Abstract
Synovial biopsies, gained either by blind needle biopsy or minimally invasive arthroscopy, offer additional information in certain clinical situations where routine assessment has not permitted a certain diagnosis. In research settings, synovial histology and modern applications of molecular biology increase our insight into pathogenesis and enable responses to treatment with new therapeutic agents to be assessed directly at the pathophysiological level. This review focuses on the diagnostic usefulness of synovial biopsies in the light of actual developments.
Similar content being viewed by others
Introduction
The possibility of utilizing information from synovial biopsies as a diagnostic and research tool has attracted substantial interest amongst rheumatologists in the past few decades [1, 2]. Its importance is likely to further increase in the near future for a number of reasons. First, early diagnosis and immediate initiation of disease-modifying antirheumatic drug (DMARD) therapy in rheumatoid arthritis was shown to significantly improve outcome parameters [3]. Early rheumatoid arthritis (RA), however, may not be unequivocally diagnosed in all cases, based on clinical and serological criteria alone [4]. Histological analysis of synovial biopsies may prove to be valuable in establishing an early diagnosis [5]. Furthermore, in addition to traditional parameters, histology and molecular markers out of synovial tissue might be useful to identify patients with poor outcome [6], provide sensitive means of assessing response to treatment [7–10], or identify those patients who most likely will respond to a certain treatment option [11]. Second, with the actual and upcoming possibilities of molecular medicine, synovial tissue is needed to conduct basic research to improve our understanding of mechanisms of action of modern biological agents and to develop new therapeutic strategies [12]. Third, images of modern non-invasive tools such as magnetic resonance imaging, single photon emission computed tomography, high resolution duplex sonography, and positron emission computed tomography may be correlated to macroscopic and histological changes using minimally invasive procedures such as needle arthroscopy, thereby providing a scientific basis for future improvements [13]. Fourth, in cases of undifferentiated arthritis, visualization of the affected joint and sampling of synovial tissue can facilitate the diagnostic process [14].
Thus, obtaining a synovial biopsy may likely be an increasingly important tool in the future, especially from joints that are involved in the early disease course, such as finger joints. Complementary to excellent previous reviews of the topic [1, 2], this review will focus on the diagnostic usage and potential of synovial biopsies.
Synovial sampling
Methods of synovial sampling
Synovial biopsies can be taken by arthroscopy (Figure 1), using blind needle techniques, or ultrasonographic guidance. The major draw-back of blind needle biopsy is the potential for sampling errors. In general, histological results are the same, regardless of the method used [15]. There is controversy, however, if biopsies from sites adjacent to cartilage, which cannot be easily biopsied by a blind procedure, display a higher degree of inflammatory changes, thereby underestimating the amount of inflammation and favouring optical guidance for targeted biopsy [15–18]. Thus, knowledge about the exact anatomical position of the biopsy may well be important. Arguments in favour of the use of closed needle biopsy are the substantially reduced costs as opposed to arthroscopic procedures and the need for only one rather than two portals into the joint. Current instrumentation and techniques of diagnostic arthroscopy have recently been reviewed elsewhere [19]. Ultrasonographic guidance of synovial biopsy is a promising new technique that allows sampling of large and small joints as well as tendon sheaths under indirect visualization requiring only one portal [20–22].
Indications in clinical practice
RA can generally be diagnosed based on clinical, serological, and radiological criteria alone and, for clinical routine purposes, does not necessitate a biopsy [1, 23]. Outside of research settings, a synovial biopsy can be justified in cases of unclear arthritis [14, 23, 24].
