Abstract
Introduction
Among the various methods for improving oxygenation while decreasing the risk of ventilation-induced lung injury in patients with acute respiratory distress syndrome (ARDS), a ventilation strategy combining prone position (PP) and recruitment manoeuvres (RMs) can be practiced. We studied the effects on oxygenation of both RM and PP applied in early ARDS patients.
Methods
We conducted a prospective study. Sixteen consecutive patients with early ARDS fulfilling our criteria (ratio of arterial oxygen partial pressure to fraction of inspired oxygen (PaO2/FiO2) 98.3 ± 28 mmHg; positive end expiratory pressure, 10.7 ± 2.8 cmH2O) were analysed. Each patient was ventilated in both the supine position (SP) and the PP (six hours in each position). A 45 cmH2O extended sigh in pressure control mode was performed at the beginning of SP (RM1), one hour after turning to the PP (RM2) and at the end of the six-hour PP period (RM3).
Results
The mean arterial oxygen partial pressure (PaO2) changes after RM1, RM2 and RM3 were 9.6%, 15% and 19%, respectively. The PaO2 improvement after a single RM was significant after RM3 only (P < 0.05). Improvements in PaO2 level and PaO2/FiO2 ratio were transient in SP but durable during PP. PaO2/FiO2 ratio peaked at 218 mmHg after RM3. PaO2/FiO2 changes were significant only after RM3 and in the pulmonary ARDS group (P = 0.008). This global strategy had a benefit with regard to oxygenation: PaO2/FiO2 ratio increased from 98.3 mmHg to 165.6 mmHg 13 hours later at the end of the study (P < 0.05). Plateau airway pressures decreased after each RM and over the entire PP period and significantly after RM3 (P = 0.02). Some reversible side effects such as significant blood arterial pressure variations were found when extended sighs were performed.
Conclusions
In our study, interventions such as a 45 cmH2O extended sigh during PP resulted in marked oxygenation improvement. Combined RM and PP led to the highest increase in PaO2/FiO2 ratio without major clinical side effects.
Similar content being viewed by others
Introduction
Acute respiratory failure is a common pathology in intensive care units. Management of acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) [1] remains a problem. Life care support such as mechanical ventilation is used to maintain or improve oxygenation. Nevertheless, as is true of many therapies, side effects such as ventilation-induced lung injury (VILI) and oxygen toxicity have been described [2, 3]. Moreover, increased mortality in ARDS patients is well established when patients are ventilated with high tidal volume (Vt) and high plateau pressure. Nowadays, low Vt and limited plateau pressure below 30 cmH2O have been associated with lower mortality and less inflammation [4–6]. Mechanical ventilation is therefore recommended as a lung-protective strategy. However, such ventilator settings are reported to induce hypoxemia, hypercapnia, alveolar derecruitment and atelectasis, which also contribute to lung injury [7, 8]. Inflated, normal, poorly aerated or nonaerated airway spaces coexist, and ventilation may induce (1) shear stress at the boundaries of these spaces, (2) inadequate cyclic opening and (3) closing of alveoli. Inflammation as well as cellular and epithelial damage may be associated with this type of ventilation [9, 10]. The "open lung concept" was developed to fight against these ventilatory side effects and to improve oxygenation [11–16]. Opening pressures used should recruit poorly aerated or nonaerated airway spaces, and once this procedure is carried out, positive end expiratory pressure (PEEP) can be applied to stabilize cyclic opening and closing of alveoli to decrease VILI and to maintain oxygenation improvement [17–21]. To reinforce this strategy, an animal study suggested that a low stretch/open lung strategy compared to a low stretch/rest lung strategy was associated with lower mortality, decreased inflammatory response, more apoptosis and less epithelial damage [22]. Prone position (PP) [23–26] and recruitment manoeuvre (RM) [27–36] have been studied, and some benefit on alveolar recruitment, VILI and oxygenation has been demonstrated [37]. In daily practice and from a practical point of view, lung-protective ventilation is recommended. In addition to this strategy, RM can be performed while patients are in supine position (SP), and they can be turned to PP if hypoxemia remains a concern. In the present study, we tested the hypothesis that RM might have a different impact on oxygenation according to whether it was performed with patient in SP or in early or late PP. We therefore conducted a prospective study to evaluate the benefits of extended sigh using 45 cmH2O airway pressure combined with PP in acute respiratory failure.
