. 10.1007/s00134-001-1132-2.
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Inouye SK, Bernard GR, Dittus RS: Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004, 291: 1753-1762. 10.1001/jama.291.14.1753.
Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW: The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004, 14: 87-98. 10.1023/B:NERV.0000028080.39602.17.
The Nature and Extent of Medical Injury in Older Patients. [http://assets.aarp.org/rgcenter/health/2000_17_injury.pdf]
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, text revision. 2000, Washington, DC: American Psychiatric Association
Justic M: Does 'ICU psychosis' really exist?. Crit Care Nurse. 2000, 20: 28-37.
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y: Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001, 27: 859-864. 10.1007/s001340100909.
Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y: Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001, 27: 1297-1304. 10.1007/s001340101017.
McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK: Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003, 51: 591-598. 10.1034/j.1600-0579.2003.00201.x.
Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, Dittus R, Ely EW: Prevalence and risk factors for development of delirium in surgical and trauma ICU patients. J Trauma. 2007,
Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW: Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005, 9: R375-R381. 10.1186/cc3729.
Ely EW, Stephens RK, Jackson JC, Thomason JW, Truman B, Gordon S, Dittus RS, Bernard GR: Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004, 32: 106-112. 10.1097/01.CCM.0000098033.94737.84.
Meagher DJ, Trzepacz PT: Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000, 5: 75-85.
Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, Ely EW: Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006, 54: 479-484. 10.1111/j.1532-5415.2005.00621.x.
O'Keeffe ST, Lavan JN: Clinical significance of delirium subtypes in older people. Age Ageing. 1999, 28: 115-119. 10.1093/ageing/28.2.115.
Ouimet S, Riker R, Bergeon N, Cossette M, Kavanagh B, Skrobik Y: Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007, 33: 1007-1013. 10.1007/s00134-007-0618-y.
Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA: Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004, 30: 1334-1339. 10.1007/s00134-004-2231-7.
Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW: Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004, 32: 955-962. 10.1097/01.CCM.0000119429.16055.92.
Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, Fang YF, Shieh MH, Kuo HP: The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004, 32: 2254-2259. 10.1097/01.CCM.0000110878.49476.42.
Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y: Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007, 33: 66-73. 10.1007/s00134-006-0399-8.
Jackson JC, Gordon SM, Girard TD, Thomason JWW, Pun BT, Dunn J, Canonico AE, Light RW, Shintani AK, Thompson JL, et al: Delirium as a risk factor for long term cognitive impairment in mechanically ventilated ICU survivors [abstract]. Am J Respir Crit Care Med. 2007, 175: A22-
Trzepacz PT: Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord. 1999, 10: 330-334. 10.1159/000017164.
Trzepacz PT: Delirium. Advances in diagnosis, pathophysiology, and treatment. Psychiatr Clin North Am. 1996, 19: 429-448. 10.1016/S0193-953X(05)70299-9.
Flacker JM, Cummings V, Mach JR, Bettin K, Kiely DK, Wei J: The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry. 1998, 6: 31-41.
Marshall JC: Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001, 29: S99-106. 10.1097/00003246-200107001-00032.
Wheeler AP, Bernard GR: Treating patients with severe sepsis. N Engl J Med. 1999, 340: 207-214. 10.1056/NEJM199901213400307.
Papadopoulos MC, Lamb FJ, Moss RF, Davies DC, Tighe D, Bennett ED: Faecal peritonitis causes oedema and neuronal injury in pig cerebral cortex. Clin Sci (Lond). 1999, 96: 461-466.
Huynh HK, Dorovini-Zis K: Effects of interferon-gamma on primary cultures of human brain microvessel endothelial cells. Am J Pathol. 1993, 142: 1265-1278.
Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L: Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol. 1984, 246: R994-R999.