Whipple disease can be diagnosed by histological evaluation of involved organs [25]. If suspected, tuberculous arthritis should lead to synovial biopsy, especially if synovial culture is negative for acid-fast bacilli, because super infections with other organisms may inhibit growth of acid-fast bacilli in synovial fluid culture [26, 27]. Non-infectious granulomatous states, such as sarcoidosis, affecting a joint can be diagnosed when typical histology of the involved synovia is demonstrated [28]. Malignant cells are sometimes observed in joint aspiration, but visualization using arthroscopy and histological evaluation should be undertaken, especially if the neoplastic process is a metastasis and the primary tumour is not known [29]. Leukemic arthritis may precede systemic onset and malignant cells are not always visible in joint aspiration, especially if hemarthros is present. Synovial biopsy may demonstrate malignant cell infiltrates in the synovia [30]. Pigmented villonodular synovitis is a differential diagnosis in mono- or oligoarticular arthritis and biopsy is required for diagnosis [31]. Pigment deposition as in amyloidosis [32], ochronosis [33], and hemochromatosis [34] can be diagnosed by special staining of the synovial biopsy. Infectious arthritis may be diagnosed by culturing of biopsy specimen. The detection of certain infections with pathogens like Neisseria, Chlamydia, fungi [14, 35], and especially infections with uncommon pathogens such as Varicella zoster virus may require a biopsy for definitive diagnosis [36]. Diagnosis of infectious arthritis based on the specific detection of nucleic acids is also an option, even though results should be interpreted cautiously, as they are not always of pathogenetic importance [37]. Deposits of gout and pseudogout can be detected in synovial tissue [38, 39] and are of diagnostic value in differentiating mass lesions due to crystals from malignant causes [39].
In rheumatic diseases, arthroscopy may also be indicated for therapeutic purposes - as in joint lavage of septic arthritis and rheumatoid arthritis - to remove crystal deposits [40], or for minimally invasive synovectomy [41]. Furthermore, there are numerous traumatologic indications for minimally invasive arthroscopy of joints, such as removal of loose bodies and fragments of cartilage, but these are beyond the scope of this review.
Complications
Synovial biopsy is generally regarded as a safe procedure [1, 2]. In a large multicenter survey of over 15,000 arthroscopies performed by rheumatologists, hemarthros was reported in 0.9%, wound and joint infection in 0.1%, and deep vein thrombosis in 0.2% of cases [42]. Complication rates in blind biopsies are equally low, with no major complications reported in over 800 blind biopsies in one series [1]. Minimally invasive joint arthroscopies and blind biopsy may thus safely be performed in an outpatient setting [17].
Macroscopic assessment of the synovial membrane
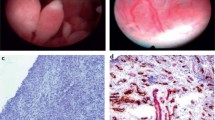
Fiberoptical visualization of joints permits assessment of bone, cartilage, synovia, and, where present, menisci and ligaments. Cartilage may be intact, impaired or destroyed; bony structures may additionally display erosions. The synovia can be assessed as to its vasculature, proliferation, presence of synovitis, and thickness (Figure 2). Furthermore, the presence of chondromatosis and fibrosis can be detected. By determining these aspects, active and chronic inflammatory processes and alterations can be differentiated.
Intraarticular images of metacarpophalangeal II joints from patients with rheumatoid arthritis. Normal cartilage and synovial membrane after methotrexate treatment (upper left), cartilage damage and starting erosions (upper right), severe synovial proliferation (lower left), and increased vascularity (lower right).
Scoring systems for the knee [17] and for metacarpophalangeal joints take into account the above mentioned alterations [43]. Macroscopic findings were shown to correlate with histological and clinical parameters [17, 44], as well as magnetic resonance imaging [13] in rheumatoid arthritis. So far, however, there has been no widely accepted method for the description or scoring of macroscopic changes and the suggested scoring systems have not been validated sufficiently. Furthermore, macroscopic features may not predict histological changes in individual patients [19].
Macroscopic aspects might help to differentiate different causes of arthritis, such as distinct vascularity patterns with straight vessels in RA as opposed to tortuous vessels or a mixed pattern in spondyloarthritis, reactive arthritis, and psoriatic arthritis [17, 45, 46]. These changes, however, are not sufficiently consistent to permit a diagnosis in individual patients [45, 46]. It is noteworthy that the pattern of vascularity might be valuable to stratify RA patients into risk groups with a worse outcome for those who display a straight vascularity pattern [47]. Further research is needed to determine the value of macroscopical scoring in arthritis and to establish standardized scoring systems.