Materials and methods
Population
From June 2002 to March 2003, we prospectively studied, during the first week of ventilation, patients with ARDS or ALI, defined according to the criteria of the ARDS American European Consensus Conference [1]. This study was approved by our local hospital ethics committee (Comité d'éthique clinique du CHU de Besançon). Written informed consent was waived. Patients were sedated, paralysed and ventilated in the volume control mode. Vasopressive drugs and fluid resuscitation were used as required to obtain a mean arterial pressure (MAP) of 75 mmHg. Patients with uncontrolled low cardiac output, a temporary pacemaker, bronchospasm or barotrauma were excluded.
Basic ventilation
A lung-protective ventilation strategy was used to maintain plateau pressure below 30 cmH2O [20]. PEEP was adjusted to obtain 92% ± 2% oxygen saturation measured via pulse oximetry (SpO2) with fraction of inspired oxygen (FiO2) between 60% and 80%. PEEP may have been increased to 6, 8, 10, 12 or 14 cmH2O to achieve the above criteria. Once these FiO2 and SpO2 criteria had been reached, ventilatory parameters were not changed. If FiO2 was still higher than 80% with a PEEP of 14 cmH2O, the increase in PEEP was interrupted and the patient was included in the study at that time. The inspiratory/expiratory (I/E) ratio was adjusted between 1:2 and 1:3. Basic ventilation was used, except when RM was performed. Mount connections were systematically removed. Heat humidifiers were used.
Recruitment manoeuvre
The RM consisted of changing the ventilatory mode to the pressure control mode and increasing pressure levels every 30 seconds to successively obtain 35, 40 and 45 cmH2O peak inspiratory pressures (PIP) (Figure 1). Once the 45 cmH2O PIP had been reached, a 30-second end-inspiratory pause was performed using the inspiratory pause function. The I/E ratio was maintained at 1:1 during RM. Respiratory frequency, PEEP and FiO2 were similar during RM. We returned to basic ventilation every 30 seconds throughout the various 30-second steps described above. At the end of the RM, previous ventilatory adjustments were applied.
Prone position
PP was maintained for six hours. FiO2 may have been temporarily increased to 100% while the patient was turned, and then it decreased back to the initial FiO2 level.
Protocol
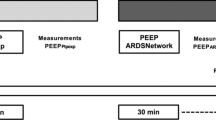
Two six-hour periods were used: one with patient in SP and one in PP. The first RM was performed at the beginning of SP (one hour after stabilization), the second one was performed one hour after turning the patient to PP and the last one was performed at the end of PP (Figure 2). Ventilatory settings, gas exchanges and haemodynamic parameters were recorded each time (from time 0 to time 8) in SP and PP: at the time of inclusion, before and immediately after each RM, before PP and one hour after turning the patient to SP.
Statistical methods
For this descriptive and analytical study, nonparametric tests were used. The Wilcoxon paired test was carried out to compare the variables before and after recruitment manoeuvres. If the number of equal variables was high, a sign test was implemented. The quantitative variables studied are reported in the tables as means ± standard deviations. A P value < 0.05 was considered statistically significant. The different analyses were carried out by using SYSTAT 8.0 software.
Results
Population
Table 1 shows the patient demographics. Sixteen ARDS patients were prospectively included, 12 with pulmonary ARDS and four with extrapulmonary ARDS. Thirteen patients completed the study, while for three patients the protocol was interrupted at some point. Pneumonia and pancreatitis were the main causes of ARDS. The patients were 63 years old on average. The mean Simplified Acute Physiology Score II was 44.7. The mean number of organ failures was about two. The mortality rate was 43.7%. Seven patients died, five as a result of pulmonary ARDS and two as a result of extrapulmonary ARDS.
Ventilatory settings
Table 2 shows the ventilator settings maintained throughout the whole study and their different effects on peak and plateau airway pressure. These decreased after each RM and over the entire PP period. The decrease in plateau pressure was significant after RM3 (P = 0.02). Plateau pressures at time 8 were lower than T0, but the decrease was not statistically significant.
Gas exchange
Table 3 shows the effects of gas exchange.
