Abstract
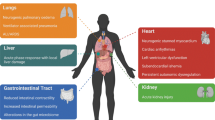
Brain and/or lung injury is the most frequent cause of admission to critical care units and patients in this setting frequently develop multiple organ dysfunction with high rates of morbidity and mortality. Mechanical ventilation is commonly used in the management of these critically ill patients and the consequent inflammatory response, together with other physiological factors, is also thought to be involved in distal organ dysfunction. This peripheral imbalance is based on a multiple-pathway cross-talk between the lungs and other organs, including the brain. Interestingly, acute respiratory distress syndrome survivors frequently present some cognitive deterioration at discharge. Such neurological dysfunction might be a secondary marker of injury and the neuroanatomical substrate for downstream impairment of other organs. Brain-lung interactions have received little attention in the literature, but recent evidence suggests that both the lungs and brain are promoters of inflammation through common mediators. This review addresses the current status of evidence regarding brain-lung interactions, their pathways and current interventions in critically ill patients receiving mechanical ventilation.
Similar content being viewed by others
Introduction
Critically ill patients frequently develop multiple organ dysfunction syndrome [1, 2] independently of the nature of the original disease. Mechanical ventilation is often an indispensable part of life support in these patients, improving gas exchange and decreasing muscle workload. Despite these therapeutic effects, however, mechanical ventilation may cause lung damage and inflammation (biotrauma) that can be propagated to distal organs. This is thought to close a feedback loop and contribute further to ventilator-induced lung injury [3, 4]. This peripheral homeostatic imbalance is based on a multiple-pathway cross-talk between the lungs and other organs, including the brain.
In critically ill patients, neurological dysfunction might be a secondary marker of damage, and the neuroanatomical substrate for downstream impairment of other organs [5, 6].
Several reports indicate that the local inflammatory response within the central nervous system may lead to altered systemic immune and inflammatory responses [7]. This bench-to-bedside review focuses on the roles the lung and brain play in the control of general homeostasis in critically ill patients.
Brain-lung interactions
From the lung to the brain
Multiple organ dysfunction syndrome is the main cause of morbidity/mortality in acute respiratory distress syndrome (ARDS) patients [8, 9]. Interestingly, most ARDS survivors show persistent cognitive deterioration at discharge [10, 11]. The underlying mechanisms are unknown, but hyperglycemia, hypotension and hypoxia/hypoxemia in the intensive care unit are significantly correlated with unfavorable neurological outcome [12–16]. The integrity of brain function depends on regular oxygen and glucose. Tight control of glycemia decreases the incidence of polyneuropathy in critically ill patients [17]. Hypoxemia is implicated in ARDS-induced brain dysfunction and in generalized cerebral atrophy. The response to hypoxia is due, in part, to the hypoxia-inducible transcription factors (HIF)-1alpha and HIF-2alpha, which regulate the expression of several genes related to angiogenesis, energy metabolism, cell survival or neural stem cell growth [18, 19]. There is currently no consensus about the actions of HIFs on neuronal survival after ischemia/hypoxia. The hypoxia-induced impairment of oxidative phosphorylation and the generation of free radicals have also been proposed as pathogenic mechanisms in chronic neurodegenerative diseases [20, 21].
Novel laboratory research questions the precise mechanisms through which acute lung injury (ALI) provokes neuronal damage. Hippocampus integrity is essential for learning, memory and cognition. In a porcine model, a higher degree of hippocampal neuronal damage was related to hypoxemia induced by lung injury rather than to that induced by a decreased oxygen supply [22]; the immune response triggered by ALI might be a plausible explanation for this [23].
The normally tight endothelia of the blood-brain barrier (BBB) and blood-lung barrier transduce signals from blood to brain or lung cells [24, 25]. Interestingly, both barriers become more permeable in some pathophysiological states, facilitating the humoral communication pathway between the brain and lungs [26]. Circulating levels of S-100B protein from astrocytes and neuronal-specific enolase are considered a good marker of brain damage [27]. S-100B levels reportedly increase with the development of encephalopathy in patients with severe sepsis and septic shock and in those with post-traumatic stress one year after suffering moderate cranio-encephalic trauma [28, 29]. In pig models of ALI and endotoxemic shock, the increase in S100-B correlates with the degree of brain damage and increased permeability of the BBB [22]. S-100B and neuronal-specific enolase might, therefore, be potential markers of cerebral damage and BBB alterations in ARDS patients [28].