Potential of histopathology derived from synovial tissue
Histological and molecular markers of inflammation in synovial tissue correlate with disease activity [48, 49], display response to treatment [7, 8] and permit estimations concerning disease outcome [6, 50] in RA. Intimal layer thickness, blood vessel proliferation and especially macrophage cell infiltrates have been pointed out as such synovial markers of disease activity [51]. Histological assessment of synovial biopsies in arthritic patients includes the determination of the thickness of the synovial layer, the density and composition of the cellular infiltrate, the presence of tumourous structures, and the presence of bacteria or crystals [52]. The vascularity of the synovial membrane can also be assessed as and aid in the differential diagnosis, especially between spondyloarthritis and RA [53]. A validated and widely used semi-quantitative scoring system that focuses on RA takes into account the degree of subintimal cellular infiltration and intimal layer thickness [49]. A more recently introduced synovitis score additionally requires the quantification of leukocyte infiltrates and permits the differentiation of inflammation into high-grade and low-grade synovitis in an attempt to distinguish between different subsets of arthritis, such as osteoarthritis (low-grade) and RA (high-grade) based on histological criteria alone [54]. Computer-assisted image analysis is constantly improving and nowadays permits a rapid and reliable analysis of tissue sections [55–57].
Histological data have been used to differentiate between patient groups with different kinds of arthritis in the past [58]. Increased vascularity [53] or the expression of distinct adhesion molecules [59] may differentiate between spondyloarthritis and RA, or the cellular composition of the infiltrate between psoriatic arthritis and RA [53]. Furthermore, the correct diagnosis in patients with undifferentiated arthritis, especially the distinction between patients with RA as opposed to other pathologies, might be facilitated by increased presence of certain cell subsets [60], molecular markers [5], or the detection of specific peptides or protein complexes. Intra-cellular citrullinated proteins and major histocompatibility complex class II/cartilage glycoprotein complexes detected by immunostaining of synovial biopsies, for instance, were demonstrated to be specific, albeit insensitive for RA [61–63] and might be a useful adjunct to conventional parameters in the diagnostic workup of patients with undifferentiated arthritis [64] or in distinguishing those with spondyloarthritis from those with RA [65] in the future. Ongoing debate about the specificity of these promising markers [66] underlines the necessity for further research in this interesting field before their routine use can be justified. [23]
Histological and molecular findings in early rheumatoid synovium as well as distinctions between histological and histochemical findings in early versus late RA have been extensively reviewed elsewhere [67]. Novel diagnostic markers can be identified using gene arrays and permit subsequent targeted research [12]. Further definitions of histological characteristics and markers to assess response to treatment in other arthritides such as spondyloarthritis and psoriatic arthritis are being developed [68, 69].
Implications for clinical trials
Synovial biopsies obtained by needle arthroscopy were used in a number of clinical trials involving various therapeutic agents and it was shown that changes in synovial histology, especially the number of CD68-positive sublining macrophages, correlate with the effectiveness of the treatment as determined by the Disease Activity Score (DAS)28 [51]. Furthermore, patients treated with placebo may present a clinical improvement or change in the DAS28, while the amount of synovial inflammation represented by inflammatory cell counts remains high [70, 71]. Consequently, the possibility of a reduction of the number of patients enrolled in early phase clinical trials using synovial histology rather than clinical, serological, and radiographic data as response criteria alone was suggested [1, 70]. Synovial biomarkers to detect responses in clinical trials have been developed [7, 8].
Conclusion
As of today, analysis of synovial biopsies in arthritis is foremost a research tool at the disposal of facilities equipped with arthroscopic instrumentation or experienced in blind biopsy procedures. The analysis of synovial biopsies with modern methods of molecular biology has increased our knowledge of disease mechanisms and permits us to correlate clinical response after the initiation of new therapeutic agents with morphological data and transcriptional changes at the cellular level. Ongoing research aims at stratifying patients in order to identify those at special risk for adverse outcome or those that most likely will profit from a certain treatment option.
In a clinical setting, synovial biopsies are helpful in certain cases where routine evaluation has failed to suggest a diagnosis, but the diagnostic spectrum is likely to expand with ongoing research. Early and specific diagnosis of RA as well as the distinction of other pathologies associated with arthritis are future goals.