Impact of RM on gas exchange
PaO2 and PaO2/FiO2 ratio increased after each RM. The mean PaO2 changes before and after RM1, RM2 and RM3 were 9.6%, 15% and 19%, respectively. The PaO2/FiO2 ratio peaked at 218 mmHg after RM3. The improvement before and after a single RM was significant after RM3 only (P < 0.05). Arterial carbon dioxide partial pressure (PaCO2) decreased after each RM (P < 0.05).
Impact of RM on gas exchange depending on body position
Improvements in PaO2 and PaO2/FiO2 ratio were transient in SP but durable during PP between RM2 and RM3. The decrease in PaCO2 after RM1 was transient in SP and durable in PP.
Impact of the global strategy on gas exchange
When patients were included, the PaO2/FiO2 ratio was 98.3 mmHg with 79% FiO2 and 10 cmH2O PEEP. At the end of the study, in SP and compared to the beginning, the PaO2/FiO2 ratio was significantly higher at 165.6 mmHg (P < 0.05). PaCO2 decreased from 39 mmHg at the beginning of the study to 36.4 mmHg at the end of the study.
Impact of RM on gas exchange depending on extrapulmonary or pulmonary ARDS
In the pulmonary ARDS group, the PaO2/FiO2 ratio improved from 115 ± 47 mmHg to 128 ± 59 mmHg after RM1, from 162 ± 83 mmHg to 196 ± 104 mmHg after RM2 and from 185 ± 83 mmHg to 230 ± 101 mmHg after RM3. In patients with extrapulmonary ARDS, the PaO2/FiO2 ratio improved from 102 ± 19 mmHg to 107 ± 22 mmHg after RM1, from 113 ± 12 mmHg to 112 ± 35 mmHg after RM2 and from 149 ± 23 mmHg to 154 ± 78 mmHg after RM3. In these subgroups, changes in PaO2/FiO2 ratio were significant only after RM3 and only in the pulmonary ARDS group (P = 0.008).
Haemodynamics
Figure 3 shows the haemodynamic effects. Vasopressive drug infusion rates were not modified throughout the entire study. A significant decrease in MAP was found when extended sighs were performed. However, they were reversible when the manoeuvre was stopped.
Complications
One patient had reversible bronchoconstriction after an extended sigh. PP could not be performed in a second patient because of heart rate disorders. PP had to be interrupted in the first few minutes for a third patient because of major desaturation related to an increase in airway pressure (above 50 cmH2O) due to abdominal compartment syndrome. RM did not cause pulmonary barotrauma. Predominant dermabrasions on the chest and the abdomen as well as facial oedema were observed after PP in four patients.
Discussion
The main findings of our early ARDS/ALI study are that there are probable combined effects of RM and PP as well as a larger PaO2 improvement when RM is performed while the patient is in PP and probably after an extended period of time.
RMs have been proved to be efficient to protect the lung while improving oxygenation [37, 38]; however, a computed tomography-based study performed during RM in an animal model indicated that there were no protective effects against hyperinflation because of persistent lung inhomogeneity during the RM procedure [39]. A recent PP meta-analysis suggested a positive result on oxygenation and mortality and that VILI may be reduced or delayed during PP [37, 40, 41]. The combination of PP and RM may be a safe strategy to use for improvement of oxygenation and to avoid VILI. However, this strategy has not been studied often in the setting of acute respiratory failure [42–45].
In an oleic acid-induced lung injury model, Cakar et al. [42] studied the combination of PP and a 60 cmH2O sustained inflation over 30 seconds. These authors observed greater oxygen improvement with reduced alveolar stress when PP was used. Three clinical studies in humans have tested the benefits of such a strategy. The findings of those studies are summarized in Table 4.
Oxygenation efficacy
Our study confirms the efficacy of RM in increasing PaO2 in SP and PP. The PaO2 improvement was transient in SP. In PP, the efficacy of RM performed after either one hour or six hours was different. First, PaO2 did not decrease between the two RMs, and PaO2 changes were larger after the second RM. PP and RM may have a combined effect on PaO2, and this PaO2 improvement would be better if RM were used, probably at different times during PP and especially at the end of PP. A benefit on PaO2 was durable one hour after the end of PP. With an extended period of PP (more than 12 hours), the beneficial effect of RM while in PP remains to be demonstrated.
