Abstract
Delirium is the most common manifestation of brain dysfunction in critically ill patients. In the intensive care unit (ICU), duration of delirium is independently predictive of excess death, length of stay, cost of care, and acquired dementia. There are numerous neurotransmitter/functional and/or injury-causing hypotheses rather than a unifying mechanism for delirium. Without using a validated delirium instrument, delirium can be misdiagnosed (under, but also overdiagnosed and trivialized), supporting the recommendation to use a monitoring instrument routinely. The best-validated ICU bedside instruments are CAM-ICU and ICDSC, both of which also detect subsyndromal delirium. Both tools have some inherent limitations in the neurologically injured patients, yet still provide valuable information about delirium once the sequelae of the primary injury settle into a new post-injury baseline. Now it is known that antipsychotics and other psychoactive medications do not reliably improve brain function in critically ill delirious patients. ICU teams should systematically screen for predisposing and precipitating factors. These include exacerbations of cardiac/respiratory failure or sepsis, metabolic disturbances (hypoglycemia, dysnatremia, uremia and ammonemia) receipt of psychoactive medications, and sensory deprivation through prolonged immobilization, uncorrected vision and hearing deficits, poor sleep hygiene, and isolation from loved ones so common during COVID-19 pandemic. The ABCDEF (A2F) bundle is a means to facilitate implementation of the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) Guidelines. In over 25,000 patients across nearly 100 institutions, the A2F bundle has been shown in a dose–response fashion (i.e., greater bundle compliance) to yield improved survival, length of stay, coma and delirium duration, cost, and less ICU bounce-backs and discharge to nursing homes.
Similar content being viewed by others
Delirium is a common problem in the ICU that is often undiagnosed if not screened for with a validated tool. It is an independent predictor of outcomes that matter, including increased health care costs, duration of ICU and hospital stay, mortality, and long-term cognitive impairment. Evidence supports the best approach to reducing the burden of this problem for our patients is not a specific drug, but rather embracing a nonpharmacological bundle of safety steps summarized in the ABCDEF (A2F) bundle. The A2F focuses on managing delirium causes, reducing sedation/ventilation/immobility, incorporating family, and rehumanizing critical care. |
Introduction and rationale
Delirium is a commonly neglected manifestation of organ dysfunction in the ICU. It is commonly unmonitored and not discussed on rounds [1]. This is often because the ICU team feels there is nothing that can be done about delirium since we are already treating the patient’s main diseases, or because it might seem logical that a sedated patient would have a cognitive dysfunction [2]. Beyond that, there seems to be a perception that the patient “needs” the sedatives and is too sick to get out of the bed anyway. On one hand the sedatives enable mechanical ventilation, but on the other they contribute to delirium development. Also, temporal, and spatial disorientation is often considered as the norm in patients sedated for several days and nights, who may be in ICU rooms without windows or with a direct view of the outside [3]. These factors lead to an indifference about this form of brain dysfunction that results in the patient’s suffering well beyond ICU discharge. It is linked to a greater risk of demise and imposes additional burden on the family and caregivers.
Objectives
In this narrative review, we aim to describe the current state of evidence for diagnostic, preventive and therapeutic measures that can mitigate the course of delirium. It is essential that as an ICU community, we consider the importance of delirium prevention and treatment in our daily management of critically ill patients. With higher acuity of patients and increased complexity of care, we must find ways to avoid over-sedation and prolonged immobilization to help patients have more complete and intact survival.
Definition and prevalence
According to the Diagnostic and statistical manual of mental disorders: DSM-5 delirium is defined as disturbance in attention (top mandatory feature) that develops over a short period of time, is associated with additional disturbances in cognition that are not better explained by another preexisting, established or evolving neurocognitive disorder, and do not occur in the context of a severely reduced level of arousal, and evidence from the history, physical examination or laboratory findings that indicate that the disturbance is a direct physiological consequence of another medical condition, substance intoxication, or withdrawal [4, 5]. If one or more of the delirium criteria are lacking, a diagnosis of subsyndromal delirium [6, 7] can be made for which the management is quite similar to that for delirium, but it is also important to consider several differential diagnoses, e.g. alcohol withdrawal syndrome [8] that usually begins with hallucinations and delusion before the well-known “delirium” tremens, interruption of antipsychotics in patients suffering from mental illness [9], isolated hallucinations associated with the use of opioids [10], as well as the sleep deprivation that is frequent in ICU patients and associated with isolated hallucinations without cognitive dysfunction experimentally [11].
Historically, delirium was reported in 60–80% of mechanically ventilated patients [12,13,14] and 20–50% of lower severity of illness ICU patients [15, 16]. With increased utilization of validated diagnostic tools globally, using translations of these tools into over 30 languages (see translations at https://cibs.webflow.io/medical-professionals/downloads/resource-language-translations), and modifications of routine management in ICUs to reduce the culture of over-sedation and immobility, delirium rates in many ICUs are now down by about 25% [12, 17, 18]. In fact, delirium prevalence was reported to be 48% in a large, 21 center, prospective study including only mechanically ventilated and shock patients, a population that for > 15 years had consistently shown delirium rates ~ 75% using the same methodology [17]. In the ICU, delirium may present as hyperactive (agitated and restless), hypoactive (flat affect, apathy, lethargy, decreased responsiveness), or mixed hyper/hypoactive states, where patients fluctuate among these states. Hypoactive delirium is the most difficult to detect. Unless a validated screening tool is used, detection can be missed due to the clinical presentation being misinterpreted as fatigue or depression. Hypoactive delirium portends more dangerous outcomes [19,20,21].
Additionally, delirium has been classified as rapidly reversible sedation-related delirium. Rapidly reversible sedation-associated delirium is defined as delirium while receiving sedation that resolved within 2 h after stopping sedation during a spontaneous awakening trial (SAT). Rapidly reversible delirium was found in 12% of the 102 patients while 75% of these patients had persistent delirium (their delirium persisted for more than 2 h after sedative interruption). Thus, assessing patient’s mental status through serial assessments of delirium throughout the day both before and after SAT will give the best picture of the patient’s mental status [22]. In the case of persistent delirium after SAT, or if SAT cannot be performed for some reason, all predisposal factors of delirium (other than analgesia sedation) should be screened and managed.
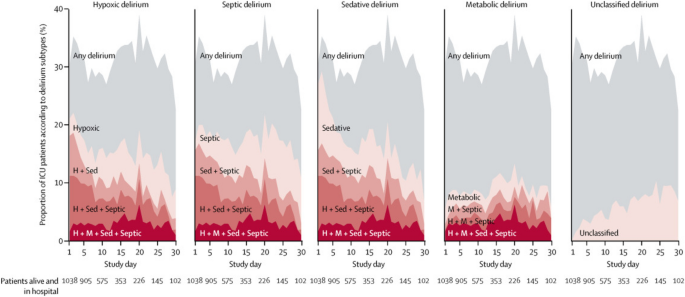
A multicenter, prospective cohort study of adult medical and surgical ICU patients with respiratory failure and/or shock within two parallel studies (BRAIN-ICU) and Delirium and Dementia in Veterans Surviving ICU Care (MIND-ICU) was conducted to determine the association between the duration of clinical phenotypes of delirium and Repeatable Battery for Assessment of Neuropsychological Status (RBANS) score, an instrument to assess global cognitive function in adults, at 3 and 12 months following critical illness [2, 23, 24]. The clinical phenotypes of delirium were defined as hypoxic, septic, sedative-associated, or metabolic (renal of liver dysfunction) delirium [13]. Sedative-associated delirium was the most common phenotype of delirium, which was present during 2634 (63%) of delirium days (Figs. 1) [2]. A worse RBANS global cognition score 12 months later was predicted by a longer duration of sedative-associated delirium after adjusting for covariates (difference in score comparing 3 days vs 0 days: − 4.03, 95% CI − 7.80 to − 0.26). Worse cognitive function at 12 months was predicted by longer durations of hypoxic delirium (− 3.76, 95% CI − 7.16 to − 0.37), septic delirium (− 3.67 − 7·13 to − 0.22), and unclassified delirium (− 4.70, − 7.16 to − 2.25). However, the duration of metabolic delirium did not predict worse cognitive function at 12 months (1.14, − 0.12–3.01) [2].