Abbreviations
- DAS:
-
Disease Activity Score
- RA:
-
rheumatoid arthritis.
References
Gerlag D, Tak PP: Synovial biopsy. Best Prac Res Clin Rheumatol. 2005, 19: 387-400. 10.1016/j.berh.2005.01.005.
Bresnihan B, Tak PP: Synovial tissue analysis in rheumatoid arthritis. Bailleres Best Prac Res Clin Rheumatol. 1999, 13: 645-659. 10.1053/berh.1999.0051.
Wiles NJ, Lunt M, Barrett EM, Bukhari MA, Silman AJ, Symmons DP, Dunn G: Reduced disability at five years with early treatment of inflammatory polyarthritis: results from a large observational cohort, using propensity models to adjust for disease severity. Arthritis Rheum. 2001, 44: 1033-1042. 10.1002/1529-0131(200105)44:5<1033::AID-ANR182>3.0.CO;2-G.
Symmons DPM, Hazes JM, Silman AJ: Cases of early inflammatory polyarthritis should not be classified as having rheumatoid arthritis. J Rheumatol. 2003, 30: 902-904.
Ali M, Veale DJ, Reece RJ, Quinn M, Henshaw K, Zanders ED, Markham AF, Emery P, Isaacs JD: Overexpression of transcripts containing LINE-1 in the synovia of patients with rheumatoid arthritis. Ann Rheum Dis. 2003, 62: 663-666. 10.1136/ard.62.7.663.
Cunnane G, Fitzgerald O, Hummel KM, Youssef P, Gay RE, Gay S, Bresnihan B: Synovial tissue protease gene expression and joint erosions in early rheumatoid arthrits. Arthritis Rheum. 2001, 44: 1744-1753. 10.1002/1529-0131(200108)44:8<1744::AID-ART309>3.0.CO;2-K.
Bresnihan B, Baeten D, Firestein GS, Fitzgerald OM, Gerlag DM, Haringman JJ, McInnes IB, Reece RJ, Smith MD, Ulfgren AK, Veale DJ, Tak PP, OMERACT 7 Special Interest Group: Synovial tissue analysis in clinical trials. J Rheumatol. 2005, 32: 2481-2482.
Kruithof E, De Rycke L, Vandooren B, De Keyser F, FitzGerald O, McInnes I, Tak PP, Bresnihan B, Veys EM: Identification of synovial biomarkers of response to experimental treatment in early-phase clinical trials in spondylarthritis. Arthritis Rheum. 2006, 54: 1795-1804. 10.1002/art.21914.
Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP: Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008, 67: 917-925. 10.1136/ard.2007.080960.
Wijbrandts CA, Dijkgraaf MG, Kraan MC, Vinkenoog M, Smeets TJ, Dinant H, Vos K, Lems WF, Wolbink GJ, Sijpkens D, Dijkmans BA, Tak PP: The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis. 2008, 67: 1139-1144. 10.1136/ard.2007.080440.
Pouw Kraan van der TC, Wijbrandts CA, van Baarsen LG, Rustenburg F, Baggen JM, Verweij CL, Tak PP: Responsiveness to anti-tumor necrosis factor alpha therapy is related to pre-treatment tissue inflammation levels in rheumatoid arthritis patients. Ann Rheum Dis. 2008, 67: 563-566. 10.1136/ard.2007.081950.
Devauchelle V, Marion S, Cagnard N, Mistou S, Falgarone G, Breban M, Letourneur F, Pitaval A, Alibert O, Lucchesi C, Anract P, Hamadouche M, Ayral X, Dougados M, Gidrol X, Fournier C, Chiocchia G: DNA microarray allows molecular profiling of rheumatoid arthritis and identification of pathophysiological targets. Genes Immun. 2004, 5: 597-608. 10.1038/sj.gene.6364132.