Pelosi et al. [43] and Oczenski et al. [44] demonstrated the efficacy of such a strategy. In Pelosi et al.'s study, sighs were used for one hour after two hours of PP. A positive PaO2 variation was found in SP and PP. In SP after RM, PaO2 returned to the baseline, whereas in PP, PaO2 remained higher than the baseline. In Oczenski et al.'s study, extended sigh was used at the end of the PP period, with a persistent increase in oxygenation while the patient was turned supine three hours later. Lim et al. [45] showed, first, with an extended sigh, an improvement in PaO2 in PP that was lower than in SP, and, second, a PEEP increase after RM prevented the after-RM decrease in PaO2/FiO2 ratio. The differences between oxygenation responses in SP and PP may be explained by two factors: Only the patients in the most severe condition with a PaO2/FiO2 ratio < 100 were turned prone in the PP group, and the basic ventilation was delivered with an 8 mL/kg Vt, which could have limited the extent of the effect of the RM [45].
Recruitment manoeuvre strategy
RM has been studied in experimental models and in clinical studies. An equivalent or superior efficacy of sigh or extended sigh has been demonstrated compared to continuous positive airway pressure (CPAP). In general, a 40 to 50 cmH2O peak alveolar pressure is sufficient for lung recruitment [46, 47]. The different RMs used in PP are summarized in Table 4 and included sigh, extended sigh and CPAP. They demonstrated a positive effect on alveolar recruitment and oxygenation in SP or PP. In our study, we practiced a RM using pressure control mode, and pressure was progressively increased in steps. The maximum pressure used was 45 cmH2O. Compared with RMs described in literature, our method presents some sufficient features to open lung [37, 48] with a gradual increase of airway pressure during sufficient time to induce progressive alveolar recruitment and more homogeneous distribution of pressure throughout lung parenchyma. PEEP probably may be increased to stabilize alveolar recruitment and PaO2 in SP.
Respiratory mechanics
In the present study, plateau pressures and PaCO2 decreased throughout the PP period and after each RM. PaCO2 decreased from 39 mmHg to 36.4 mmHg, and plateau pressure decreased from 24.6 cmH2O to 23 cmH2O. These results indirectly suggest changes in compliance and alveolar recruitment. Pelosi et al. [43] confirmed the benefit of such a ventilatory strategy: In their study, PaCO2 showed a decreasing pattern and end expiratory lung volume in PP was higher after RM than it was in SP (277 ± 198 mL vs. 68 ± 83 mL). Compliance followed the same improvement [43].
Complications
In our study, the protocol had to be interrupted once for arrhythmia and once for bronchoconstriction. Transient hypotension was noted, but MAP remained normal at the end of RM. In a systematic RM review, hypotension (12%) and desaturation (9%) were the most common adverse events. Serious adverse events (barotrauma and arrhythmia) were uncommon [49]. In an experimental model, a decrease in cardiac output was observed [50]. Nielsen et al. [51] tested the impact of RM in hypovolemia, normovolemia and hypervolemia. Lung RMs significantly decreased left ventricular end diastolic volume as well as cardiac output during hypovolemia. Caution should be taken, and volemia should be evaluated before starting a RM.
Methodological considerations and limitations
This study has several limitations. We are unable to argue for the long-lasting effect of the RM and PP combination on PaO2 and the benefit of such a strategy performed in all early ALI/ARDS groups. These questions require the enrolment of patients in a crossover study and follow-up of PaO2 while the patient is returned to SP. Such a study remains to be done. However, the response with regard to PaO2 is quite substantial and already has clinical significance. Because of the relatively small number of patients in our study, we were unable to sort patients according to the type of ARDS (lobar, patchy or diffuse ARDS).
The mechanisms of PaO2 improvement cannot be emphasized in our study. With the observed change in plateau pressure for a given Vt, an increase in compliance and an improvement in residual capacity are likely. It would be interesting to measure alveolar recruitment and compliance. As the RM was considered part of daily care, Swan-Ganz catheterisation and cardiac ultrasonography were not systematically performed during the procedure. We do not have the data to analyse the transient haemodynamic instability which occurred during some RMs.