Breslow MJ, Miller CF, Parker SD, Walman AT, Traystman RJ: Effect of vasopressors on organ blood flow during endotoxin shock in pigs. Am J Physiol. 1987, 252: H291-H300.
Van Der Mast RC: Pathophysiology of delirium. J Geriatr Psychiatry Neurol. 1998, 11: 138-145.
Lipowski ZJ: Delirium: Acute Confusional States. Rev edition. 1990, New York: Oxford University Press
Engel GL, Romano J: Delirium, a syndrome of cerebral insufficiency. J Chronic Dis. 1959, 9: 260-277. 10.1016/0021-9681(59)90165-1.
Fink MP, Evans TW: Mechanisms of organ dysfunction in critical illness: report from a Round Table Conference held in Brussels. Intensive Care Med. 2002, 28: 369-375. 10.1007/s00134-001-1191-4.
Wurtman RJ, Hefti F, Melamed E: Precursor control of neurotransmitter synthesis. Pharmacol Rev. 1980, 32: 315-335.
Inouye SK, Charpentier PA: Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996, 275: 852-857. 10.1001/jama.275.11.852.
Ely EW, Girard TD, Shintani AK, Jackson JC, Gordon SM, Thomason JW, Pun BT, Canonico AE, Light RW, Pandharipande P, et al: Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007, 35: 112-117. 10.1097/01.CCM.0000251925.18961.CA.
Aldemir M, Ozen S, Kara IH, Sir A, Bac B: Predisposing factors for delirium in the surgical intensive care unit. Crit Care. 2001, 5: 265-270. 10.1186/cc1044.
Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW: Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006, 104: 21-26. 10.1097/00000542-200601000-00005.
Schor JD, Levkoff SE, Lipsitz LA, Reilly CH, Cleary PD, Rowe JW, Evans DA: Risk factors for delirium in hospitalized elderly. JAMA. 1992, 267: 827-831. 10.1001/jama.267.6.827.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, et al: Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002, 30: 119-141. 10.1097/00003246-200201000-00020.
Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G: The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998, 114: 541-548. 10.1378/chest.114.2.541.
Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, Katz N, Cook EF, Orav EJ, Lee TH: The relationship of postoperative delirium with psychoactive medications. JAMA. 1994, 272: 1518-1522. 10.1001/jama.272.19.1518.
Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, Strauss E, Siu AL: Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003, 58: 76-81.
Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, et al: Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000, 9: 335-352. 10.1046/j.1365-2869.2000.00225.x.
Aurell J, Elmqvist D: Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed). 1985, 290: 1029-1032.
Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ: Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000, 117: 809-818. 10.1378/chest.117.3.809.
Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ: Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003, 167: 708-715. 10.1164/rccm.2201090.
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al: Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001, 286: 2703-2710. 10.1001/jama.286.21.2703.
The Confusion Assessment Method for the ICU (CAM-ICU) training manual. [http://www.icudelirium.org/delirium/CAM-ICU-Training.html]
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI: Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990, 113: 941-948.
Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK: Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001, 29: 1370-1379. 10.1097/00003246-200107000-00012.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK: The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002, 166: 1338-1344. 10.1164/rccm.2107138.
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al: Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003, 289: 2983-2991. 10.1001/jama.289.22.2983.
Inouye SK, Bogardus ST, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM: A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999, 340: 669-676. 10.1056/NEJM199903043400901.
Marcantonio ER, Flacker JM, Wright RJ, Resnick NM: Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001, 49: 516-522. 10.1046/j.1532-5415.2001.49108.x.
Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y: A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005, 53: 622-628. 10.1111/j.1532-5415.2005.53210.x.
Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH: Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999, 27: 2609-2615. 10.1097/00003246-199912000-00001.
Kress JP, Pohlman AS, O'Connor MF, Hall JB: Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000, 342: 1471-1477. 10.1056/NEJM200005183422002.
American Psychiatric Association: Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999, 156: 1-20.