Ostendorf B, Peters R, Dann P, Becker A, Scherer A, Wedekind F, Friemann J, Schulitz K-P, Mödder U, Schneider M: Magnetic resonance imaging and miniarthroscopy of the metacarpophalangeal joints. Arthritis Rheum. 2001, 44: 2492-2502. 10.1002/1529-0131(200111)44:11<2492::AID-ART429>3.0.CO;2-X.
Saaibi DL, Schumacher HRJ: Percutaneous needle biopsy and synovial histology. Bailleres Best Prac Res Clin Rheumatol. 1996, 10: 535-554. 10.1016/S1521-6942(96)80012-X.
Youssef PP, Kraan M, Breedveld F, Bresnihan B, Cassidy N, Cunnane G, Emery P, Fitzgerald O, Kane D, Lindblad S, Reece R, Veale D, Tak PP: Quantitative microscopis analysis of the inflammation in rheumatoid arthritis arthritis synovial membrane samples selected at arthroscopy compared with samples obtained blindly by needle biopsy. Arthritis Rheum. 1998, 41: 663-669. 10.1002/1529-0131(199804)41:4<663::AID-ART13>3.0.CO;2-L.
Smeets TJ, Kraan MC, Galjaard S, Youssef PP, Smith MD, Tak PP: Analysis of the cellular infiltrate and expression of matrix metalloproteinases and granzyme B in paired synovial biopsy specimens from the cartilage-pannus junction in patients with RA. Ann Rheum Dis. 2001, 60: 561-565. 10.1136/ard.60.6.561.
Baeten D, Bosch Van den F, Elewaut D, Stuer A, Veys EM, De Keyser F: Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol. 1999, 18: 434-441. 10.1007/s100670050134.
Kirkham B, Portek I, Lee CS, Stavros B, Lenarczyk A, Lassere M, Edmonds J: Intraarticular variability of synovial membrane histology, immunohistology, and cytokine mRNA expression in patients with rheumatoid arthritis. J Rheumatol. 1999, 26: 777-784.
Gerlag DM, Tak PP: How to perform and analyse synovial biopsies. Best Prac Res Clin Rheumatol. 2009, 23: 221-232. 10.1016/j.berh.2009.01.006.
Mc Gonagle D, Gibbon W, O'Connor P, Blythe D, Wakefield R, Green M, Veale DJ, Emery P: A preliminary study of ultrasound aspiration of bone erosion in early rheumatoid arthrits. Rheumatology. 1999, 38: 329-331. 10.1093/rheumatology/38.4.329.
Scirè CA, Epis O, Codullo V, Humby F, Morbini P, Manzo A, Caporali R, Pitzalis C, Montecucco C: Immunhistological assessment of the synovial tissue in small joints in rheumatoid arthritis: validation of a minimally invasive ultrasound-guided synovial biopsy procedure. Arthritis Res Ther. 2007, 9: R101-10.1186/ar2302.
Koski JM, Helle M: Ultrasound guided synovial biopsy using portal and forceps. Ann Rheum Dis. 2005, 64: 926-929. 10.1136/ard.2004.027409.
Gerlag DM, Tak PP: How useful are synovial biopsies for the diagnosis of rheumatic diseases?. Nat Clin Pract Rheumatol. 2007, 3: 248-249. 10.1038/ncprheum0485.
Chaturvedi V, Chopra GS, Dutta V, Singal VK, Chawla ML, Rai R, Bharadwaj JR, Lahiri AK, Shahi BN: Medical arthroscopy-emerging era of rheumatological intervention. J Ass Phys In. 2004, 52: 279-282.
Lange U, Teichmann J: Whipple arthritis: diagnosis by molecualr analysis of synovial fluid - current status of diagnosis and therapy. Rheumatology. 2003, 42: 473-480.
Jacobs JC, Li SC, Ruzal-Shapiro C, Kiernan H, Parisien M, Shapiro A: Tuberculous arthritis in children: diagnosis by needle biopsy of the synovium. Clin Pediatr (Phila). 1994, 33: 344-348. 10.1177/000992289403300606.
Opara TN, Gupte CM, Liyanage SH, Poole S, Beverly MC: Tuberculous arthritis of the knee with Staphylococcus superinfection. J Bone Joint Surg [Br]. 2007, 89: 664-666.