“CONFIRM or EXCLUDE DELIRIUM: To diagnose any organ dysfunction it is necessary to identify the fact, the degree and the causes of this dysfunction. With brain dysfunction, active screening for delirium, using the CAM-ICU or ICDSC is critical. In this case, it is necessary to identify if the patient can pay attention and organize thoughts. Assessments of inattention, such as falling asleep in the middle of a conversation or missing details of the conversation can be used. Then ask the patient to hold up two fingers of one hand and repeat this action with the other hand. Failing to perform these easy tasks is a highly specific screen for delirium. The next step is to identify the cause of brain dysfunction.” [25]
Prevalence of delirium phenotypes. Each plot area (on the y-axis) shows the percentage of the study participatnets in the hospital who had any delirium, a single delirium phenotype, or a combination of multiple delirum phenotypes according to the study day (shown on the x-axis). The grey shading indicates the overall percentage of participants with delirium on each study day. The red lines and shaded area represent the numbe of phenotypes of delirum present, with darker reds respresenting the presence of more phenotypes of delirum. The lighetest red regions show the percentage of participants with a given single phenotype (H hypoxic, M metabolic, Sed sedative, Sep septic)
Delirium detection
Using rigorous psychometric evaluation, the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) Guidelines recommend routine monitoring of delirium in adult ICU patients and using with either the Confusion Assessment Method for the ICU (CAM-ICU) or the Intensive Care Delirium Screening Checklist (ICDSC) [25, 26]. The CAM-ICU was originally validated in 96 adult patients at Vanderbilt University Medical Center (USA) in a medical or coronary ICU. Critical care study nurses performed 471 paired evaluations and compared with assessments by delirium experts using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. Compared with the reference standard used for diagnosing delirium, the CAM-ICU had a sensitivity of 100% and 93%, specificity of 98% and 100%, and high interrater reliability (κ = 0.96; 95% CI, 0.92–0.99) [12]. The CAM-ICU can be conducted in under 1 min, including in non-verbal patients, has been modified and validated in pediatric, emergency department, and neurocritical care patients, and has been translated into over 30 languages [27,28,29]. The CAM-ICU provides a result for delirium at the time of the test performance. It should be conducted at least once per shift, and if at all possible, each time changes in consciousness occur (e.g., before and after sedation cessation) [30]. The updated version of the CAM-ICU that was published in 2014 was also validated against the fifth version of DSM-5 using strict and standardized neuropsychological evaluation [3]. The ICDSC was originally validated in 93 patients at Hôpital Maisonneuve-Rosemont in Montreal, Quebec, Canada. A psychiatry evaluation was compared to an ICU physician evaluation. The sensitivity and specificity of the ICDSC was evaluated using a receiver operating characteristic (ROC) analysis. The area under the ROC curve was 0.9017; the ICDSC’s predicted sensitivity was 99% and specificity was 64% [15]. The ICDSC is well suited to patients that are non-communicative and includes data obtained during routine bedside care over the whole nursing shift. Both the CAM-ICU and the ICDSC can recognize patients that have subsyndromal delirium (i.e., have some abnormal features in their delirium assessment, but not meeting all criteria for delirium diagnosis). A recent systematic review of studies in ICU patients using the CAM-ICU demonstrated pooled sensitivity of 80% and specificity of 96%, while the ICDSC demonstrated a pooled sensitivity of 74% and specificity of 82% [31]. However, the sensitivity of both tools many decrease when performed by bedside personnel as compared to researchers [32]. This highlights the importance of education [30]. These two tools, the CAM-ICU and the ICDSC, are widely used in all types of critical care settings all over the world (i.e., medical, surgical, neurological, and cardiac).
Lastly, there are a small number of assessment tools designed to quantify the severity of delirium, which has been linked to increased mortality and the possibility of a nurse home placement [33,34,35]. While mainly used for research in the ICU population, delirium severity scores have been used in both in clinical settings and in research outside of the ICU. The Delirium Rating Scale (DRS) [33], CAM-S [34] and the CAM-ICU-7 [35] are the three tools used to rate the severity of delirium. The DRS [33] was designed for research, while the CAM-S [34] has been validated in general medicine and in elective, major, noncardiac surgery patients. The CAM-ICU-7, a severity scoring adapted from the CAM-ICU assessment, is an ICU delirium severity score that was validated in 518 adult medical, surgical, and progressive ICUs at three academic medical centers. Patients received the CAM-ICU and Richmond Agitation-Sedation Scale (RASS) assessments twice daily. Patients were assessed with both the CAM-ICU and the Delirium Rating Scale-Revised (DRS-R)-98. A 7-point scale rated 0–7 was derived from responses to the CAM-ICU and RASS assessments. High internal consistency (Cronbach’s alpha = 0.85) and good correlation with DRS-R-98 scores (correlation coefficient = 0.64) was found with the CAM-ICU-7. Good predictive validity was found with the CAM-ICU-7 showing higher odds (OR = 1.47; 95% CI = 1.30–1.66) of in-hospital mortality, and lower odds (OR = 0.8; 95% CI = 0.72–0.9) of being discharged home after adjusting for co-factors. Increased length of ICU stay was also associated with higher CAM-ICU-7 scores (p = 0.001). Further studies need to be conducted with this tool to determine if it could be utilized to correlate delirium severity with long-term complications of delirium. Additionally, studies should compare this tool to other delirium severity measures and provide validation in a various populations of critically ill patients prior to utilization in clinical practice [35].
The Prediction Model for Delirium (PRE-DELIRIC), The Early Prediction Model for Delirium (E-PRE-DELIRIC), and the Lanzhou model are 3 prediction models that could aid clinicians in preventing or treating delirium. The PRE-DELIRIC includes 10 predictors [age, APACHE II score, admission group (medical, surgical, trauma, neurologic), emergency admission, infection, coma, sedation, morphine use, urea level, and metabolic acidosis], the E-PRE-DELIRIC includes 9 predictors [age, history of cognitive impairment, history of alcohol abuse, blood urea nitrogen, admission group (medical, surgical, trauma, neurologic), emergency admission, mean arterial blood pressure, use of corticosteroids, and respiratory failure], and the Lanzhou Model includes 11 predictors (age, APACHE II score, mechanical ventilation, emergency surgery, coma, multiple trauma, metabolic acidosis, history of hypertension, history of delirium, history of dementia, and use of dexmedetomidine). A prospective observational study of 455 ICU patients validated these predictive models in routine clinical practice. The PRE-DELIRIC showed an area under the receiver operating characteristic (AUROC) curve of 0.79 (95% CI, 0.75–0.83), the E-PRE-DELIRIC showed an AUROC curve of 0.72 (95% CI, 0.67–0.77), and the Lanzhou Model showed an AUROC curve of 0.77 (95% CI, 0.72–0.81). However, the outputs from these models are often not pragmatic and make real time action by clinicians limited, especially if calculated in patients that have been in the ICU more than 24 h [36]. They can be used for screening for high-risk delirium patients before enrollment in clinical trials on delirium management, and/or for comparing baseline characteristics of these patients.
The role of magnetic resonance imagining (MRI) in the evaluation and management of delirium is unclear [37]. MRIs may provide diagnostic information for structural problems such as strokes or abscesses that guides therapy. MRI may also provide information on long-term cognitive prognosis [38]. Pre-operative deep and white matter and thalamic abnormalities on diffusion tension imaging have been shown in elderly patients with postoperative delirium [39]. A case series of 8 patients that underwent MRI showed white mater hyperintensities (WMH) and atrophy in 6 patients. Smaller WMH were found in younger patients. Six patients had a 3-month neuropsychological follow up which showed memory impairment, executive dysfunction, and attention impairment [40]. There is no obvious role of MRI in standard delirium care, as scanning adds burden for patients, uses a lot of resources and is likely to yield motion artifacts. However, in case of persistent delirium after having managed all potential causes, MRI can be indicated to look for any brain injury that cannot be seen with brain CT (small ischemic stroke, bleeding, encephalitis, etc.) By contrast, MRI is an excellent tool for research purposes in delirium.