Conclusions
In clinical practice, and when RM may be used to improve PaO2 and decrease VILI, RM may be useful during PP and probably needs to be performed when the patient has been in PP for some time to obtain a full response. Whether a better response is obtained after a longer period of time in PP remains to be demonstrated. The pressure control mode used in our study was as efficient as other methods. However, the place of this strategy needs to be determined in ARDS patients who fail to respond to usual treatment so as not to delay the use of rescue treatments such as extracorporeal membrane oxygenation.
Key messages
-
RM can be used in SP or PP to improve oxygenation.
-
A pressure control mode was as efficient as other RMs.
-
A probable combined effect on oxygenation exists between PP and RM.
-
The combination of PP and RM may be assessed several times, preferably when the patient has been in PP for a few hours.
-
No significant side effects were encountered in our study.
Abbreviations
- ALI:
-
acute lung injury
- ARDS:
-
acute respiratory distress syndrome
- CPAP:
-
continuous positive airway pressure
- FiO2:
-
fraction of inspired oxygen
- MAP:
-
mean arterial pressure
- PaO2:
-
arterial oxygen partial pressure
- PaO2/FiO2 ratio:
-
ratio of arterial oxygen partial pressure to fraction of inspired oxygen
- PaCO2:
-
arterial carbon dioxide partial pressure
- Paw:
-
peak airway pressure
- PEEP:
-
positive end expiratory pressure
- PIP:
-
peak inspiratory pressure
- PP:
-
prone position
- Pplat:
-
plateau pressure
- RM:
-
recruitment manoeuvre
- RR:
-
respiratory rate
- SAPS II:
-
Simplified Acute Physiology Score II
- SP:
-
supine position
- V t :
-
tidal volume.
References
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy L, Legall JR, Morris A, Spragg R: The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994, 149: 818-824.
Dreyfuss D, Saumon G: Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998, 157: 294-323.
Ricard JD, Dreyfuss D, Saumon G: Ventilator induced lung injury. Eur Respir J Suppl 2003, 42: 2s-9s.
Brochard L, Roudot-Thoraval F, Roupie E, Declaux C, Chastre J, Fernandez-Mondéjar E, Clémenti E, Mancebo J, Matamis D, Ranieri M, Blanch L, Rodi G, Mentec H, Dreyfuss D, Ferrer M, Brun-Buisson C, Tobin M, Lemaire F: Tidal volume reduction for prevention of ventilation-induced lung injury in acute respiratory distress syndrome. Am J Respir Crit Care Med 1998, 158: 1831-1838.
The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000, 342: 1301-1308.
Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P: Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med 2009, 151: 566-576. Erratum: Ann Intern Med 2009, 151: 897
Hubmayr RD: Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med 2002, 165: 1647-1653. 10.1164/rccm.2001080-01CP
Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L: Influence of tidal volume on alveolar recruitment: respective role of PEEP and a recruitment maneuver. Am J Respir Crit Care Med 2001, 163: 1609-1613.
Lapinsky S, Mehta S: Bench-to-bedside review: recruitment and recruiting maneuvers. Crit Care 2005, 9: 60-65.
Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo S, Cornejo R, Bugedo G, Carlesso E, Russo R, Caspani L, Gattinoni L: Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med 2010, 181: 578-586. 10.1164/rccm.200905-0787OC
Suárez-Sipmann F, Böhm S, Lachmann B: Clinical perspectives of "the open lung concept.". Minerva Anestesiol 1999, 65: 310-312.
Kacmarek RM, Schwartz DR: Lung recruitment. Respir Care Clin N Am 2000, 6: 597-623. 10.1016/S1078-5337(05)70090-3
Kacmarek RM: Strategies to optimize alveolar recruitment. Curr Opin Crit Care 2001, 7: 15-20. 10.1097/00075198-200102000-00003
Papadakos PJ, Lachmann B: The open lung concept of alveolar recruitment can improve outcome in respiratory failure and ARDS. Mt Sinai J Med 2002, 69: 73-77.
Hess DR, Bigatello LM: Lung recruitment: the role of recruitment maneuvers. Respir Care 2002, 47: 308-317.
Kacmarek RM, Kallet RH: Should recruitment maneuvers be used in the management of ALI and ARDS? Respir Care 2007, 52: 622-631.
