Abstract
The aim of the present study was to perform an immunohistological assessment of the synovial tissue from involved small joints in rheumatoid arthritis (RA) and to explore the reliability of a mini-invasive ultrasound (US)-guided technique of small joint synovial biopsy for the histopathological assessment. Synovial tissue collected during arthrotomic surgery of small joints in nine patients served as the gold standard for the validation of the histological assessment. Small hand-joint synovial biopsies from an additional nine patients with erosive RA were obtained by a mini-invasive US-guided procedure, performed percutaneously by the portal and rigid forceps technique. Using digital image analysis, the area fractions of synovial macrophages (CD68 cells), T cells (CD3 cells) and B cells (CD20 cells) were measured in all high-power fields of every sample at different cutting levels. The representative sample was defined as the minimal number of high-power fields whose mean area fraction would reflect the overall mean area fraction within a percentage mean difference of 10%. For each patient, a range of three to five large samples for surgical biopsies and a range of 8–12 samples for US-guided biopsies were collected and analysed. In arthrotomic samples, the analysis of a randomly selected tissue area of 2.5 mm2 was representative of the overall value for CD68, CD3 and CD20 cells. US-guided samples allowed histological evaluation in 100% of cases, with a mean valid area of 18.56 mm2 (range 7.29–38.28 mm2). The analysis of a cumulative area of 2.5 mm2 from eight randomly selected sections (from different samples or from different cutting levels) allowed to reduce the percentage mean difference to less than 10% for CD68, CD3 and CD20 cells. In conclusion, US-guided synovial biopsy represents a reliable tool for the assessment of the histopathological features of RA patients with a mini-invasive approach.
Similar content being viewed by others
Introduction
A number of approaches to the assessment of the synovial membrane have been proposed in an attempt to establish the degree of inflammation and the phenotypic characterization of infiltrating cell subsets [1, 2]. Of the cell types found in the synovium, the intensity of CD68-positive macrophage infiltration at baseline has been associated with progressive joint damage [3, 4], and has been confirmed as an optimal biomarker of clinical response in several randomized clinical trials of both disease-modifying antirheumatic drugs and biologic agents [5–8].
The importance of the evaluation of the synovial membrane, particularly for clinical trials, has been reinforced by work demonstrating that changes in the synovial membrane are more reliable than clinical assessments, such as the disease activity score, when determining response to treatment [9]. In addition, the development of techniques such as digital image analysis has made the rapid reliable assessment of large areas of tissue a realistic proposition [10, 11].
There are several possible approaches to the acquisition of synovial tissue, but arthroscopic biopsy is generally accepted as the gold standard [12], allowing for good quality, sizeable biopsy specimens. The knee joint has been the favourite biopsy site owing to the ease of arthroscopic access and to the knowledge that it appears representative microscopically of other synovial joints [13]. Several validation studies demonstrated the reliability of a multiple sampling of synovial membrane for immunohistological studies, inferring that synovial sampling from clinically involved knee joints might provide a picture of the disease for each patient at every disease phase [1, 12, 14–19].
The knee joint, however, is only involved in a subset of patients with early arthritis, and knee involvement at onset would appear to identify a cohort of patients with a more aggressive disease course [20]. Studies based exclusively on knee biopsy, at least in early arthritis, would therefore be inherently biased towards recruitment of patients with a worse prognosis. In addition, as the small joints of the hands and feet are most commonly involved in early arthritis and since associated outcome measures of erosive burden are assessed here, acquisition of the synovial membrane from these joints would appear imperative for high-quality translational research.
For these reasons, different biopsy techniques have been developed to acquire synovial tissue from small joints, both by needle and by arthroscopic approach [21, 22]. The recent development of ultrasound (US)-guided synovial biopsy may help to overcome the blindness of the needle biopsy and the invasiveness of arthroscopic biopsy of small joints [23].
US-guided synovial biopsy of small joints is not, however, presently a standard technique in clinical practice – predominantly because of several still unanswered important issues regarding the pathologic variability in small joints and the validity of the technique to produce meaningful biological specimens.