EEG is a potentially useful tool to assess for delirium. Inflammatory mediators cross the blood–brain barrier and increase vascular permeability and result in EEG changes [41, 42]. A prospective cohort of non-intubated patients underwent delirium assessment with the 3D-CAM within 1 h of an EEG. Generalized theta or delta slowing was the EEG finding most strongly associated with delirium (odds ratio 10.3, 95% CI 5.3–20.1). Prevalence of delirium severity correlated with overall delirium severity (R2 = 0.907) and each of the individual features of the CAM. After adjustment for delirium presence or severity, EEG slowing was associated with longer hospitalizations, worse functional outcomes, and increased mortality. However, larger studies need to be conducted to confirm these findings [43]. EEG is also indicated to eliminate non-convulsive seizures that can be associated with delirium, especially in ICU septic patients [44].
ICU delirium and patient outcomes
“DELIRIUM IS A MANIFESTATION OF BRAIN DYSFUNCTION: The longer a patient suffers from organ dysfunction, the greater is the chance for prolonged and irreversible impairment. This holds true for delirium as a marker of acute brain dysfunction.” [23]
Delirium in hospitalized patients is a strong independent predictor of mortality, increased hospital length of stay, subsequent hospitalizations, long-term cognitive impairment, and cost of care. A prospective cohort study of 275 adult medical and coronary ICUs sought to determine the effect of delirium on mortality and length of stay. During the ICU stay, 183 (81.7%) patients developed delirium. Following adjustment for age, severity of illness, comorbid conditions, coma, and use of sedation or analgesia, delirium was independently associated with higher 6-month mortality (adjusted hazard ratio [HR], 3.2; 95% confidence interval [CI], 1.4–7.7; p = 0.008), longer hospital stay (adjusted HR, 2.0; 95%CI, 1.4–3.0; p < 0.001), and a longer post-ICU stay (adjusted HR, 1.6; 95% CI, 1.2–2.3; p = 0.009) [14]. The true attributable risk of mortality to delirium has been evaluated in other studies [45] specifically evaluating the differential severity of illness prior to delirium onset demonstrating that delirium is not casually related to mortality. This study specifically found that in patients with > 2 days of delirium in the ICU, there was a true risk of mortality attributable to delirium. This study brings to light the differences between associations and causality. Causality requires the following criteria: strength of association, consistency, temporality, biological gradient, plausibility, and lastly an experiment demonstrating that treatment of delirium decreases mortality.
Further the incident risk of delirium mortality was recently evaluated in 1495 critically ill adults. Incident delirium and days spent with delirium were not significantly associated with mortality. Both, days spent with coma and days spent with delirium or coma were significantly associated with mortality [46]. A retrospective cohort study of 6323 ICU patients evaluated the association between delirium subtypes and 90-day mortality following adjustment for covariates. Only mixed delirium, not hyperactive, hypoactive, or rapidly reversible delirium was associated with 90-day mortality [1.57 (95%CI: 1.51–2.14)] [47].
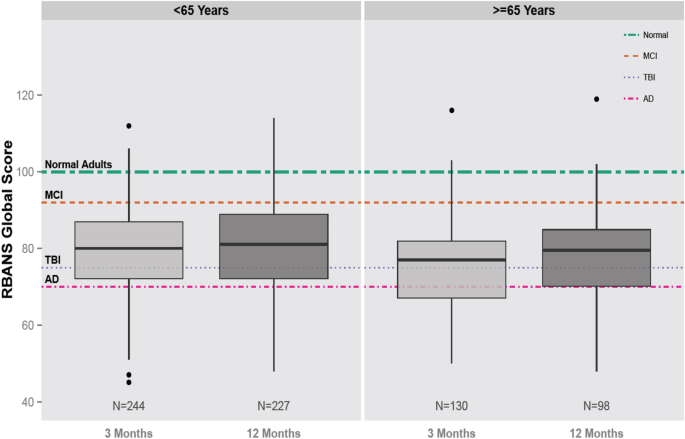
The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study, a large, multicenter, prospective observational cohort study of 821 adult medical ICU and surgical ICU patients with respiratory failure, cardiogenic or septic shock was conducted to determine the prevalence of long-term cognitive impairment following critical illness. At 3 months following discharge, RBANS score similar to Alzheimer’s disease was found in 26% of patients, and a score similar to moderate traumatic brain injury was found in 40% of patients (Fig. 2). Both young and older adults, with and without comorbidities, experienced these impairments, which were still present at 12 months in 24% and 34% of these individuals [23]. A subgroup analysis of 402 patients that received surgery with anesthesia exposure had similar global cognition scores to those who did not, at 3 and 12 months even after in-hospital or baseline covariates [48]. Delirium was the strongest independent predictor of cognitive impairment in this cohort. Delirium does not always precede cognitive impairment and there are no randomized, clinical trials to date demonstrating that long-term cognitive impairment is improved through treatment of delirium [49].
Delirium also has a high cost. In a subgroup analysis within the BRAIN-ICU study, the patient-level 30-day cumulative cost attributable to higher resource utilization of ICU delirium was $17,838 (95% confidence interval, $11,132–$23,497). This cost could have been even higher if not for the early mortality associated with delirium in some patients that resulted in a reduction in cost of care of $4654 (95% confidence interval, $2056–7869) [50]. Direct 1-year health care costs associated with delirium are predicted to range between $143 to $152 billion, assuming delirium occurs in 20% of elderly patients hospitalized annually [51].
Prevention and management of ICU delirium
“NO SINGLE PHARMACOLOGICAL AGENT CAN PREVENT DELIRIUM: No single pharmacological agent can prevent brain dysfunction in the form of delirium. It is necessary to actively monitor for delirium and pay attention to the details that may put patients at risk for delirium.” [53]
Pharmacologic prevention of ICU delirium
The neurotransmitter hypothesis has led to studies that have evaluated the benefit of antipsychotic medications in delirium. Haloperidol primarily acts by blocking dopamine and atypical antipsychotics block serotonin, dopamine, alpha-1 adrenergic receptors, and histamine. Multiple studies have been conducted targeting this hypothesis, as well as other central nervous receptors, yet none have consistently demonstrated a significant reduction in delirium. The PADIS guidelines therefore suggest not using haloperidol, atypical antipsychotics, dexmedetomidine, statins, or ketamine to prevent delirium in all critically ill adults [17]. The main studies supporting this recommendation are described below.
A double-blind placebo-controlled randomized trial (HOPE ICU) was conducted to determine the effect of haloperidol on the prevention of delirium. Patients were randomized to receive haloperidol 2.5 mg or 0.9% saline placebo intravenously every 8 h if receiving mechanical ventilation within 7 h of admission. The number of days alive and without delirium and coma were similar between the haloperidol group and the placebo group (median 5 days [IQR 0–10] vs. 6 days [0–11] days; p = 0.53) [52].
The Prophylactic Haloperidol Use for Delirium in Patients at High Risk for Delirium (REDUCE) trial was a randomized, double-blind, placebo-controlled study of 1789 critically ill patients who received prophylactic haloperidol 1 mg, haloperidol 2 mg, or placebo. The 1-mg haloperidol group was prematurely stopped because of futility. No difference occurred in the median survival during 28 days in the 2-mg haloperidol group compared with 28 days in the placebo group (95% CI, 0–0; p = 0.93) with a hazard ratio of 1.003 (95% CI, 0.78–1.30; p = 0.82). None of the 15 secondary outcomes were statistically different between the three groups. These outcomes included delirium incidence (mean difference 1.5%; 95% CI, − 3.6% to 6.7%), delirium- and coma-free days (mean difference 0 days; 95% CI, 0–0 days), and duration of mechanical ventilation, ICU, and hospital length of stay (mean difference 0 days; 95% CI, 0–0 days for all three measures). Adverse events did not differ between the groups [53].