Rimensberger PC, Cox PN, Frndova H, Bryan AC: The open lung during small tidal volume ventilation: concepts of recruitment and "optimal" positive end-expiratory pressure. Crit Care Med 1999, 27: 1946-1952. 10.1097/00003246-199909000-00038
Hickling K: Low tidal volume ventilation: a PEEP at the mechanisms of derecruitment. Crit Care Med 2003, 31: 318-320. 10.1097/00003246-200301000-00057
Halter J, Steinberg J, Schiller H, DaSilva M, Gatto M, Landas S, Nieman G: Positive end expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 2003, 167: 1620-1626. 10.1164/rccm.200205-435OC
Giris K, Hamed H, Khater Y, Kacmarek R: A decremental PEEP trial identifies the PEEP level that maintains oxygenation after lung recruitment. Respir Care 2006, 51: 1132-1139.
Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L, Expiratory Pressure Study (Express) Group: Acute lung injury and acute respiratory distress positive end-expiratory pressure setting in adults with syndrome: a randomized controlled trial. JAMA 2008, 299: 646-655. 10.1001/jama.299.6.646
Fanelli V, Mascia L, Puntorieri V, Assenzio B, Elia V, Fornaro G, Martin EL, Bosco M, Delsedime L, Fiore T, Grasso S, Ranieri M: Pulmonary atelectasis during low stretch ventilation: "open lung" versus "lung rest" strategy. Crit Care Med 2009, 37: 1046-1053. 10.1097/CCM.0b013e3181968e7e
Guerin C, Badet M, Rosselli S, Heyer L, Sab JM, Langevin B, Philit F, Fournier G, Robert D: Effects of prone position on alveolar recruitment and oxygenation in acute lung injury. Intensive Care Med 1999, 25: 1222-1230. 10.1007/s001340051050
Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R, Prone-Supine Study Group: Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001, 345: 568-573. 10.1056/NEJMoa010043
Pelosi P, Caironi P, Taccone P, Brazzi L: Pathophysiology of prone positioning in the healthy lung and in ALI/ARDS. Minerva Anestesiol 2001, 67: 238-247.
Mackenzie CF: Anatomy, physiology, and pathology of the prone position and postural drainage. Crit Care Med 2001, 29: 1084-1085. 10.1097/00003246-200105000-00046
Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS: Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med 1999, 25: 1297-1301. 10.1007/s001340051061
Grasso S, Puntillo F, Mascia L, Cafarelli A, Trotta T, Capobianco G, Ancona G, Bruno F, Slutsky A, Ranieri VM: Volume recruiting maneuver (VRM) in ARDS patients. Am J Respir Crit Care Med 1999, 159: 1172-1178.
Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS: Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med 1999, 25: 1297-1301. 10.1007/s001340051061
Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L: Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med 1999, 159: 872-880.
Foti G, Cereda M, Sparacino ME, De Marchi L, Villa F, Pesenti A: Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intensive Care Med 2000, 26: 501-507. 10.1007/s001340051196
Richards G, White H, Hopley M: Rapid reduction of oxygenation index by employment of recruitment technique in patients with severe ARDS. Intensive Care Med 2001, 16: 193-199. 10.1046/j.1525-1489.2001.00193.x
Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, Marini JJ, Gattinoni L: Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 2001, 164: 131-140.
Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Ranieri VM: Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 2002, 96: 795-802. 10.1097/00000542-200204000-00005
Constantin JM, Jaber S, Futier E, Cayot-Constantin S, Verny-Pic M, Jung B, Bailly A, Guerrin R, Bazin JE: Respiratory effects of different recruitment maneuvers in acute respiratory distress syndrome. Crit Care 2008, 12: R50. 10.1186/cc6869
Badet M, Bayle F, Richard JC, Guerin C: Comparison of optimal positive end-expiratory pressure and recruitment maneuvers during lung-protective mechanical ventilation in patients with acute lung injury/acute respiratory distress syndrome. Respir Care 2009, 54: 847-854. 10.4187/002013209793800448
Pelosi P, Gama de Abreu M, Rocco P: New and conventional strategies for lung recruitment in acute respiratory distress syndrome. Crit Care 2010, 14: 210-216. 10.1186/cc8851
Kacmarek R, Villar J: Lung recruitment maneuvers during respiratory distress syndrome: is it useful? Minerva Anestesiol 2011, 77: 85-89.