Endotoxin administration in rats appears to induce systemic inflammation together with activation of central nervous system microglia and astroglia. This is followed by cell death in different regions of the brain, with the hippocampus being one of the most vulnerable regions [30, 31]. In patients in septic shock, breakdown of the BBB, assessed by magnetic resonance imaging, has been observed. It is also associated with a poor outcome and sepsis-associated delirium in these patients [32, 33]. Such evidence suggests that ALI might have implications on brain dysfunction after intensive care unit stay, but such involvement is as yet poorly understood.
From the brain to the lung
It is fairly well established that brain injury itself and its neurological sequels are the main causes of death or disability in this setting. Nevertheless, emerging evidence shows that extracerebral dysfunctions, mainly respiratory failure, are common and increase morbimortality [34]. Two studies have reported that one-third of acute brain injured patients developed ALI, worsening clinical outcome [34–37], but the causes remain obscure. The mechanisms include neurogenic lung edema, inflammatory mediators, nosocomial infections and adverse effects of neuroprotective therapies [34]. Brain injury might also increase lung vulnerability to subsequent injurious mechanical or ischemia-reperfusion insults, thereby increasing the risk of subsequent lung failure. In massive brain injury rabbits, we observed increased susceptibility of lungs to ventilator-induced lung injury when compared with intact brain animals at similar ventilatory settings [38].
Neurogenic pulmonary edema is a well-recognized complication of central nervous system insult [5, 6]. It has been attributed to a massive catecholamine release after massive brain injury [1, 39], causing a hypertensive crisis and followed by neurogenic hypotension. Avlonitis and colleagues [40] prevented inflammatory lung injury in rats by preventing the hypertensive response by means of alpha-adrenergic antagonist pretreatment. This strategy reduced systemic inflammation and preserved capillary-alveolar membrane integrity. In the same study, control of neurogenic hypotension with noradrenaline improved the systemic inflammatory response and oxygenation [40]. Since up-regulation of pro-inflammatory mediators could occur in all organs, early anti-inflammatory treatment and vasoactive agents might be warranted in the management of the brain-dead donor.
Brain microglia and astrocytes become the main source of inflammatory mediators during acute brain injury. Increased BBB permeability in this scenario allows the passage of mediators from brain to periphery, provoking a transcranial gradient that can originate secondary complications and multiorganic dysfunction [41–44]. Experimentally induced cerebral hemorrhage injury increased the expression of intercellular adhesion molecules and tissue factor in both brain and lungs, and lungs showed a progressive neutrophil recruitment with disruption of alveolar structures [39]. Moreover, traumatic brain injury in rats progressively damaged intra-cellular membranes in type II pneumocytes and persistently increased lipid peroxydation in the lung [45]. Immune defense of the airways might also be altered in the very early stages of brain injury. Interestingly, early ultrastructural damage to the tracheobronchial epithelium, with further progression over time, has been described in a rat model of traumatic brain injury [34, 46]. These findings might suggest that early alterations in the airway defense mechanisms are partly responsible for the high incidence of ventilator-associated pneumonia in brain injured patients [47, 48].
Lungs and brain share some identical biochemical mediators of inflammation that can be released to the bloodstream and sensed at a distance through the interaction with specific receptors [49]. In the lung, local activation and recruitment of defence cells causes extravasation of circulating leukocytes [50], contributing to the release of chemokines and cell adhesion molecules and ultimately leading to altered tissue remodelling [51, 52]. In this regard, Skrabal and colleagues [53] found increased plasma (protein) and lung (protein and mRNA) tumor necrosis factor-α, IL-1β and IL-6 levels in brain-dead pigs. Peripheral organs or blood cells cannot be ruled out as the main source of cytokines, but the damaged brain might be an important site of cytokine production and distribution.