The present study aimed to address a number of these issues: whether the analysis of randomly selected synovial tissue collected from small joints in rheumatoid arthritis (RA) patients could be representative of the overall inflammatory status of the joint; whether adequate synovial tissue could be obtained by US-guided synovial biopsy of small joints with regard to a series of standard immune (CD3 cells, CD20 cells, CD68 cells) and histological parameters; and to determine the minimum number of synovial biopsies under US guidance required to achieve reliable measurements of the above immune histological features.
For this purpose, we first analysed RA patient synovial membranes collected from surgical procedures to assess the minimum area of synovial tissue representative of the overall joint status for a series of standardized immunohistological parameters. We next tested the availability of that area in our synovial samples collected by the novel US-guided procedure, and evaluated in US-guided biopsies the variability of the main immune-phenotypic features to assess the number of samples needed to achieve reliable measurements.
Materials and methods
Patients
To assess the immunohistological features of rheumatoid synovitis, surgical synovial tissue specimens were obtained during arthroplasty or synovectomy from clinically involved (swollen) small joints of nine patients with longstanding erosive RA who fulfilled the 1987 American Rheumatism Association classification criteria [24].
To validate US-guided synovial biopsy, the procedure was performed in an additional nine patients with longstanding erosive RA before starting biologic agent treatment.
All patients gave their informed consent for biopsy, and the study was approved by the Local Ethical Committee. The main demographic and clinical characteristics of the patients as well as the biopsy sites are presented in Table 1.
Ultrasound-guided synovial biopsies
US-guided synovial biopsy was performed using the portal and forceps technique [23]. Briefly, US examination was performed with the Toshiba Nemio (Toshiba America Medical Systems, Inc. Tustin, CA, USA) using a multifrequency linear transducer (Hockey Stick Linear 8–14 MHz; Toshiba America Medical Systems). The transducer was used for standard Doppler-sonographic evaluation of the joint and for direct assistance of the biopsy procedure. Effusion and synovitis were identified and distinguished according to the following definition: effusion was defined as hypoechoic or anechoic compressible intra-articular material, within synovial recesses. Synovitis was defined as echogenic noncompressible intra-articular tissue, within synovial recesses. Power-Doppler variables were adjusted to the lowest permissible pulse repetition frequency to maximize the sensitivity. Low wall filters were used. The colour gain was set just below the level at which colour noise appeared underlying bone (no flow should be visualized at the bony surface). [25]
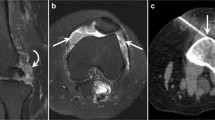
Under sterile conditions, US dorsal longitudinal guidance was used for metacarpophalangeal and proximal interphalangeal biopsies. The skin and subcutaneous tissue and the synovial space were infiltrated with 1–3 ml local anaesthetic (Xilonest 1%; Astrazeneca, Wedel, Germany) using a 25-gauge needle. After 2 minutes a 14-gauge needle was inserted into the planned area under direct US vision. A 6 F percutaneous sheath introducer (Cordis Corporation, Miami, FL, USA) was inserted into the joint under US guidance following a flexible wire. The flexible wire was then removed, and a rigid Hartmann's ear forceps (Medicon, Tuttlingen, Germany) was used for the biopsy procedures through the portal. Several independent samples (at least eight) could be taken through the same portal (Figure 1) from the hypertrophic synovium including power-Doppler-positive areas. The whole procedure was completed within 30 minutes and it was generally well tolerated. No procedure-related adverse events were recorded in our series.
Synovial samples
Biopsy specimens were immediately fixed in 4% formalin for up to 24 hours. After paraffin embedding, 20 serial sections 5 μm thick (100 μm interval) from each sample at three different cutting levels were mounted onto glass slides.
With regard to the synovial surgical biopsies, slides obtained from all available specimens were analysed at three different cutting levels. Given the higher homogeneity of the cellular reaction in the upper subsynovial layer and the sampling potential of the US-guided biopsy, the proximal 600 μm of synovial sublining immediately adjacent to the lining layer was considered for the purpose of histomorphometric analysis [26].