Grasso S, Stripoli T, Sacchi M, Trerotoli P, Staffieri F, Franchini D, De Monte V, Valentini V, Pugliese P, Crovace A, Driessen B, Fiore T: Inhomogeneity of lung parenchyma during the open lung strategy: a computed tomography scan study. Am J Respir Crit Care Med 2009, 180: 415-423. 10.1164/rccm.200901-0156OC
Gattinoni L, Carlesso E, Taccone P, Polli F, Guérin C, Mancebo J: Prone positioning improves survival in severe ARDS: a pathophysiological review and individual patient meta-analysis. Minerva Anestesiol 2010, 76: 448-454.
Abroug F, Ouanes-Besbes L, Dachraoui F, Ouanes I, Brochard L: An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Crit Care 2011, 15: R6. 10.1186/cc9403
Cakar N, Van der Kloot T, Youngblood M, Adams A, Nahum A: Oxygenation response to a recruitment maneuver during supine and prone positions in an oleic acid-induced lung injury model. Am J Respir Crit Care Med 2000, 161: 1949-1956.
Pelosi P, Bottino N, Chiumello D, Caironi P, Panigada M, Gamberoni C, Colombo G, Bigatello LM, Gattinoni L: Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med 2003, 167: 521-527. 10.1164/rccm.200203-198OC
Oczenski W, Hörmann C, Keller C, Lorenzl N, Kepka A, Schwarz S, Fitzgerald R: Recruitment maneuvers during prone positioning in patients with acute respiratory distress syndrome. Crit Care Med 2005, 33: 54-61. 10.1097/01.CCM.0000149853.47651.F0
Lim CM, Jung H, Koh Y, Lee JS, Shim TS, Lee SD, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD: Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit Care Med 2003, 31: 411-418. 10.1097/01.CCM.0000048631.88155.39
Lim SC, Adams AB, Simonson DA, Dries DJ, Broccard AF, Hotchkiss JR, Marini JJ: Intercomparison of recruitment maneuver efficacy in three models of acute lung injury. Crit Care Med 2004, 32: 2371-2377. 10.1097/01.CCM.0000147445.73344.3A
Ianuzzi M, De Sio A, De Robertis E, Piazza O, Servillo G, Tuffano R: Different patterns of lung recruitment maneuvers in primary acute respiratory distress syndrome: effects on oxygenation and central hemodynamics. Minerva Anestesiol 2010, 76: 692-698.
Albert S, DiRocco J, Gilman A, Bates J, Lafollette R, Kubiak B, Fischer J, Maroney S, Nieman G: The role of time and pressure on alveolar recruitment. J Appl Physiol 2009, 106: 757-765. 10.1152/japplphysiol.90735.2008
Fan E, Wilcow ME, Brower R, Steward T, Mehta S, Lapinsky S, Meade M, Ferguson N: Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med 2008, 178: 1156-1163. 10.1164/rccm.200802-335OC
Lim SC, Adams AB, Simonson DA, Dries DJ, Broccard AF, Hotchkiss JR, Marini JJ: Transient hemodynamic effects of recruitment maneuvers in three experimental models of acute lung injury. Crit Care Med 2004, 32: 2378-2384. 10.1097/01.CCM.0000147444.58070.72
Nielsen J, Nilsson M, Fredén F, Hultman J, Alström U, Kjærgaard J, Hedenstierna G, Larsson A: Central hemodynamics during lung recruitment maneuvers at hypovolemia, normovolemia and hypervolemia: a study by echocardiography and continuous pulmonary artery flow measurements in lung-injured pigs. Intensive Care Med 2006, 32: 585-594. 10.1007/s00134-006-0082-0
Acknowledgements
The authors thank the physicians and nursing staff in the intensive care unit for their cooperation in the management of patients during the study. We are grateful to Melanie Cole and Delphine Roussely for their help in writing this article. This work was supported by Don du souffle.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GR and GC contributed to study conception and design. GR, GC, JCN, EB and CP contributed to patient recruitment into the study. GR contributed to the acquisition of data. NF contributed to the statistical analysis. All investigators commented on, critically revised and read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rival, G., Patry, C., Floret, N. et al. Prone position and recruitment manoeuvre: the combined effect improves oxygenation. Crit Care 15, R125 (2011). https://doi.org/10.1186/cc10235
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc10235