North AFJ, Fink CW, Gibson WM, Levinson JE, Schuchter SL, Howard WK, Johnson NH, Harris K: Sarcoid arthritis in children. Am J Med. 1970, 48: 449-455. 10.1016/0002-9343(70)90044-6.
Metyas SK, Lum CA, Raza AS, Vaysburd M, Forrester DM, Quismorio FPJ: Inflammatory arthritis secondary to metastatic gastric cancer. J Rheumatol. 2003, 30: 2713-2715.
Evans TI, Nercessian BM, Sanders KM: Leukemic arthritis. Semin Arthritis Rheum. 1994, 24: 48-56. 10.1016/0049-0172(94)90099-X.
Van Linthoudt D, Schmumacher HR: Acute monosynovitis or oligoarthritis in patients with quiescent rheumatoid arthritis: some possible causes. J Clin Rheumatol. 1995, 1: 46-53. 10.1097/00124743-199502000-00010.
de Ruiter EA, Ronday HK, Markusse HM: Amyloidosis mimiching rheumatoid arthritis. Clin Rheumatol. 1998, 17: 409-411. 10.1007/BF01450905.
Melis M, Onori P, Aliberti G, Vecci E, Gaudio E: Ochronoic arthropathy. Structural and ultrastructural features. Ultrastructura Pathol. 1994, 18: 467-471. 10.3109/01913129409023221.
Schumacher HR, Straka PC, Krikker MA, Dudley AT: The arthropathy of hemochromatosis. Ann NY Acad Sci. 1988, 526: 224-233. 10.1111/j.1749-6632.1988.tb55508.x.
Scheinberg MA, Cohen M: Clinical images: arthritis induced by South American blastomysis. Arthritis Rheum. 2001, 44: 490-492. 10.1002/1529-0131(200102)44:2<492::AID-ANR75>3.0.CO;2-0.
Luna-Pizarro D, Rodríguez-Castillo A, Pérez-Hernández E, Pérez-Hernández J, Hérnandez-Salgado A, Escobar-Gutiérrez A: Monoarthritis of the knee with unusual lesions in adults associated with varicella-zoster virus infection. Arthroscopy. 2008, 25: 106-108.
Schmid S, Bossart W, Michel BA, Brühlmann P: Outcome of patients with arthritis and parvovirus B19 DNA in synovial membranes. Rheumatol Int. 2007, 27: 747-751. 10.1007/s00296-007-0337-2.
Schumacher HR: Ultrastructura findings in chondrocalcinosis and pseudogout. Arthritis Rheum. 1976, 19 (Supp 3): 413-425. 10.1002/1529-0131(197605/06)19:3+<413::AID-ART1780190715>3.0.CO;2-8.
Staub-Zähner T, Garzoni D, Fretz C, Lampert C, Öhlschlegel C, Wüthrich RP, Fehr T: Pseudotumor of gout in the patella of a kidney transplant recipient. Nat Clin Pract Nephrol. 2007, 3: 345-349. 10.1038/ncpneph0494.
Wang CC, Lien SB, Huang GS, Pan RY, Shen HC, Kuo CL, Shen PH, Lee CH: Arthroscopic elimination of monosodium urate deposition of the first metatarsophalangeal joint reduces the recurrence of gout. Arthroscopy. 2009, 25: 153-158.
Tanaka N, Sakahashi H, Sato E, Ishii S: Immunhistological indication for arthroscopic synovectomy in rheumatoid knees: analysis of synovial symples obtained by needle arthroscopy. Clin Rheumatol. 2002, 21: 46-51. 10.1007/s100670200011.
Kane D, Veale DJ, FitzGerald O, Reece R: Survey of arthroscopy performed by rheumatologists. Rheumatology (Oxford). 2002, 41: 210-215. 10.1093/rheumatology/41.2.210.