Tissue sections from US-guided biopsy were considered valid for histological analysis only if an intact lining was visible on H & E-stained sections. All sections were coded and systematically examined throughout their entire area at three different cutting levels by a single, blinded observer (CAS).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated through graded ethanol solutions, and were immunostained as previously reported [27]. Briefly, once hydrated the sections were heated for 35 minutes at 96°C in DAKO Target Retrieval Solution (S1699; DAKO, Carpinteria, CA, USA). The sections were then washed in Tris-buffered saline (pH 7.6) and incubated for 10 minutes with Protein Block Serum Free (X0909; DAKO). The following primary antibodies were used (2 hours of incubation): rabbit anti-human CD3 polyclonal antibody (immunoglobulin, A0452; DAKO), mouse anti-human CD20 antibody (IgG2a, clone L26; DAKO), and mouse anti-human CD68 antibody (IgG3, clone PG-M1; DAKO). Sections were then incubated with the appropriate biotinylated secondary antibody (E0431 swine anti-rabbit immunoglobulin, or Z0259 rabbit anti-mouse immunoglobulin; DAKO) for 30 minutes, followed by streptavidin biotin–alkaline phosphatase complex (K0391; DAKO) for an additional 30 minutes. Reactions were then developed using the New Fuchsin Substrate Kit (K0698; DAKO) and sections were counterstained with Meyer's Haematoxylin 0.1% (MHS-16; Sigma Diagnostics, St Louis, MO, USA) and mounted with Aquamount mounting medium (BDH, Poole, UK). Primary and secondary antibodies were diluted in DAKO Antibody Diluent (S3022; DAKO).
Microscopic analysis
All high-power (× 40) microscopic fields (HPFs) were examined on an Olympus microscope (BX51; Olympus, Tokyo, Japan), captured using a digital camera (Olympus) and transferred to a computer platform. The resultant colour images were of dimension 2,048 × 1,536 pixels, RGB format, with a 24-bit-per-pixel resolution. For each acquisition session, the microscope, camera and computer were calibrated according to a standardized procedure. The images obtained were stored in an uncompressed TIFF format and were examined using the image-analysis system ImageJ 1.35 s (National Institutes of Health, Bethesda, MD, USA). Image segmentation was performed by RGB colour discrimination using threshold ranges such that a binary overlay was created covering only the positively stained areas. This threshold was determined by two distinct observers and was kept constant for all measurements for the same marker. A separate binary mask was created that identified the total tissue area in each image, so the final parameter of analysis was the area fraction. To speed up the image analysis process, all procedures were performed using an ad hoc macro program for each marker.
Statistical analysis
The area fraction of immunoreactivity for each marker was measured on multiple HPFs for each patient. The interfield variability was determined as the percentage difference between the mean area fraction when all HPFs from each patient were considered and the mean area fraction calculated from randomly selected individual HPFs. For surgical biopsies, this analysis was performed with multiple sets of data (10 sets) of an increasing number of randomly chosen HPFs from all available specimens of each patient.
For US-guided biopsies the same analysis was performed with an increasing number of samples at different cutting levels. To increase the sampling efficiency, the number of HPFs required to reduce the percentage mean difference to less than 10% of the total sample mean was used as the variability threshold for the assay, as previously reported [26].
The variance of the measurements was reduced into its contributing factors (patient, sample and cutting level) and was analysed by analysis of variance using a general linear model and nested design. All samples at three different cutting levels were analysed for the computations. Both samples and cutting levels were nested in the patient variable. The F values for each analysis were provided. Differences were considered significant at P < 0.05. This variance component analysis was carried out using the STATISTICA data analysis software system (version 7.1, 2005; StatSoft, Inc., Tulsa, OK, USA).
Results
Determination of maximum sampling efficiency on surgical synovial samples
Figure 2 shows the effects of an increasing sample (HPF) number on the estimate of the overall sample mean for each immunostain. As the number of fields increases (and hence the fraction of the total sampling population used to create the area fraction calculation increases), the proximity of the sample mean to the overall mean area fraction is asymptotic. The number of HPFs required to reduce the sample mean to within 10% of the overall sample mean was set as the efficiency threshold for the analysis. Any further sampling analysis after this point would lead to an insignificant gain in the estimate of the mean area fraction for the particular tissue marker being examined.
Evaluation of the minimum area required for quantitative analyses of CD68, CD3 and CD20 cells. For each of the tissue markers studied, a plot was generated to show the effects of increasing sample size (that is, the number of high-power fields examined) and the proximity of this sample mean from the overall determination of area fraction. Data represent the mean values of all cases. As the number of high-power fields examined increases, the difference between the sampling mean and the overall mean reduces. A threshold value of 10% of the overall mean (arrows) was set as providing a reasonable estimator of the true sample mean.