The autonomic nervous system should also be considered in this neuro-immune crosstalk. Systemic inflammation is controlled, in part, by the vagus nerve (the cholinergic anti-inflammatory pathway), and, in the critical care scenario, such control might be influenced by both acute brain injury and sedation [31, 54, 55]. Sympathetic nervous system activation may be involved in 'remote' ischemic preconditioning [56]. Ischemic preconditioning is an endogenous mechanism that can protect different organs (for example, brain or lungs) through the development of an adaptive local or remote response to ischemia. The mechanism of ischemic preconditioning involves both triggers and mediators and a complex second messenger chain that includes adenosine, nitric oxide, heat shock proteins, mitogen-activated protein kinases, and mitochondrial ATP-dependent potassium channels. Oxygen free radicals also appear to be involved, playing a paradoxical protective role [57, 58]. The exact signalling pathway of this response is still under investigation but it may open a new field in therapeutic strategies in the clinical context of the critically ill patient submitted to mechanical ventilation.
Therapeutic implications
Prevention of neurological disorders secondary to ARDS is of paramount importance, but information that could influence the clinical management of these patients is, unfortunately, scarce. Avoiding hypoxemia and maintaining appropriate arterial pressure and glycemia should have a positive effect on neurological outcome. Prevention of secondary ischemic insults after severe head injury is a commonly used therapeutic approach. High vascular flow is a recognized experimental factor that promotes lung injury [59], and this can be further aggravated if the lungs are predisposed. Robertson and colleagues [60] found that the incidence of ARDS was five-fold higher in severely head-injured patients when they were treated with a protocol that preserved cerebral blood flow by maintaining cerebral blood pressure above 70 mmHg. Several aspects of ventilation management in neurocritical care have implications in both brain and lung injured patients. Koutsoukou and colleagues [61] found deterioration in lung elastance on day 5 in brain-damaged patients mechanically ventilated without positive end-expiratory pressure (PEEP), again suggesting a seeding effect of brain injury in distant organs. In a similar context, the application of PEEP levels that inadvertently increase alveolar dead space may alter cerebral hemodynamics. Mascia and colleagues [62] found that PEEP-induced overdistension and associated elevation of arterial carbon dioxide tension (PaCO2) was followed by an increase in intracranial pressure. Although specific evidence-based recommendations on how to set ventilators in acute lung and brain injury are lacking, clinicians must ensure protection for both organs. Further studies of optimal ventilatory patterns are clearly warranted in mechanically ventilated patients with mild to severe brain injury and also in possible organ donors [63, 64].
Conclusion
In patients with brain injury and acute lung failure, prevention of inadvertent ischemic brain insults and the use of protective lung strategies are mandatory. Since the cross-talk between the brain and lungs may occur through different pathways, greater control of physiological variables might be important to protect the brain, even when it is not the primary injured organ. Brain damage after acute lung injury may constitute an exciting new field of research to reduce morbidity and mortality of critically ill patients.
References
Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ: Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med 2005, 33: 654-660. 10.1097/01.CCM.0000155911.01844.54
Plötz FB, Slutsky AS, van Vught AJ, Heijnen CJ: Ventilator-induced lung injury and multiple organ failure: a critical review of facts and hypotheses. Intensive Care Med 2004, 30: 1865-1872. 10.1007/s00134-004-2363-9
Kono H, Fujii H, Amemiya H, Asakawa M, Hirai Y, Maki A, Tsuchiya M, Matsuda M, Yamamoto M: Role of Kupffer cells in lung injury in rats administered endotoxin1. J Surg Res 2005, 129: 176-189. 10.1016/j.jss.2005.06.009
Pannu N, Mehta RL: Effect of mechanical ventilation on the kidney. Best Prac Res Clin Anaesthesiol 2004, 18: 189-203. 10.1016/j.bpa.2003.08.002
Malik AB: Mechanisms of neurogenic pulmonary edema. Circ Res 1985, 57: 1-18.
Ducker TB: Increased intracranial pressure and pulmonary oedema. Part 1: clinical study of 11 patients. J Neurosurg 1968, 28: 112-117.