Kalisvaart KJ, de Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, Eikelenboom P, van Gool WA: Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005, 53: 1658-1666.
Skrobik YK, Bergeron N, Dumont M, Gottfried SB: Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004, 30: 444-449. 10.1007/s00134-003-2117-0.
Seitz DP, Gill SS, van Zyl LT: Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007, 68: 11-21.
Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA: Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005, 353: 2335-2341. 10.1056/NEJMoa052827.
Schneider LS, Dagerman KS, Insel P: Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005, 294: 1934-1943. 10.1001/jama.294.15.1934.
Milbrandt EB, Kersten A, Kong L, Weissfeld LA, Clermont G, Fink MP, Angus DC: Haloperidol use is associated with lower hospital mortality in mechanically ventilated patients. Crit Care Med. 2005, 33: 226-229. 10.1097/01.CCM.0000150743.16005.9A.
Maldonado JR, van der Starre PJ, Wysong A: Post-operative sedation and the incidence of ICU delirium in cardiac surgery patients [abstract]. Anesthesiology. 2003, 99: A465-
Pandharipande P, Pun B, Herr D, Girard T, Miller R, Thompson J, Shintani A, Maze M, Bernard G, Ely E: Double blind randomized controlled trial comparing dexmedetomidine vs. lorazepam to reduce duration of delirium and coma in mechanically ventilated (MV) patients [abstract]. Am J Respir Crit Care Med. 2007, 175: A508-
Disclaimer
This article is part of Critical Care Volume 12 Supplement 3: Analgesia and sedation in the ICU. Publication of the supplement has been funded by an unrestricted grant from GlaxoSmithKline. GlaxoSmithKline has had no editorial control in respect of the articles contained in this publication.The opinions and views expressed in this publication are those of the authors and do not constitute the opinions or recommendations of the publisher or GlaxoSmithKline. Dosages, indications and methods of use for medicinal products referred to in this publication by the authors may reflect their research or clinical experience, or may be derived from professional literature or other sources. Such dosages, indications and methods of use may not reflect the prescribing information for such medicinal products and are not recommended by the publisher or GlaxoSmithKline. Prescribers should consult the prescribing information approved for use in their country before the prescription of any medicinal product.Whilst every effort is made by the publisher and editorial board to see that no inaccurate or misleading data, opinion, or statement appear in this publication, they wish to make it clear that the data and opinions appearing in the articles herein are the sole responsibility of the contributor concerned.Accordingly, the publishers, the editor and editorial board, GlaxoSmithKline, and their respective employees, officers and agents accept no liability whatsoever for the consequences of such inaccurate or misleading data, opinion or statement.
Acknowledgements
Dr Girard is a Hartford Geriatrics Health Outcomes Research Scholar and a Vanderbilt Physician-Scientist Development Program Scholar. Dr Pandharipande is the recipient of a Mentored Research Training Grant from the Foundation for Anesthesia Education and Research. Dr Ely is the Associate Director for Research of the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC), VA Service, Department of Veterans Affairs Medical Center, Nashville, Tennessee, USA. He receives support from the VA Clinical Science Research and Development Service (VA Merit Review Award) and the National Institutes of Health (AG0727201).
This article is part of Critical Care Volume 12 Supplement 3: Analgesia and sedation in the ICU. The full contents of the supplement are available online at http://ccforum.com/supplements/12/S3.
Publication of the supplement has been funded by an unrestricted grant from GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
TDG and PPP have received honoraria from Hospira, Inc. EWE has received grant support and honoraria from Hospira, Inc., Pfizer, Inc., and Eli Lilly and Company.
Rights and permissions
About this article
Cite this article
Girard, T.D., Pandharipande, P.P. & Ely, E.W. Delirium in the intensive care unit. Crit Care 12 (Suppl 3), S3 (2008). https://doi.org/10.1186/cc6149
Published:
DOI: https://doi.org/10.1186/cc6149