It can be seen that the analysis of a cumulative area of 2.5 mm2 (randomly selected from all available samples) is sufficient to reduce the variability of the estimate to within 10% of the total sample mean for all markers. Given the more homogeneous distribution of CD68-positive cells within the sublining, a variation of less than 10% was typically obtained by evaluation of only 1.2 mm2, while CD3 and CD20 cells needed a larger area because of their focal distribution in the sublining layer. These results provide essential baseline data for the comparative evaluation of different biopsy regimes and methodologies, and they represent a 'gold standard' for the development of a morphometric protocol for the biopsy of synovial tissue from the minor joints.
Efficacy of ultrasound-guided small joint biopsies
Qualitative histological examination of H & E-stained specimens showed that good quality synovial tissue was available in all cases of US-guided biopsy. A representative synovial sample obtained by US-guided biopsy from a small joint of a RA patient is illustrated in Figure 3. The histological validity and the amount of valuable synovial tissue are detailed in Table 1.
The success rate of the synovial sampling (that is, valid samples/total collected samples) ranged from 25% to 83%, with a mean value of 63% (95% confidence interval, 52–75%). The mean valuable area for each valid sample at a single cutting level was 0.88 mm2 (95% confidence interval, 0.80–0.97 mm2), ranging from 0.16 to 3.19 mm2. By analysing three different cutting levels for each valid sample, the minimum useful area (2.5 mm2) for quantitative analysis was achieved in all patients (Table 1).
Quantitative analysis in ultrasound-guided samples
The overall number of sections for each patient ranged from 9 to 30 (three cutting levels for each valid sample).
We first analysed the variability of each immunohistological parameter between patients, and between samples and cutting levels. Table 2 summarizes the results of the components of variance analysis. The observed differences were mainly due to interpatient differences, and secondarily to differences between samples or cutting levels in a similar way.
To estimate the number of sections to analyse in each patient to minimize intrapatient variability, we calculated the difference between the mean values in all sections and those obtained from a 2.5 mm2 randomly selected area from an increasing number of sections.
The outcome of such analysis is depicted in Figure 4. From this figure it can be concluded that the percentage mean difference for the staining of a marker decreases below ± 10% when a minimum of eight samples are considered in the evaluation.
Number of ultrasound-guided biopsy sections required for quantitative analysis. Evaluation of the number of ultrasound-guided biopsy sections required for quantitative analyses of CD68, CD3 and CD20 cells. Reduction in the percentage mean difference can be obtained by studying 2.5 mm2 from an increasing number of sections. Arrows, number of sections that allow one to achieve a percentage mean difference lower than 10%. x axis, number of sections studied; y axis, percentage mean difference.
Discussion
The results presented here show that the analysis of a small amount of synovial tissue is also representative of the joint status in small joints of RA patients, show that US-guided synovial biopsy at this site represents a reliable approach for good quality tissue collection, and show that quantitative immunohistological studies are feasible through the examination of multiple specimens obtained by US-guided biopsy of small joints.
Several studies have addressed the heterogeneity of cellular and molecular marker expression in RA synovial biopsies, investigating the amount of tissue or the number of samples needed to obtain reliable, reproducible results capable of detecting small changes within the synovial membrane [15–19, 26]. One of the first studies that attempted to address this question was performed on synovial tissue from RA patients undergoing joint surgery [26], using a semiquantitative approach to estimate the degree of cellularity. The study demonstrated that the analysis of a cumulative area of 2.5 mm2 from at least three biopsy specimens gave an accurate estimate of the overall joint. Analogous results have been reported on the synovium obtained from the knee joint either arthroplastically, arthroscopically or using blind needle biopsies, when considering T-cell infiltration, lining layer thickness or vascularity scores [15]. Our analysis focusing on synovial tissue obtained surgically from small joints reproduces these findings, demonstrating that the analysis of a 2.5 mm2 tissue section allows an estimate of the number of macrophages (CD68-positive cells), T cells (CD3-positive cells) and B cells (CD20-positive cells) within 10% of the mean for the overall tissue. Although we limited our study to the examination of only these three cell subsets, it is widely recognized that the combination of CD68 cells, CD3 cells and CD20 cells, which are highly variable focal parameters, gives a biologically relevant assessment of the overall cellular infiltration within the synovium.