Avlonitis VS, Fisher AJ, Kirby JA, Dark JH: Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation 2003, 75: 1928-1933. 10.1097/01.TP.0000066351.87480.9E
Dos Santos CC, Slutsky AS: The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 2006, 68: 585-618. 10.1146/annurev.physiol.68.072304.113443
Slutsky AS, Tremblay LN: Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998, 157: 1721-1725.
Hopkins RO, Brett S: Chronic neurocognitive effects of critical illness. Curr Opin Crit Care 2005, 11: 369-375. 10.1097/01.ccx.0000166399.88635.a5
Miltbrand EB, Angus DC: Potential mechanisms and markers of critical illness-associated cognitive dysfunction. Curr Opin Crit Care 2005, 11: 355-359. 10.1097/01.ccx.0000170508.63067.04
Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lorh V: Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 1999, 160: 50-56.
Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF: Two-year cognitive, emotional and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005, 171: 340-347. 10.1164/rccm.200406-763OC
Thal SC, Engelhard K, Werner C: New cerebral protection strategies. Curr Opin Anaesthesiol 2005, 18: 490-495. 10.1097/01.aco.0000182561.32680.30
Rovlias A, Kotsou S: The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000, 46: 335-342. 10.1097/00006123-200002000-00015
Panickar KS, Nonner D, Barrett JN: Overexpression of Bcl-xl protects septal neurons from prolonged hypoglycaemia and from acute ischemia-like stress. Neuroscience 2005, 135: 73-80. 10.1016/j.neuroscience.2005.02.052
Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ: Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 2005, 64: 1348-1353.
Semenza GL: Oxygen-regulated transcription factors and their role in pulmonary disease. Respir Res 2000, 1: 159-162. 10.1186/rr27
Chavez JC, Baranova O, Lin J, Pichiule P: The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 2006, 26: 9471-9481. 10.1523/JNEUROSCI.2838-06.2006
Hopkins RO, Gale SD, Weaver LK: Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj 2006, 20: 263-271. 10.1080/02699050500488199
Incalzi RA, Marra C, Giordano A, Calcagni ML, Cappa A, Basso S, Pagliari G, Fuso L: Cognitive impairment in chronic obstructive pulmonary disease. A neuropsychological and SPECT study. J Neurol 2003, 250: 325-332. 10.1007/s00415-003-1005-4
Fries M, Bickenbach J, Henzler D, Beckers S, Dembinski R, Sellhaus B, Rossaint R, Kuhlen R: S-100 protein and neuro-histopathologic changes in a porcine model of acute lung injury. Anesthesiology 2005, 102: 761-767. 10.1097/00000542-200504000-00011
Raabe A, Wissing H, Zwissler B: Brain cell damage and S100B increase after acute lung injury. Anesthesiology 2005, 102: 713-714. 10.1097/00000542-200504000-00003
Blatteis CM: The afferent signalling of fever. J Physiol 2000, 526: 470.
Rabinovitch M: Elastase and cell matrix interactions in the pathobiology of vascular disease. Acta Paediatr Jpn 1995, 37: 657-666.
Lou J, Donati YR, Juillard P, Giroud C, Vesin C, Mili N, Grau GE: Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am J Pathol 1997, 151: 1397-1405.
Ingebrigtsen T, Romner B: Biochemical serum markers for brain damage: a short review with emphasis on clinical utility in mild head injury. Restor Neurol Neurosci 2003, 21: 171-176.
Nguyen DN, Spapen H, Su F, Schiettecate J, Shi L, Hachimi-Idrissi S, Huyghens L: Elevated serum levels of S100B protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med 2006, 34: 1-8. 10.1097/01.CCM.0000217218.51381.49
Ambros JT, Herrero-Fresneda I, Borau OG, Boira JM: Ischemic preconditioning in solid organ transplantation: from experimental to clinics. Transpl Int 2007, 20: 219-229. 10.1111/j.1432-2277.2006.00418.x
Semmler A, Okulla T, Sastre M, Demitrescu-Ozimek L, Heneka MT: Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 2005, 30: 144-157. 10.1016/j.jchemneu.2005.07.003
Vallés A, Martí O, Armario A: Mapping the areas sensitive to long-term endotoxin tolerance in the rat brain: a c-fos mRNA study. J Neurochem 2005, 93: 1177-1188. 10.1111/j.1471-4159.2005.03100.x
Ebersoldt M, Sharshar T, Annane D: Sepsis-associated delirium. Intensive Care Med 2007, 33: 941-950. 10.1007/s00134-007-0622-2
Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, de la Grandmaison GL, Aboab J, Gray F, Menon D, Annane D: Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med 2007, 33: 798-806. 10.1007/s00134-007-0598-y
Pelosi P, Severgnini P, Chiaranda M: An integrated approach to prevent and treat respiratory failure in brain-injured patients. Curr Opin Crit Care 2005, 11: 37-42. 10.1097/00075198-200502000-00006
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R: The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med 1994, 149: 818-824.
Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, Erickson VR, Pittet JF: The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma 2003, 55: 106-111.
Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD: Acute lung injury in patients with subarachnoid hemorrhage: Incidence, risk factors and outcome. Crit Care Med 2006, 34: 196-202. 10.1097/01.CCM.0000194540.44020.8E
López-Aguilar J, Villagrá A, Bernabé F, Murias G, Piacentini E, Real J, Fernandez-Segoviano P, Romero PV, Hotchkiss JR, Blanch L: Massive brain injury enhances lung damage in an isolated lung model of ventilator-induced lung injury. Crit Care Med 2005, 33: 1077-1083. 10.1097/01.CCM.0000162913.72479.F7
Wu S, Fang CX, Kim J, Ren J: Enhanced pulmonary inflammation following experimental intracerebral hemorrhage. Exp Neurol 2006, 200: 245-249.
Avlonitis VS, Wigfield CH, Kirby JA, Dark JH: The hemodynamic mechanisms of lung injury and systemic inflammatory response following brain death in the transplant donor. Am J Transplant 2005, 5: 684-693. 10.1111/j.1600-6143.2005.00755.x
Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR: Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci 2007, 25: 231-238. 10.1111/j.1460-9568.2006.05275.x
Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T: Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care 2002, 8: 101-105. 10.1097/00075198-200204000-00002
McKeating EG, Andrews PJ, Signorini DF, Mascia L: Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth 1997, 78: 520-523.
Rhodes JK, Andrews PJ, Holmes MC, Seckl JR: Expression of interleukin-6 messenger RNA in a rat model of diffuse axonal injury. Neurosci Lett 2002, 335: 1-4. 10.1016/S0304-3940(02)00811-X
Yildirim E, Solaroglu I, Okutan O, Ozisik K, Kaptanoglu E, Sargom MF, Sakinci U: Ultrastructural changes in tracheobronchial epithelia following experimental traumatic brain injury in rats: protective effect of erythropoietin. J Heart Lung Transplant 2004, 23: 1423-1429. 10.1016/j.healun.2003.10.006
Yildirim E, Solaroglu I, Okutan O, Ozisik K, Kaptanoglu E, Sargom MF, Sakinci U: Ultrastructural changes in tracheobronchial epithelia following experimental traumatic brain injury in rats: protective effect of erythropoietin. J Heart Lung Transplant 2004, 23: 1423-1429. 10.1016/j.healun.2003.10.006
Fabregas N, Torres A: Pulmonary infection in the brain injured patient. Minerva Anestesiol 2002, 68: 285-290.
Rincon-Ferrari MD, Flores-Cordero JM, Leal-Noval SR, Murillo-Cabezas F, Cayuelas A, Munoz-Sanchez MA, Sanchez-Olmedo JI: Impact of ventilator-associated pneumonia in patients with severe head injury. J Trauma 2004, 57: 1234-1240.
Masek K, Slansky J, Petrovicky P, Hadden JW: Neuroendocrine immune interactions in health and disease. Int Immunopharmacol 2003, 3: 1235-1246. 10.1016/S1567-5769(03)00015-8
Kalsotra A, Zhao J, Anakk S, Dash PK, Strobel HW: Brain trauma leads to enhanced lung inflammation and injury: evidence for role of P4504Fs in resolution. J Cereb Blood Flow Metab 2007, 27: 963-974.