A randomized, double-blind, placebo-controlled study was conducted to determine the effect of risperidone on postoperative delirium following cardiac surgery in 126 patients. Patients were randomized to receive 1 mg of risperidone or placebo. The incidence of postoperative delirium was lower in the risperidone group than the placebo group (11.1% vs. 31.7%, p = 0.009, relative risk = 0.35, 95% confidence interval = 0.16–0.77) [54]. A randomized, double-blind, placebo-controlled trial was conducted in 700 elderly patients admitted to the ICU after non-cardiac surgery at two tertiary-care hospitals in China. Patients were randomized to receive either dexmedetomidine from ICU admission on the day of surgery until 0800 h on postoperative day 1 or placebo. During the first seven days postoperatively, the incidence of delirium was significantly lower in the dexmedetomidine group (32 [9%] of 350 patients) as compared to the placebo group (79 [23%] of 350 patients; odds ratio [OR] 0.35, 95% CI 0.22–0.54; p < 0.0001) [55]. A multicenter, double-blind, placebo-controlled trial randomized 100 critically ill patients without delirium to nocturnal dexmedetomidine or placebo. A greater proportion of patients in the dexmedetomidine group remained delirium-free during the ICU stay (dexmedetomidine [40 (80%) of 50 patients] compared to placebo [27 (54%) of 50 patients]; relative risk, 0.44; 95% confidence interval, 0.23–0.82; p = 0.006) [56]. The authors of the PADIS guidelines considered the delirium incidence and duration, duration of mechanical ventilation, ICU length of stay, and mortality the most critical outcomes. Although there was a consistent decrease in delirium incidence, the PADIS guideline committee deemed that none of the studies reported a meaningful difference for any of the other important clinical outcomes. Additionally, many of these studies contained primarily surgical patients that have a lower severity of illness than medical patients. Given the strong association of delirium and severity of illness, and that many critically ill patients may have delirium when admitted to the ICU, further research needs to be conducted to assess pharmacologic prevention of delirium [21, 26].
The association between statin cessation during critical illness and an increased occurrence of delirium has been shown in three cohort studies [56,57,58]. Conversely, a randomized study in cardiac surgery patients found that pre-operative atorvastatin did not decrease delirium [59]. Similarly, delirium was not decreased in older adults following major surgery in a large, randomized study following a single dose of ketamine [60]. However, a double-blinded RCT in 162 patients (26% surgical) compared ketamine to placebo in order to reduce the dose of remifentanil used for analgosedation (primary outcome) and reported unexpectedly significant lower incidence and duration of delirium with ketamine but no other differences in patients’ outcomes, which deserves further investigation [61].
Pharmacologic treatment of ICU delirium
“NO SINGLE PHARMACOLOGICAL AGENT CAN TREAT DELIRIUM: No single pharmacological agents can treat delirium. However, currently, clinicians need to focus on predisposal factors of delirium. Non-pharmacologic strategies should be used. There may be situations where the use of drugs may be necessary to manage hyperactive behavior of delirious patients, but it is essential to realize that it is not treating the delirium.” [17]
Similar to the data on ICU delirium prevention, no large trials have shown that the use of any pharmacologic agents can treat delirium in the ICU stetting thus leading the PADIS guidelines to suggest against routine using haloperidol, atypical antipsychotics, or statins to treat delirium. The guidelines do underscore that there may be situations where the use of these drugs may be warranted to manage the hyperactive behavior of delirious patients, or stress related symptoms (anxiety, hallucinations, delusion, fear, etc.), but it is essential to realize that it is not treating the delirium. If antipsychotics are chosen for these situations, it should be in the smallest doses and shortest duration that is necessary [24, 26]. It is also important to note the conceptual limitation that disambiguating delirium prevention from delirium treatment is exceptionally challenging in the real-world setting.
The MIND study, a randomized, double-blind placebo-controlled trial compared the use of haloperidol, ziprasidone, or placebo every 6 h for up to 14 days in 101 mechanically ventilated ICU patients. Patients in the haloperidol group had a similar number of days alive without delirium or coma compared to the ziprasidone and placebo groups (14 [6,7,8,9,10,11,12,13,14,15,16,17,18] days, 15 [9.1–18] days, 12.5 [1.2–17.2]) [62].
The Modifying the Impact of the ICU-Associated Neurological Dysfunction-USA (MIND USA) Study, a multicenter, randomized, placebo-controlled study of 566 patients with acute respiratory failure or shock compared haloperidol, ziprasidone, and placebo for the treatment of delirium. The adjusted median number of days alive without delirium or coma was 8.5 (95% CI, 5.6–9.9) in the placebo group, as compared with 7.9 (95% CI, 4.4–9.6) in the haloperidol group and 8.7 (95% CI, 5.9–10.0) in the ziprasidone group, p = 0.26. Within the study, 60% of patients had hypoactive delirium and 40% of patients had hyperactive delirium at some point in the study. There was no difference between the groups in mechanical ventilation duration, ICU or hospital length of stay, days to ICU readmission, death at 30 days, or death at 90 days compared with placebo. Arrhythmias, Parkinsonism (extrapyramidal symptoms), neuroleptic malignant syndrome, study drug discontinuation, and other safety concerns were extremely low across all three groups [17].
However, the results of a multinational European cohort study by Collet et al. [63], the AID-ICU study, including 1260 patients from 99 ICUs in 13 countries have shown that haloperidol was the most common pharmacological intervention for delirium regarding delirium subtype. In this study the use of haloperidol within 24 h of ICU admission [aOR 1.2 (0.5–2.5); p = 0.66] and within 72 h of ICU admission [aOR 1.9 (1.0–3.9); p = 0.07], was not associated with increased 90-day mortality, yet at 72 h after admission to the ICU the use of haloperidol was associated with the need for circulatory support [aOR 2.6 (1.1–6.9)].
Antipsychotics remain viable for the short-term control of severe agitation to prevent the risk of patient’s self-removing of ICU devices, fall, or aggressive behavior against the ICU team, severe anxiety with the need to avoid respiratory suppression (e.g., heart failure, COPD, or asthma), or symptomatic delirium features such as hallucinations or delusions [26]. If an antipsychotic is initiated, low starting doses should be considered, and daily review of drug interactions, adverse effects, dosing titration, and need for the antipsychotic should be completed.
In addition to antipsychotics, other drugs have been investigated to treat delirium such as statins and dexmedetomidine. A randomized, double-blind placebo-controlled trial of 142 patients found that high dose simvastatin (80 mg daily) does not increase days alive without delirium and without coma at day 14 (5.7 days (SD 5.1) with simvastatin and 6.1 days (5.2) with placebo (mean difference 0.4 days, 95% CI-1.3–2.1; p = 0.66) [64]. While there is no recommendation for statin use, the PADIS guidelines recommend using dexmedetomidine for patients with delirium in which agitation is preventing extubation or weaning off the ventilator [26]. The Dexmedetomidine to Lessen ICU Agitation (DAHLIA) study was a double-blind placebo-controlled trial in 15 ICUs in Australia and New Zealand in which 39 patients were randomized to dexmedetomidine and 32 to receive placebo. In the first 7 days after study randomization, dexmedetomidine was associated with a small increase in ventilator-free hours compared to placebo (median, 144.8 h vs. 127.5 h, 95% CI 4–33.2 h, p = 0.01). Dexmedetomidine use did not affect ICU or hospital length of stay or the patient’s discharge disposition. Patients did not receive opioids commonly and the prevalence of alcohol withdrawal was not reported [65]. Future studies need to evaluate the role of dexmedetomidine in patients with hypoactive delirium or those in which agitation is not preventing extubation, as well as in non-intubated patients [66].
Future studies evaluating pharmacologic therapy of delirium need to concentrate on long-term cognitive and functional outcomes. Additionally, agents such a valproic acid, which have only been included in small studies, need to be thoroughly evaluated in prospective randomized trials [22].