Macrophage infiltration is currently regarded as the main histopathological marker of activity and severity in RA [6, 28, 29]. In addition, despite the rapidly growing interest in the pathogenic role of B cells within the synovial membrane [30], the present study is the first to specifically address the question of what constitutes a representative synovial sample analysis for B-cell infiltration.
There have been numerous previous studies attempting to standardize the quantity of synovial tissue required to achieve a representative measure of the overall joint, the majority using an arthroscopic approach to obtain synovial tissue from knee joints and hence able to determine the number of biopsies from exact sites within the joint to allow accurate histopathologcial evaluation of the synovial membrane [12]. The recent development of a novel minimally invasive technique of US-guided synovial biopsy has been reported in the literature [23, 31], and includes assessment of the small joint biopsy with success rates in acquisition of histologically reliable tissue ranging from 89% to 93%. Among 120 US-guided biopsies, however, only one report is made of metacarpophalangeal and metatarsophalangeal joint biopsy. Our study therefore describes the largest case series of small joint synovial US-guided biopsies in RA. The collection of several independent samples (up to 12 samples) is feasible and allows a high histological success rate (100% in our series). Since the procedure is minimally invasive, repeated biopsies could be planned to monitor the disease course and/or the response to therapy.
A basic stereological rule for the analysis of all tissue states that the degree of variation is greatest between individuals and is least between sections from the same biopsy. This stereological rule was elegantly demonstrated for synovial tissue by Dolhain and colleagues, who looked at the degree of T-cell infiltration within and between multiple biopsy sites [14]. We used a similar approach to address the problem of variability in cellular infiltrates in biopsies obtained under US guidance from small joints, and we came to similar conclusions demonstrating that the main component of the variance was due to the differences between patients rather than between samples or cutting levels of the same sample. We concluded that eight different sections obtained from either different samples or different cutting levels are required to reduce the sampling error to less than 10% for a reliable analysis of CD68, CD3 and CD20 cells. In our series, considering one cutting level in five out of nine cases, two cutting levels in eight out of nine cases, and three cutting levels in all cases produced a reliable result.
The limitation of the methodology used in this study mainly results from the analysis of a heterogeneous group of RA patients and from the application of results derived from surgical biopsies (from a different set of patients) to US-guided biopsies. Performing US-guided biopsy and surgery on the same patient, however, can be easily appreciated as far from simple, from both a practical point of view and from an ethical point of view. In addition, the benefit of our approach is that we provide data applicable to synovial samples from patients with different disease durations and different pharmacological treatments, which maximizes differences between patients [1], thus increasing the representativeness of our study. Moreover, the bias in the evaluations of joint replacement synovial tissue is limited in our series because, as in US-guided biopsies, synoviectomy or arthroplasty in small joints were performed in active diseases, differing from large joint surgery where it is generally performed in end-stage disease [3].
Conclusion
In summary, the present study shows that US-guided biopsy of synovial hand joints in RA patients is a reliable tool for histological evaluation. If 12 different samples are taken, a valid assessment at least for CD20 cells, CD3 cells and CD68 cells is possible.
These findings are comparable with those obtained when synovial tissue from the knee is examined, and are the first attempt to standardize the minimum requirements for analysis of the small joints of the hands. The study of the synovial tissue from small joints can be a valuable research tool, allowing for this tissue to be incorporated into future trial designs, which is critical for further understanding of the pathogenesis of this disease and for assessing marker changes in the course of disease or in response to targeted therapies.
Abbreviations
- H & E:
-
= haematoxylin and eosin
- HPF:
-
= high-power field
- RA:
-
= rheumatoid arthritis
- US:
-
= ultrasound.
References
Rooney M, Condell D, Quinlan W, Daly L, Whelan A, Feighery C, Bresnihan B: Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988, 31: 956-963. 10.1002/art.1780310803.