Ostendorf B, Dann P, Wedekind F, Brauckmann U, Friemann J, Koebke J, Schulitz K-P, Schneider M: Miniarthroscopy of metacarpophalangeal joints in rheuamtoid arthritis. Rating of diagnostic value in synovitis staging and efficiency of synovial biopsy. J Rheumatol. 1999, 26: 1901-1908.
Ostendorf B, Dann P, Wedekind F, Brauckmann U, Friemann J, Schulitz K-P, Schneider M: Korrelation von Makroskopie und histologischen Befunden miniarthroskopischer Biopsien des MCP-Gelenkes bei Rheumatoider Arthritis. Z Rheumatol. 1998, 57 (Suppl 1): F32-
Reece R, Canete JD, Parsons WJ, Emery P, Veale DJ: Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 1999, 7: 1481-1484. 10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E.
Canete JD, Rodríguez JR, Salvador G, Gómez-Centeno A, Munoz-Gómez J, Sanmartí R: Diagnostic usefulness of synovial vascular morphology in chronic arthrits. A systematic survey of 100 cases. Semin Arthritis Rheum. 2003, 32: 378-387. 10.1053/sarh.2002.50004.
Salvador G, Sanmartí R, Gil-Torregrosa B, García-Peiró A, Rodríguez-Cros JR, Canete JD: Synovial vascular patterns and angiogenic factors expression in synovial tissue and serum of patients with rheumatoid arthritis. Rheumatology. 2006, 45: 966-971. 10.1093/rheumatology/kel043.
Joosten LAB, Netea MG, Kim S-H, Yoon D-Y, Oppers-Walgreen B, Radstake TRD, Barrera P, Loo van den FAJ, Dinarello CA, Berg Van den WB: IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006, 103: 3298-3303. 10.1073/pnas.0511233103.
Tak PP, Versendaal J, Jonker M, Bresnihan B, Post WJ, 't Hart BA: Analysis of the synovial cell infiltrate in early rheuamtoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997, 40: 217-225. 10.1002/art.1780400206.
Kraan MC, Haringman JJ, Weedon H, Barg EC, Smith MD, Ahern MJ, Smeets TJ, Breedveld FC, Tak PP: T cells, fibroblast-like synoviocytes, and granzyme B+ cytotoxic cells are associated with joint damage in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004, 63: 483-488. 10.1136/ard.2003.009225.
Haringman JJ, Gerlag D, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D: Synovial tissue macrophages: highly sensitive bio-markers for response to treatment in rheumatoid arthritis patients. Ann Rheum Dis. 2005, 64: 834-838. 10.1136/ard.2004.029751.
Koizumi F, Matsuno H, Wakakaki K: Synovitis in rheumatoid arthritis: Scoring of characteristic histopathological features. Pathol Int. 1999, 49: 298-304. 10.1046/j.1440-1827.1999.00863.x.
Baeten D, Demetter P, Cuvelier C, Bosch Van den F, Kruithof E, Van Damme N, Verbruggen G, Mielants H, Veys EM, De Keyser F: Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann Rheum DIs. 2000, 59: 954-953. 10.1136/ard.59.12.945.
Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, Haupl T: Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006, 49: 358-364. 10.1111/j.1365-2559.2006.02508.x.
Cunnane G, Björk L, Ulfgren A, Lindblad S, Fitzgerald O, Bresnihan B, Andersson U: Quantitative analysis of synovial membrane inflammation: a comparison between automated and conventional microscopic measurements. Ann Rheum Dis. 1999, 58: 493-499. 10.1136/ard.58.8.493.
Rooney T, Bresnihan B, Andersson U, Gogarty M, Kraan MC, Schumacher HR, Ulfgren A-K, Veale DJ, Youssef PP, Tak PP: Microscopic measurement of inflammation in synovial tissue: inter-observer agreement for manual quantitative, semiquantitative and computerized digital image analysis. Ann Rheum Dis. 2007, 66: 1656-1660. 10.1136/ard.2006.061143.
Haringman JJ, Vinkenoog M, Gerlag DM, Smeets TJ, Zwinderman AH, Tak PP: Reliability of computerized image analysis for the evaluation of serial synovial biopsies in randomized controlled trials in rheumatoid arthritis. Arthritis Res Ther. 2004, 7: R862-867. 10.1186/ar1757.