Nonpharmacologic prevention and management
While no pharmacologic agents have been shown to significantly impact delirium, bundling of non-pharmacologic strategies have and thus this bundle concept has become a mainstay of ICU care. One mnemonic to consider when thinking about the differential causes of delirium is DR.DRE, composed of Disease Remediation, Drug Removal (screening for both drug related delirium and withdrawal syndromes), and Environment. The use of this mnemonic helps consider the most common risk factors and may be particularly useful for communication within the whole therapeutic team (medical, nursing, physiotherapy, pharmacology personnel). However, the use of mnemonics depends on the ICU culture and clinicians’ preference and should not replace an exhaustive screening for all frequent causes of delirium (e.g., bladder retention, hypoglycemia, lack of bowel movement). Bright light therapy, family participation in care, and psychoeducational programs are the three single-component interventions that have been evaluated in the ICU. Three studies evaluated the effects of bright light therapy and did not find a reduction in delirium or ICU length of stay [67,68,69], so the PADIS guidelines made a conditional recommendation against its use. The PADIS guidelines recommend using multicomponent interventions such reorientation, cognitive stimulation, use of clocks, sleep enhancement, increased wakefulness, early mobility, and use of hearing aids and eyeglasses when indicated. Many multicomponent bundles have shown improved outcomes in critically ill adults including reduction in delirium, ICU length of stay and hospital mortality [67, 70,71,72,73,74].
One example of a multi-component strategy is the A2F bundle (A, assess, prevent, and manage pain; B, both spontaneous awakening and spontaneous breathing trials; C, choice of analgesic and sedation; D, delirium: assess, prevent, and manage; E, early mobility, and exercise; and F, family engagement). This easy to memorize bundle is a 6-step approach, created to facilitate implementation of the recommendations of multiple guidelines [24,25,26, 75]. This bundle has been shown to improve a spectrum of patient outcomes in a single center study, a multiple hospital/single regional system study, and a large nationwide collaborative. However, notably all the below discussed trials are non-randomized and did not have concurrent controls. While it is widely believed to be effective, there is currently not a single RCT demonstrating the benefit of the A2F bundle which is the gold standard in terms of demonstration of therapeutic efficacy.
A prospective, cohort quality improvement study in ventilated and non-ventilated patients was conducted in 6,064 patients at seven community hospitals. Patients had a 7% higher odds of hospital survival for every 10% increase in total bundle compliance (odds ratio, 1.07; 95% CI, 1.04–1.11; p < 0.001). Patients had a 15% higher hospital survival for every 10% increase in partial bundle compliance (odds ratio, 1.15; 95% CI, 1.09–1.22; p < 0.001). With total bundle compliance (incident rate ratio, 1.02; 95% CI, 1.01–1.04; p = 0.004) and partial bundle compliance (incident rate ratio, 1.15; 95% CI, 1.09–1.22; p < 0.001), patients had more days alive and free of delirium and coma [76].
In a prospective, multicenter, quality improvement collaborative from 68 academic, community, and federal ICUs during a 20-month collection period, performance of the complete A2F bundle was associated with a lower likelihood of death within 7 days (HR 0.32; CI, 0.17–0.62), next-day mechanical ventilation (OR 0.28; CI, 0.22–0.36), coma (OR 0.35; CI, 0.22–0.56), delirium (OR 0.60; CI, 0.49–0.72), physical restraint use (OR 0.37; CI,0.30–0.46), ICU readmission (OR 0.54; CI, 0.37–0.79), and discharge to a facility other than home (OR 0.64; CI. 0.510.80). There was a dose response between a higher proportional bundle performance and improvement in each clinical outcome (p < 0.002). Pain was more commonly reported as bundle performance increased (p = 0.0001), probably because more patients were awake [77]. Members of the collaborative faculty published two subsequent papers to aid in implementation of the A2F bundle [78, 79].
Lastly, a prospective cohort study of 1855 mechanically ventilated patients was conducted to evaluate staged implementation of the A2F bundle. Implementation of the full versus partial bundle resulted in reduced mechanical ventilation duration (− 22.3%; 95% CI, − 22.5% to − 22.0%; p < 0.001), ICU length of stay (− 10.3%; 95% CI, − 15.6% to − 4.7%; p = 0.028), and hospital length of stay (− 7.8%; 95% CI, − 8.7% to − 6.9%; p = 0.006) after adjustment for patient-level covariates. ICU and hospital costs were also decreased by 24.2% (95% CI, − 41.4% to − 2.0%; p = 0.03) and 30.2% (95% CI, − 46.1% to − 9.5%; p = 0.007), respectively [80].
Quite contrary in a recent meta-analysis of randomized controlled trials by Bannon et al. [81], concentrating on the effectiveness of non-pharmacological interventions versus standard care in reducing the incidence and duration of delirium in the ICU the authors identified 15 trials (2812 participants) with results indicating that current evidence is too weak to support the use of non-pharmacological interventions (principally single interventions) in reducing incidence and duration of delirium in critically ill patients. However, to support the importance of the F (Family) element of the A2F bundle, a trial of reorientation using a family voice showed a beneficial effect [n = 30, MD (days) − 1.30, 99% CI − 2.41 to − 0.19, p = 0.003 [81]. The future goals to be achieved in ICU Delirium research and care have been identified recently and should include all the above mentioned non-pharmacological interventions and practices, including the A2F bundle [49].
Important to note, the “A” element of the A2F bundle stands for assess, prevent, and treat pain. Untreated pain can predispose patients to delirium. However, utilization of opioids can also result in delirium [ 82 ]. This highlights the importance of using validated tool such as the Numeric Rating Scale, Critical Care Pain Observational Tool, or the Behavioral Pain Scale to diagnose pain in critically ill patients [ 26 ].
Also, important to note, the “C’ element of the A2F bundle stands for choice of sedation and focuses on constant vigilance to ensure that patients receive the best sedative agent at the least amount. The PADIS guidelines suggest using either propofol or dexmedetomidine over benzodiazepines for sedation in critically ill mechanically ventilated adults [83]. These recommendations were based on observational studies demonstrating increased risk of delirium when receiving benzodiazepines [84, 85], and comparator studies of either propofol or dexmedetomidine versus benzodiazepines, where each of the studies showed worse outcomes in the benzodiazepine group [86,87,88,89]. No significant differences have been found in time to extubation or other important secondary outcomes in three randomized trials containing a total of 850 patients comparing dexmedetomidine and propofol [86,87,88,89].
SPICE III is an open label, randomized controlled trial comparing dexmedetomidine as primary sedation to usual care (propofol, midazolam or other sedation) in patients receiving less than 12 h of mechanical ventilation who are expected to be mechanically ventilated for at least one additional day that was conducted following publication of the PADIS guidelines. The target RASS goal was − 2 to + 1. The target RASS goal was − 2 to + 1. Death at 90 days occurred in 569 of 1956 (29.1%) of the usual care group and 566 of 1956 (29.1%) in the dexmedetomidine group (adjusted risk difference, 0.0 percentage points; 95% confidence interval, − 2.9 to 2.8). In the dexmedetomidine group, 64% of the patients received propofol, 3% received midazolam, and 7% received both during the first 2 days following randomization. Noteworthy, 60% of patients received propofol, 12% received midazolam, and 20% received both in the dexmedetomidine group. Given the multiple sedatives administered in both groups, the application of the results of this study is difficult. In the dexmedetomidine group, bradycardia and hypotension occurred in 5.1% and 2.7% patients respectively. The median number of days free from coma or delirium was 1 day longer in the dexmedetomidine as compared to the usual care group 24 (11–26) vs. 23 (10–26), adjusted risk difference, 1 (95% confidence interval, 0.5–1.5) [90].
The Maximizing the Efficacy of Sedation and Reducing Neurological Dysfunction and Mortality in Septic Patients with Acute Respiratory Failure (MENDS 2), is a multicenter, double-blind, randomized controlled trial of 432 patients randomized to dexmedetomidine or propofol for up to 14 days or extubation. No difference was found between dexmedetomidine and propofol in the number of days alive without delirium or coma (odds ratio [OR], 0.96; 95% CI, 0.74–1.26), ventilator-free days (OR, 0.98; 95% CI, 0.63–1.51), or death at 90 days (HR, 1.06; 95% CI, 0.74–1.52) [91].