Koizumi F, Matsuno H, Wakaki K, Ishii Y, Kurashige Y, Nakamura H: Synovitis in rheumatoid arthritis: scoring of characteristic histopathological features. Pathol Int. 1999, 49: 298-304. 10.1046/j.1440-1827.1999.00863.x.
Tarner IH, Harle P, Muller-Ladner U, Gay RE, Gay S: The different stages of synovitis: acute vs chronic, early vs late and non-erosive vs erosive. Best Pract Res Clin Rheumatol. 2005, 19: 19-35. 10.1016/j.berh.2004.08.002.
Mulherin D, Fitzgerald O, Bresnihan B: Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996, 39: 115-124. 10.1002/art.1780390116.
Haringman JJ, Kraan MC, Smeets TJ, Zwinderman KH, Tak PP: Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Ann Rheum Dis. 2003, 62: 715-721. 10.1136/ard.62.8.715.
Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D, McInnes IB, Bresnihan B, Tak PP: Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005, 64: 834-838. 10.1136/ard.2004.029751.
Smeets TJ, Barg EC, Kraan MC, Smith MD, Breedveld FC, Tak PP: Analysis of the cell infiltrate and expression of proinflammatory cytokines and matrix metalloproteinases in arthroscopic synovial biopsies: comparison with synovial samples from patients with end stage, destructive rheumatoid arthritis. Ann Rheum Dis. 2003, 62: 635-638. 10.1136/ard.62.7.635.
Gerlag DM, Haringman JJ, Smeets TJ, Zwinderman AH, Kraan MC, Laud PJ, Morgan S, Nash AF, Tak PP: Effects of oral prednisolone on biomarkers in synovial tissue and clinical improvement in rheumatoid arthritis. Arthritis Rheum. 2004, 50: 3783-3791. 10.1002/art.20664.
Baeten D, Houbiers J, Kruithof E, Vandooren B, Van den Bosch F, Boots AM, Veys EM, Miltenburg AM, De Keyser F: Synovial inflammation does not change in the absence of effective treatment: implications for the use of synovial histopathology as biomarker in early phase clinical trials in rheumatoid arthritis. Ann Rheum Dis. 2006, 65: 990-997. 10.1136/ard.2005.047852.
Haringman JJ, Vinkenoog M, Gerlag DM, Smeets TJ, Zwinderman AH, Tak PP: Reliability of computerized image analysis for the evaluation of serial synovial biopsies in randomized controlled trials in rheumatoid arthritis. Arthritis Res Ther. 2005, 7: R862-R867. 10.1186/ar1757.
Humby F, Manzo A, Kirkham B, Pitzalis C: The synovial membrane as a prognostic tool in rheumatoid arthritis. Autoimmun Rev. 2007, 6: 248-252. 10.1016/j.autrev.2006.08.013.
Smith MD, Baeten D, Ulfgren AK, McInnes IB, Fitzgerald O, Bresnihan B, Tak PP, Veale D, OMERACT synovial special interests group: Standardisation of synovial tissue infiltrate analysis: how far have we come? How much further do we need to go?. Ann Rheum Dis. 2006, 65: 93-100. 10.1136/ard.2005.036905.
Kraan MC, Reece RJ, Smeets TJ, Veale DJ, Emery P, Tak PP: Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: Implications for pathogenesis and evaluation of treatment. Arthritis Rheum. 2002, 46: 2034-2038. 10.1002/art.10556.
Dolhain RJ, Ter Haar NT, De Kuiper R, Nieuwenhuis IG, Zwinderman AH, Breedveld FC, Miltenburg AM: Distribution of T cells and signs of T-cell activation in the rheumatoid joint: implications for semiquantitative comparative histology. Br J Rheumatol. 1998, 37: 324-330. 10.1093/rheumatology/37.3.324.
Bresnihan B, Cunnane G, Youssef P, Yanni G, Fitzgerald O, Mulherin D: Microscopic measurement of synovial membrane inflammation in rheumatoid arthritis: proposals for the evaluation of tissue samples by quantitative analysis. Br J Rheumatol. 1998, 37: 636-642. 10.1093/rheumatology/37.6.636.
Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS: Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003, 5: R352-R360. 10.1186/ar1004.