Smeets TJ, Dolhain RJEM, Breedved F, Tak PP: Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J Pathol. 1998, 186: 75-81. 10.1002/(SICI)1096-9896(199809)186:1<75::AID-PATH142>3.0.CO;2-B.
Elewaut D, De Keyser F, Bosch Van den F, Lazarovits AI, De Vos M, Cuvelier C, Verbruggen G, Mielants H, Veys EM: Enrichment of T cells carrying beta7 integrins in inflamed synovial tissue from patients with early spondyloarthropathy, compared to rheumatoid arthritis. J Rheumatol. 1998, 25: 1932-1937.
Kraan MC, Haringman JJ, Post WJ, Versendaal J, Breedved F, Tak PP: Immunhistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology. 1999, 38: 1074-1080. 10.1093/rheumatology/38.11.1074.
Baeten D, Peene I, Meheus L, Sebbag M, Serre G, Veys EM, De Keyser F: Specific presence of intracellular citrullinated proteints in rheumatoid arthritis synovium. Arthritis Rheum. 2001, 44: 2255-2262. 10.1002/1529-0131(200110)44:10<2255::AID-ART388>3.0.CO;2-#.
De Rycke L, Nicholas AP, Cantaert T, Kruithof E, Echols JD, Vandekerckhove B, Veys EM, De Keyser F, Baeten D: Synovial intra-cellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis-specific humoral activity. Arthritis Rheum. 2006, 52: 2323-2330. 10.1002/art.21220.
Steenbakkers PGA, Baeten D, Rovers E, Veys EM, Rijnders AWM, Meijerink J, De Keyser F, Boots AMH: Localization of MHC class II/human cartilage glycoprotein-39 complexes in synovia of rheumatoid arthritis patients using complex-specific monoclonal antibodies. J Immunol. 2003, 170: 5719-5727.
Baeten D, Kruithof E, De Rycke L, Vandooren B, Wyns B, Boullart L, Hoffman IEA, Boots AM, Veys EM, De Keyser F: Diagnostic classification of spondyloarthropathy and rheumatoid arthritis by synovial histopathology. Arthritis Rheum. 2004, 50: 2931-2941. 10.1002/art.20476.
Kruithof E, Baeten D, De Rycke L, Vandooren B, Foell D, Roth J, Canete JD, Boots AM, Veys EM, De Keyser F: Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res Ther. 2005, 7: R569-580. 10.1186/ar1698.
Vossenaar ER, Smeets TJM, Kraan MC, Raats JM, van Venrooij WJ, Tak PP: The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum. 2004, 50: 3485-3494. 10.1002/art.20584.
Hitchon CA, El-Gabalawy HS: The histopathology of early synovitis. Clin Exp Rheumatol. 2003, 21 (Suppl 31): S28-S36.
van Kuijk AWR, Reinders-Blankert P, Smeets TJM, Dijkmans BAC, Tak PP: Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. 2006, 65: 1551-1557. 10.1136/ard.2005.050963.
Baeten D, Kruithof E, De Rycke L, Boots AM, Mielants H, Veys EM, De Keyser F: Inflitration of the synovial membrane with macrophage subsets and polymorphnuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res Ther. 2004, 7: 359-369. 10.1186/ar1501.
Baeten D, Houbiers J, Kruithof E, Vandooren B, Bosch Van den F, Boots AM, Veys EM, Miltenburg AMM, De Keyser F: Synovial inflammation does not change in the absence of effective treatment: implications for the use of synovial histopathology as biomarkers in early phase clinical trials in rheumatoid arthritis. Ann Rheum Dis. 2006, 65: 990-997. 10.1136/ard.2005.047852.
Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP: Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: impications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007, 56: 3869-3871. 10.1002/art.22964.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Vordenbäumen, S., Joosten, L.A., Friemann, J. et al. Utility of synovial biopsy. Arthritis Res Ther 11, 256 (2009). https://doi.org/10.1186/ar2847
Published:
DOI: https://doi.org/10.1186/ar2847