Finally, the whole A2F bundle is driven toward a reduction of sedatives use. In this way, the best non-pharmacological prevention of delirium could be to completely avoid sedation when unnecessary. A RCT in 137 postoperative ICU patients mostly admitted for peritonitis and septic shock reported as secondary outcomes a significant reduction in delirium incidence (72% vs. 43%; − 29 (− 50 to − 14)%, p < 0.001) and delirium duration [2 (0–4) days vs. 0 (0–2); − 0.5 (− 1.0–0.0) days, p = 0.003] in the group where the sedatives were immediately stopped compared to the group where a moderate sedation (RASS -3) was sustained for one day and half [92].
The optimal sedation strategies for mechanically ventilated patients with severe respiratory failure and adult respiratory distress syndrome (ARDS) and prevention of ICU delirium have become especially important in the light of COVID-19 pandemic [93]. The recently published COVID-D study, an observational cohort of 2,088 COVID-19 positive ICU patients from 69 sites and 14 countries, reported 81% of patients had coma for a median of 10 [IQR 6–15] days, and 55% were delirious for a median of 3 [IQR 2–6] days [94]. Deep sedative levels and prolonged sedatives infusions while on mechanical ventilation was common with 64% receiving benzodiazepines for 7 [4,5,6,7,8,9,10,11,12] days and 71% receiving propofol for 7 [4,5,6,7,8,9,10,11] days and the median RASS score was − 4 [− 5 to − 3]. Benzodiazepines were associated with increased risk of delirium development and fewer days alive without either delirium or coma. Additionally, visits (in-person or virtual) with family or friends were associated with decreased risk of delirium. These data support the management goals behind the A2F Bundle in every clinical and epidemiological situation in the ICU: use of lighter sedation, avoidance of benzodiazepines, involvement of family, friends, and caregivers to provide targeted humane care, even in patients with ARDS, including patients suffering from COVID-19.
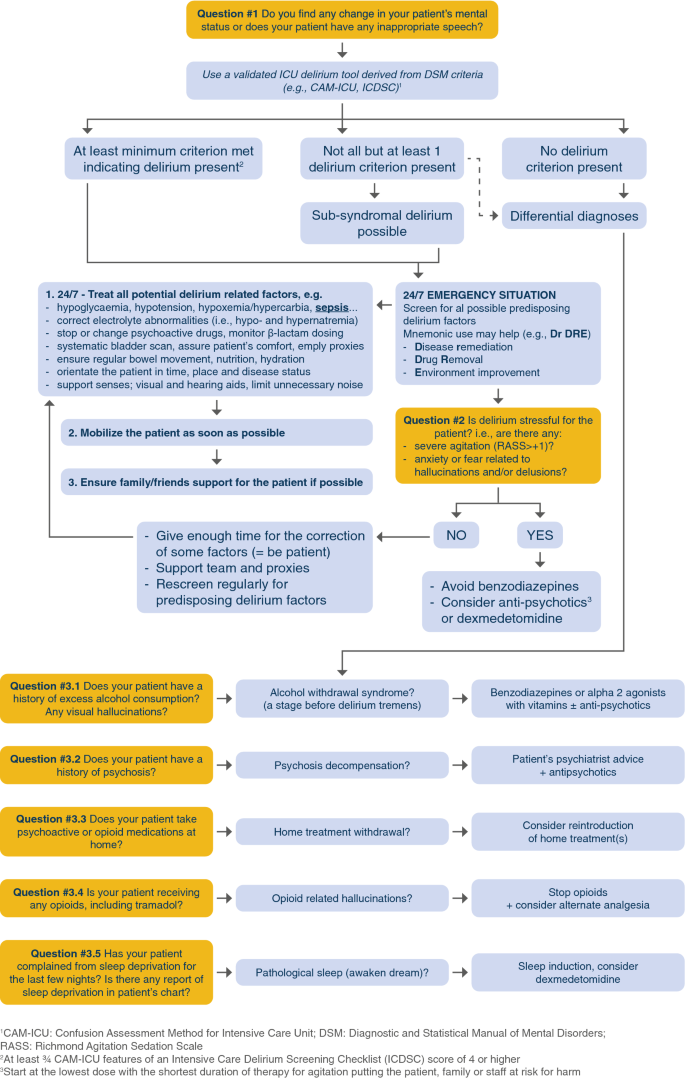
Recently, an expert panel recommended the A2F bundle for these patients and proposed to update the bundle adding a R for respiratory drive control (A2F-R bundle) [95]. In the A2F perspective to prefer non pharmacological intervention in order to reduce sedatives and the risk of cognitive dysfunction, it would be especially meaningful in mechanically ventilated patients to prioritize the optimization of the ventilator setting, preferring a more comfortable ventilation mode, allowing for reducing opioids and sedatives, along with the screening and management of patient’s associated factors of high respiratory demand (metabolic acidosis, fever, stressful symptoms, e.g., pain, anxiety, dyspnea). This strategy would benefit from patient’s outcomes as well as the sparing of analgesics, sedatives and neuromuscular blocking agents which is crucial in times of pandemics and high requirement of ICU resources. Figure 3 is an algorithm to guide clinicians in evaluating patients exhibiting an acute change in mental status.
Future directions
Knowledge gaps and research agenda for the next 10 years
-
1.
Validation and development of objective tools for delirium diagnosis such as EEG or computer-based apps.
-
2.
Mastering delirium pathophysiology and its association with long-term cognitive impairment.
-
3.
Development of new delirium phenotyping models.
-
4.
Beyond casual inference, understanding the association of delirium with outcomes.
-
5.
Further understanding delirium biomarkers and their practical use in predictive models.
-
6.
Conduction of large, randomized clinical trials in critically ill patients evaluating the effects of sleep optimization, cognitive/physical training, alternate safety practices, and family engagement/non-pharmacological interventions on delirium and long-term outcomes [49].
Future directions include assessing diagnostic tools including EEG, CSF studies, and imaging studies (MRI) and utilizing prediction models in diverse patient populations. Additionally, studies need to assess the effects of antipsychotics on the symptoms of hallucinations and delusions in ICU patients with delirium. Lastly, larger, randomized studies need to be conducted to assess the non-pharmacologic prevention and treatment of delirium and its burden including clinically meaningful and long-term outcomes.
Conclusion
Delirium is an acute organ dysfunction independently predictive of mortality and multiple morbidities including increased ICU and hospital length of stay, cognitive dysfunction, and cost. Multiple tools have been validated for diagnosis of delirium in the ICU. The CAM-ICU and the ICDSC are the two tools that are recommended for diagnosing delirium in the ICU by PADIS guidelines. Antipsychotics, dexmedetomidine, statins, and ketamine are not recommended to prevent delirium. Antipsychotics are also not recommended for use to treat delirium. However, antipsychotics can be considered for short-term control of severe agitation or stressful symptoms (anxiety, hallucinations, delusion, fear). Non-pharmacologic therapy including the A2F Bundle is the main means of delirium prevention or treatment, including the suggestion of adding a “R” to underline the role of better management of Respiratory drive control and ventilator setting in patients with ARDS and more generally in all mechanically ventilated patients. Diagnostic tools, effects of antipsychotics on hallucinations and delusions, and non-pharmacological prevention and treatment of delirium and association with long-term outcomes are some of the top study areas to be conquered next in the field of ICU delirium.