Crotti TN, Ahern MJ, Lange K, Weedon H, Coleman M, Roberts-Thomson PJ, Haynes DR, Smith MD: Variability of RANKL and osteoprotegerin staining in synovial tissue from patients with active rheumatoid arthritis: quantification using color video image analysis. J Rheumatol. 2003, 30: 2319-2324.
Youssef PP, Triantafillou S, Parker A, Coleman M, Roberts-Thomson PJ, Ahern MJ, Smith MD: Variability in cytokine and cell adhesion molecule staining in arthroscopic synovial biopsies: quantification using color video image analysis. J Rheumatol. 1997, 24: 2291-2298.
Lindberg J, af Klint E, Ulfgren AK, Stark A, Andersson T, Nilsson P, Klareskog L, Lundeberg J: Variability in synovial inflammation in rheumatoid arthritis investigated by microarray technology. Arthritis Res Ther. 2006, 8: R47-10.1186/ar1903.
Linn-Rasker SP, Van der Helm-van Mil AH, Breedveld FC, Huizinga TW: Arthritis of the large joints, in particular the knee, at first presentation is predictive for a high level of radiological destruction of the small joints in rheumatoid arthritis. Ann Rheum Dis. 2007, 66: 646-50. 10.1136/ard.2006.066704.
Arayssi TK, Schumacher HR: Evaluation of a modified needle for small joint biopsies. J Rheumatol. 1998, 25: 876-878.
Sekiya I, Kobayashi M, Taneda Y, Matsui N: Arthroscopy of the proximal interphalangeal and metacarpophalangeal joints in rheumatoid hands. Arthroscopy. 2002, 18: 292-297.
Koski JM, Helle M: Ultrasound guided synovial biopsy using portal and forceps. Ann Rheum Dis. 2005, 64: 926-929. 10.1136/ard.2004.027409.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al: The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31: 315-324. 10.1002/art.1780310302.
Taylor P: The value of sensitive imaging modalities in rheumatoid arthritis. Arthritis Res Ther. 2003, 5: 210-213. 10.1186/ar794.
Kennedy TD, Plater-Zyberk C, Partridge TA, Woodrow DF, Maini RN: Representative sample of rheumatoid synovium: a morphometric study. J Clin Pathol. 1988, 41: 841-846. 10.1136/jcp.41.8.841.
Bugatti S, Caporali R, Manzo A, Vitolo B, Pitzalis C, Montecucco C: Involvement of subchondral bone marrow in rheumatoid arthritis: lymphoid neogenesis and in situ relationship to subchondral bone marrow osteoclast recruitment. Arthritis Rheum. 2005, 52: 3448-3459. 10.1002/art.21377.
Yanni G, Whelan A, Feighery C, Bresnihan B: Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994, 53: 39-44.
Jahangier ZN, Jacobs JW, Kraan MC, Wenting MJ, Smeets TJ, Bijlsma JW, Lafeber FP, Tak PP: Pretreatment macrophage infiltration of the synovium predicts the clinical effect of both radiation synovectomy and intra-articular glucocorticoids. Ann Rheum Dis. 2006, 65: 1286-1292. 10.1136/ard.2005.042333.
Vos K, Thurlings RM, Wijbrandts CA, van SD, Gerlag DM, Tak PP: Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum. 2007, 56: 772-778. 10.1002/art.22400.
Marin F, Lasbleiz J, Albert JD, Askri A, Werner-Leyval S, Duval H, Duvauferrier R: Synovial biopsy under US guidance: technical considerations and results. J Radiol. 2006, 87: 561-565.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CAS substantially contributed to the conception of the study, and the acquisition, analysis and interpretation of data. OE substantially contributed to acquisition of tissue specimens. VC contributed to the acquisition and interpretation of the results. FH participated in drafting the manuscript. PM substantially participated in the methodological aspect of the study. AM substantially contributed to interpretation of the data. RC contributed to interpretation of the data and to critical review of the manuscript. CP substantially contributed to the critical review of the manuscript. CM provided final approval of the version to be published.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Scirè, C.A., Epis, O., Codullo, V. et al. Immunohistological assessment of the synovial tissue in small joints in rheumatoid arthritis: validation of a minimally invasive ultrasound-guided synovial biopsy procedure. Arthritis Res Ther 9, R101 (2007). https://doi.org/10.1186/ar2302
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/ar2302