Matsumoto T, Yokoi K, Mukaida N, Harada A, Yamashita J, Watanabe Y, Matsushima K: Pivotal role of interleukin-8 in the acute respiratory distress syndrome and cerebral reperfusion injury. J Leukoc Biol 1997, 62: 581-587.
Sasaki K, Tateoka N, Ando H, Yoshizaki F: Effect of flavones on rat brain and lung matrix metalloproteinase activity measured by film in-situ zymography. J Pharm Pharmacol 2005, 57: 459-465. 10.1211/0022357055588
Skrabal CA, Thompson LO, Potapov EV, Southard RE, Joyce DL, Youker KA, Noon GP, Loebe M: Organ-specific regulation of pro-inflammatory molecules in heart, lung and kidneys following brain death. J Surg Res 2005, 123: 118-125. 10.1016/j.jss.2004.07.245
Tracey KJ: Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest 2007, 117: 289-296. 10.1172/JCI30555
Dantzer R, Konsman JP, Bluthe RM, Kelley KW: Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci 2000, 85: 60-65. 10.1016/S1566-0702(00)00220-4
Peralta C, Hotter G, Closa D, Gelpi E, Bulbena O, Rosello-Catafau J: Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology 1997, 25: 934-937. 10.1002/hep.510250424
Olguner C, Koca U, Akan M, Karci A, Elar Z: The safe limits of mechanical factors in the apnea testing for the diagnosis of brain death. Tohoku J Exp Med 2007, 211: 115-120. 10.1620/tjem.211.115
Sojka P, Stalnacke BM, Bjornstig U, Karlsson K: One-year follow-up of patients with mild traumatic brain injury: occurrence of post-traumatic stress-related symptoms at follow-up and serum levels of cortisol, S-100B and neuron-specific enolase in acute phase. Brain Inj 2006, 20: 613-620.
Lopez-Aguilar J, Piacentini E, Villagra A, Murias G, Pascotto S, Saenz-Valiente A, Fernandez-Segoviano P, Hotchkiss JR, Blanch L: Contributions of vascular flow and pulmonary capillary pressure to ventilator-induced lung injury. Crit Care Med 2006, 34: 1106-1112. 10.1097/01.CCM.0000205757.66971.DA
Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, Cormio M, Uzura M, Grossman RG: Prevention of secondary ischemic insults after severe head injury. Crit Care Med 1999, 27: 2086-2095. 10.1097/00003246-199910000-00002
Koutsoukou A, Perraki H, Raftopoulou A, Koulouris N, Sotiropoulou C, Kotanidou A, Orfanos S, Roussos C: Respiratory mechanics in brain-damaged patients. Intensive Care Med 2006, 32: 1947-1954. 10.1007/s00134-006-0406-0
Mascia L, Grasso S, Fiore T, Bruno F, Berardino M, Ducati A, Mascia L: Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med 2005, 31: 373-379. 10.1007/s00134-004-2491-2
Martinez-Perez M, Bernabé F, Pena R, Fernandez R, Nahum A, Blanch L: Effects of expiratory tracheal gas insufflation in patients with severe head trauma and acute lung injury. Intensive Care Med 2004, 30: 2021-2027. 10.1007/s00134-004-2439-6
Salim A, Miller K, Dangleben D, Cipolle M, Pasquale M: High-frequency percussive ventilation: an alternative mode of ventilation for head-injured patients with adult respiratory distress syndrome. J Trauma 2004, 57: 542-546.
Acknowledgements
Supported by Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica and Instituto de Salud Carlos III – Fondo de Investigación Sanitaria (FIS) PI-04/2365, Fundació Parc Taulí CIR 2002. RETICS G03/063 (Red-Gira). Ministerio de Educación y Ciencia: BFU2006-07124/BFI. JL-A holds a Senior Researcher grant FIS 99/3091 and is supported by the Program for researchers stabilization from Direcció d'Estratègia i Coordinació del Departament de Salut de la Generalitat de Catalunya and ISCIII.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Gonzalvo, R., Martí-Sistac, O., Blanch, L. et al. Bench-to-bedside review: Brain-lung interaction in the critically ill – a pending issue revisited. Crit Care 11, 216 (2007). https://doi.org/10.1186/cc5930
Published:
DOI: https://doi.org/10.1186/cc5930