References
Morandi A, Piva S, Ely EW, Myatra SN, Salluh JIF, Amare D, Azoulay E, Bellelli G, Csomos A, Fan E et al (2017) Worldwide survey of the “assessing pain, both spontaneous awakening and breathing trials, choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment” (ABCDEF) bundle. Crit Care Med 45(11):e1111–e1122
Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, Hughes CG, Chandrasekhar R, Pun BT, Boehm LM et al (2018) Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med 6(3):213–222
Chanques G, Ely EW, Garnier O, Perrigault F, Eloi A, Carr J, Rowan CM, Prades A, de Jong A, Moritz-Gasser S et al (2018) The 2014 updated version of the confusion assessment method for the intensive care unit compared to the 5th version of the diagnostic and statistical manual of mental disorders and other current methods used by intensivists. Ann Intensive Care 8(1):33
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association
Slooter AJC, Otte WM, Devlin JW, Arora RC, Bleck TP, Claassen J, Duprey MS, Ely EW, Kaplan PW, Latronico N et al (2020) Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med 46(5):1020–1022
Brummel NE, Boehm LM, Girard TD, Pandharipande PP, Jackson JC, Hughes CG, Patel MB, Han JH, Vasilevskis EE, Thompson JL et al (2017) Subsyndromal delirium and institutionalization among patients with critical illness. Am J Crit Care 26(6):447–455
Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y (2007) Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med 33(6):1007–1013
Wood E, Albarqouni L, Tkachuk S, Green CJ, Ahamad K, Nolan S, McLean M, Klimas J (2018) Will this hospitalized patient develop severe alcohol withdrawal syndrome?: The rational clinical examination systematic review. JAMA 320(8):825–833
Salomon C, Hamilton B, Elsom S (2014) Experiencing antipsychotic discontinuation: results from a survey of Australian consumers. J Psychiatr Ment Health Nurs 21(10):917–923
Sivanesan E, Gitlin MC, Candiotti KA (2016) Opioid-induced hallucinations: a review of the literature, pathophysiology, diagnosis, and treatment. Anesth Analg 123(4):836–843
Babkoff H, Sing HC, Thorne DR, Genser SG, Hegge FW (1989) Perceptual distortions and hallucinations reported during the course of sleep deprivation. Percept Mot Skills 68(3 Pt 1):787–798
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R et al (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286(21):2703–2710
Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA et al (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 371(9607):126–134
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291(14):1753–1762
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y (2001) Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 27(5):859–864
Guenther U, Popp J, Koecher L, Muders T, Wrigge H, Ely EW, Putensen C (2010) Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care 25(1):144–151
Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, Malhotra A, Owens RL, Feinstein DJ et al (2018) Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 379(26):2506–2516
Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, Serafim RB, Stevens RD (2015) Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ 350:2538
Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, Ely EW (2006) Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc 54(3):479–484
Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman Pun B, Dittus R, Ely EW (2007) Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med 33(10):1726–1731
Hayhurst CJ, Marra A, Han JH, Patel MB, Brummel NE, Thompson JL, Jackson JC, Chandrasekhar R, Ely EW, Pandharipande PP et al (2020) Association of hypoactive and hyperactive delirium with cognitive function after critical illness. Crit Care Med 48(6):e480–e488
Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP (2014) Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med 189(6):658–665
Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK et al (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369(14):1306–1316
Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, Gan TJ, McEvoy MD, Miller TE (2020) Perioperative quality initiative w: american society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg 130(6):1572
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM et al (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41(1):263–306
Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B et al (2018) Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 46(9):e825–e873
Han JH, Wilson A, Shintani AK, Graves AJ, Schnelle J, Vernon J, Dittus RS, Storrow AB, Ely EW (2011) The validation of the brief confusion assessment method in older emergency department patients. Ann Emerg Med 60(Suppl):S28
Smith HA, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Savage S, Rutherford MT, Denton D, Thompson JL, Chandrasekhar R et al (2016) The preschool confusion assessment method for the ICU: valid and reliable delirium monitoring for critically Ill infants and children. Crit Care Med 44(3):592–600
Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, Eden SK, Terrell MK, Boswell T, Wolfram K et al (2011) Diagnosing delirium in critically ill children: validity and reliability of the pediatric confusion assessment method for the intensive care unit. Crit Care Med 39(1):150–157
Pun B, Devlin J (2013) Delirium monitoring in the ICU: strategies for initiating and sustaining screening efforts. Semin Respir Crit Care Med 34(02):179–188
Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC (2012) The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 16(4):R115
van Eijk MM, van den Boogaard M, van Marum RJ, Benner P, Eikelenboom P, Honing ML, van der Hoven B, Horn J, Izaks GJ, Kalf A et al (2011) Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med 184(3):340–344
Trzepacz PT (1999) The delirium rating scale - its use in consultation-liaison research. Psychosomatics 40(3):193–204
Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Jones RN (2014) The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 160(8):526–533
Khan BA, Perkins AJ, Gao S, Hui SL, Campbell NL, Farber MO, Chlan LL, Boustani MA (2017) The confusion assessment method for the ICU-7 delirium severity scale: a novel delirium severity instrument for use in the ICU. Crit Care Med 45(5):851–857
Green C, Bonavia W, Toh C, Tiruvoipati R (2018) Prediction of ICU delirium: validation of current delirium predictive models in routine clinical practice. Crit Care Med 47(3):428–435
Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, Maclullich AM (2008) Neuroimaging studies of delirium: a systematic review. J Psychosom Res 65(3):239–248
Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, Thompson J, Shintani AK, Geevarghese S, Miller RR 3rd et al (2012) The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med 40(7):2022–2032
Shioiri A, Kurumaji A, Takeuchi T, Matsuda H, Arai H, Nishikawa T (2010) White matter abnormalities as a risk factor for postoperative delirium revealed by diffusion tensor imaging. Am J Geriat Psyc 18(8):743–753
Morandi A, Gunther ML, Vasilevskis EE, Girard TD, Hopkins RO, Jackson JC, Pandharipande P, Ely EW (2010) Neuroimaging in delirious intensive care unit patients: a preliminary case series report. Psychiatry (Edgmont) 7(9):28–33
Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L (1984) Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol 246(6 Pt 2):R994-999
van der Kooi AW, Zaal IJ, Klijn FA, Koek HL, Meijer RC, Leijten FS, Slooter AJ (2015) Delirium detection using EEG: what and how to measure. Chest 147(1):94–101
Kimchi EY, Neelagiri A, Whitt W, Sagi AR, Ryan SL, Gadbois G, Groothuysen D, Westover MB (2019) Clinical EEG slowing correlates with delirium severity and predicts poor clinical outcomes. Neurology 93(13):e1260–e1271
Gilmore EJ, Gaspard N, Choi HA, Cohen E, Burkart KM, Chong DH, Claassen J, Hirsch LJ (2015) Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med 41(4):686–694
Klein Klouwenberg PM, Zaal IJ, Spitoni C, Ong DS, van der Kooi AW, Bonten MJ, Slooter AJ, Cremer OL (2014) The attributable mortality of delirium in critically ill patients: prospective cohort study. BMJ 349:g6652
Duprey MS, van den Boogaard M, van der Hoeven JG, Pickkers P, Briesacher BA, Saczynski JS, Griffith JL, Devlin JW (2020) Association between incident delirium and 28- and 90-day mortality in critically ill adults: a secondary analysis. Crit Care 24(1):161
Rood PJT, van de Schoor F, van Tertholen K, Pickkers P, van den Boogaard M (2019) Differences in 90-day mortality of delirium subtypes in the intensive care unit: a retrospective cohort study. J Crit Care 53:120–124
Hughes CG, Patel MB, Jackson JC, Girard TD, Geevarghese SK, Norman BC, Thompson JL, Chandrasekhar R, Brummel NE, May AK et al (2017) Surgery and anesthesia exposure is not a risk factor for cognitive impairment after major noncardiac surgery and critical illness. Ann Surg 265(6):1126–1133
Pandharipande PP, Ely EW, Arora RC, Balas MC, Boustani MA, La Calle GH, Cunningham C, Devlin JW, Elefante J, Han JH et al (2017) The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med 43(9):1329–1339
Vasilevskis EE, Chandrasekhar R, Holtze CH, Graves J, Speroff T, Girard TD, Patel MB, Hughes CG, Cao A, Pandharipande PP et al (2018) The cost of ICU delirium and coma in the intensive care unit patient. Med Care 56(10):890–897
Leslie DL, Inouye SK (2011) The importance of delirium: economic and societal costs. J Am Geriatr Soc 59(Suppl 2):S241-243
Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, Jackson J, Perkins GD, McAuley DF (2013) Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 1(7):515–523
van den Boogaard M, Slooter AJC, Bruggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW, Pretorius D, de Koning J, Simons KS, Dennesen PJW et al (2018) Effect of haloperidol on survival among critically Ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 319(7):680–690
Prakanrattana U, Prapaitrakool S (2007) Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care 35(5):714–719
Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D (2016) Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 388(10054):1893–1902
Skrobik Y, Duprey MS, Hill NS, Devlin JW (2018) Low-dose nocturnal dexmedetomidine prevents ICU delirium a randomized, placebo-controlled trial. Am J Respir Crit Care Med 197(9):1147–1156
Morandi A, Hughes CG, Girard TD, McAuley DF, Ely EW, Pandharipande PP (2011) Statins and brain dysfunction a hypothesis to reduce the burden of cognitive impairment in patients who are critically III. Chest 140(3):580–585
Mather JF, Corradi JP, Waszynski C, Noyes A, Duan Y, Grady J, Dicks R (2017) Statin and its association with delirium in the medical ICU. Crit Care Med 45(9):1515–1522
Billings FTT, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ (2016) High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA 315(9):877–888
Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, Veselis RA, Grocott HP, Emmert DA, Rogers EM et al (2017) Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 390(10091):267–275
Perbet S, Verdonk F, Godet T, Jabaudon M, Chartier C, Cayot S, Guerin R, Morand D, Bazin JE, Futier E et al (2018) Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth Crit Care Pain Med 37(6):589–595
Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY et al (2010) Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med 38(2):428–437
Collet MO, Caballero J, Sonneville R, Bozza FA, Nydahl P, Schandl A, Woien H, Citerio G, van den Boogaard M, Hastbacka J et al (2018) Prevalence and risk factors related to haloperidol use for delirium in adult intensive care patients: the multinational AID-ICU inception cohort study. Intensive Care Med 44(7):1081–1089
Page VJ, Casarin A, Ely EW, Zhao XB, McDowell C, Murphy L, McAuley DF (2017) Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MoDUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 5(9):727–737
Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, Davies A, Delaney A, Ghosh A, van Haren F et al (2016) Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA 315(14):1460–1468
Louis C, Godet T, Chanques G, Bourguignon N, Morand D, Pereira B, Constantin JM (2018) network A: effects of dexmedetomidine on delirium duration of non-intubated ICU patients (4D trial): study protocol for a randomized trial. Trials 19(1):307
Ono H, Taguchi T, Kido Y, Fujino Y, Doki Y (2011) The usefulness of bright light therapy for patients after oesophagectomy. Intensive Crit Care Nurs 27(3):158–166
Taguchi T, Yano M, Kido Y (2007) Influence of bright light therapy on postoperative patients: a pilot study. Intensive Crit Care Nurs 23(5):289–297
Simons KS, Laheij RJ, van den Boogaard M, Moviat MA, Paling AJ, Polderman FN, Rozendaal FW, Salet GA, van der Hoeven JG, Pickkers P et al (2016) Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: a randomised controlled trial. Lancet Respir Med 4(3):194–202
Foster J, Kelly M (2013) A pilot study to test the feasibility of a nonpharmacologic intervention for the prevention of delirium in the medical intensive care unit. Clin Nurse Spec 27(5):231–238
Moon KJ, Lee SM (2015) The effects of a tailored intensive care unit delirium prevention protocol: a randomized controlled trial. Int J Nurs Stud 52(9):1423–1432
Colombo R, Corona A, Praga F, Minari C, Giannotti C, Castelli A, Raimondi F (2012) A reorientation strategy for reducing delirium in the critically ill. Results of an interventional study. Minerva Anestesiol 78(9):1026–1033
Hanison J, Conway D (2015) A multifaceted approach to prevention of delirium on intensive care. BMJ Qual Improv Rep 4(1).
Rivosecchi RM, Kane-Gill SL, Svec S, Campbell S, Smithburger PL (2016) The implementation of a nonpharmacologic protocol to prevent intensive care delirium. J Crit Care 31(1):206–211
Davidson JE, Aslakson RA, Long AC, Puntillo KA, Kross EK, Hart J, Cox CE, Wunsch H, Wickline MA, Nunnally ME et al (2017) Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med 45(1):103–128
Barnes-Daly MA, Phillips G, Ely EW (2017) Improving hospital survival and reducing brain dysfunction at seven california community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6064 patients. Crit Care Med 45(2):171–178
Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, Byrum D, Carson SS, Devlin JW, Engel HJ et al (2019) Caring for critically Ill patients with the ABCDEF BUNDLE: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 47(1):3–14
Stollings JL, Devlin JW, Pun BT, Puntillo KA, Kelly T, Hargett KD, Morse A, Esbrook CL, Engel HJ, Perme C et al (2019) Implementing the ABCDEF bundle: top 8 questions asked during the ICU liberation ABCDEF bundle improvement collaborative. Crit Care Nurse 39(1):36–45
Balas MC, Pun BT, Pasero C, Engel HJ, Perme C, Esbrook CL, Kelly T, Hargett KD, Posa PJ, Barr J et al (2019) Common challenges to effective ABCDEF bundle implementation: the ICU liberation campaign experience. Crit Care Nurse 39(1):46–60
Hsieh SJ, Otusanya O, Gershengorn HB, Hope AA, Dayton C, Levi D, Garcia M, Prince D, Mills M, Fein D et al (2019) Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med 47(7):885–893
Bannon L, McGaughey J, Verghis R, Clarke M, McAuley DF, Blackwood B (2019) The effectiveness of non-pharmacological interventions in reducing the incidence and duration of delirium in critically ill patients: a systematic review and meta-analysis. Intensive Care Med 45(1):1–12
Duprey MS, Dijkstra-Kersten SMA, Zaal IJ, Briesacher BA, Saczynski JS, Griffith JL, Devlin JW, Slooter AJC (2021) Opioid use increases the risk of delirium in critically ill adults independently of pain. Am J Respir Crit Care Med.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM et al (2002) Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 30(1):119–141
Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA Jr, Dittus R, Ely EW (2008) Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 65(1):34–41
Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW (2006) Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 104(1):21–26
Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA et al (2007) Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 298(22):2644–2653
Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW et al (2009) Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 301(5):489–499
Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J (2012) Dexmedetomidine for long-term sedation I: dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 307(11):1151–1160
Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, Bourdet S, Ivanova A, Henderson AG, Pohlman A et al (2006) A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med 34(5):1326–1332
Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, Bin Kadiman S, McArthur CJ, Murray L, Reade MC et al (2019) Early sedation with dexmedetomidine in critically Ill patients. N Engl J Med 380(26):2506–2517
Hughes CG, Mailloux PT, Devlin JW, Swan JT, Sanders RD, Anzueto A, Jackson JC, Hoskins AS, Pun BT, Orun OM et al (2021) Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med 384(15):1424–1436
Chanques G, Conseil M, Roger C, Constantin JM, Prades A, Carr J, Muller L, Jung B, Belafia F, Cisse M et al (2017) Immediate interruption of sedation compared with usual sedation care in critically ill postoperative patients (SOS-Ventilation): a randomised, parallel-group clinical trial. Lancet Respir Med 5(10):795–805
Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW (2020) COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care 24(1):176
Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, Simpson BK, Wilson-Linville S, Hinojal OB, Vallejo de la Cueva A et al (2021) Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D) a multicentre cohort study. Lancet Respir Med 9(3):239–250
Chanques G, Constantin JM, Devlin JW, Ely EW, Fraser GL, Gelinas C, Girard TD, Guerin C, Jabaudon M, Jaber S et al (2020) Analgesia and sedation in patients with ARDS. Intensive Care Med 46(12):2342–2356
Acknowledgements
Dr Ely has received honoraria for CME activities sponsored by Pfizer, Orion, and Abbott. Dr Pandharipande has received a research grant from Pfizer (previously Hospira Inc.). Dr Chanques received fees for speaker (Orion Pharma, Aspen Medical) and for expert board (Orion Pharma). None of the other authors have any conflicts of interest to disclose.
Funding
BTP is supported in part by National Heart Lung and Blood Institute (R01HL14678-01). EWE is currently receiving grant funding from National Institute on Aging (1R01AG058639-02A1 and 3R01AG058639-02S1) and the Veteran's Administration. PPP is supported by the National Institute of Health AG061161, AG058639, AG054259 and GM120484.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stollings, J.L., Kotfis, K., Chanques, G. et al. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med 47, 1089–1103 (2021). https://doi.org/10.1007/s00134-021-06503-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-021-06503-1