Retraction
This article was mistakenly published twice. For this reason this duplicate article has now been retracted. For citation purposes please cite the original:http://www.inljournal.com/?_action=articleInfo&article=19
Abstract
Polycrystalline NiFe2O4 was prepared by solid-state reaction from nanosize powders of NiO and Fe2O3 which were synthesized by wet chemical method. Enhancement of apparent density of the sample has been observed with the increase in sintering temperature and has great influence on the transport properties of NiFe2O4. Decrease in resistivity with increasing temperature confirms the semiconducting behavior of the prepared ferrites. Also, it was found that the value of resistivity at room temperature decreased with the increase in sintering temperature. Variation of activation energy has been found for different sintering temperatures. Dielectric constant shows the normal behavior of the ferrite materials which can be explained on the basis of Koops' two-layer model and Maxwell-Wagner polarization theory. Also, the increased value of dielectric constant (κ) has been observed with the increase in sintering temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The study of spinel ferrite is of great importance from both the fundamental and the applied research points of view. High resistivity, low dielectric losses, mechanical toughness, and chemical stability make them very promising candidates for high-frequency applications[1–4]. Also, as most of the properties needed for ferrite application are not intrinsic but extrinsic, the preparation of ferrites with optimized properties has always demanded delicate handling and cautious approach. The ferrite is not completely defined by its chemistry and crystal structure. Thus, in order to reduce the losses, many parameters such as density, grain size, porosity, and their intra- and intergranular distributions must be controlled[5]. Optimum sintering temperature is of great importance as sintering influences both densification and grain growth, and many magnetic and electrical properties benefit from both a high relative density and a small grain size. This opens the doors for tailoring given properties by careful synthesis of the building blocks (atoms and molecules) and their assembly to fabricate functional materials with improved properties.
This new class of materials is used in important applications such as high-frequency transformers, ferrofluids, pigments in paints and ceramics, biomedical applications like drug delivery system, hyperthermia, NMR, high-density magnetic recording, varistors, and dye-sensitized solar cells[6–10]. In this study, the effects of sintering temperature on apparent density and transport properties of the NiFe2O4 are investigated.
Methods
Polycrystalline NiFe2O4 was prepared through the solid-state reaction using conventional double sintering ceramic technique from nanosize powder of NiO and Fe2O3 which were synthesized by wet chemical method. After thorough mixing, the powder was presintered at 800°C for 3 h. The presintered ferrite powder was crushed and mixed with 1 wt.% polyvinyl alcohol (PVA) as a binder and uniaxially pressed into toroid and pellets. The compacts were successively sintered in a muffle furnace in air from the temperature range of 1,000°C to 1,400°C for 4 h to eliminate the PVA, and finally, the furnace was cooled down to room temperature.
The bulk densities of the pellets were determined by measuring the volume and mass after sintering. DC electrical resistivity was measured up to 300°C temperature using Keithley model 6514 electrometer (Keithley Instruments Inc., Cleveland, OH, USA). Frequency-dependent dielectric constant of the pellet samples has been measured by an inductance analyzer named WAYNE KERR INDUCTANCE ANALYZER 3255B (Wayne Kerr Electronics Inc., Woburn, MA, USA).
Results and discussion
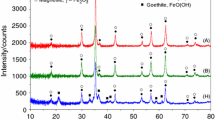
Figure1 shows the X-ray diffraction (XRD) pattern of the synthesized NiFe2O4 for various sintering temperatures. The X-ray lines show considerable broadening, indicating the fine particle nature of the ferrite. The observed peaks at (200), (311), (400), (422), (511), (440), and (533) confirmed the spinel structure of the samples. No extra peak other than NiFe2O4 has been found up to the calcination temperature 600°C which indicates the single phase of the prepared sample. However, further increase in calcination temperature multiphase component of NiFe2O4 has been observed. Figure2 shows the dependence of apparent density of the samples on sintering temperature. It is clearly observed that the density increases with increasing the sintering temperature and saturates at 1,200°C, and consequently, the pores decrease through diffusion kinetics, which is clear from the scanning electron microscope (SEM) micrographs of the prepared samples in Figure3.
High resistivity is a prerequisite for high-frequency applications to counter the eddy current losses, and ferrites have higher resistance than metals by several orders of magnitude, which degrade the ferrite performance. In Figure4, DC electrical resistivity, as a function of inverse temperature, has been presented which shows the semiconducting behavior of the prepared ferrite.
In Figure5, the resistivity is seen to vary with sintering temperature. Decrease in resistivity with increase in sintering temperature may be attributed to the microstructural factors such as grain size, porosity, grain boundary area, as well as conversion of trivalent Fe3+ ions to the divalent Fe2+ state. The smaller the grain size, the more the number of grain boundaries. Thus, the bulk of the resistivity is contributed by the insulating grain boundaries as the ferrite grains are conducting. An increase in sintering temperature results in greater density and grain growth which decreases the porosity and the number of grain boundaries[11]. Since the pores are nonconductive, the charge carriers will face less pores on their way for higher sintering temperature and thereby lead to a decrease in resistivity. In addition to this, with increasing sintering temperature, partial reduction of trivalent Fe3+ ions to the divalent Fe2+ takes place. The Fe ions at the A sites contribute little to conduction due to the larger distances between them, but the formation of Fe2+ ions gives rise to the fact that conduction of ferrite due to electron hopping between Fe3+ and Fe2+ ions coexist at the closer spaced B sites in the spinel lattice and, thereby, decrease the resistivity[12].
The activation energy has been calculated and is shown in Table 1. It is observed that the activation energy decreases with increasing sintering temperature. The decrease of activation energy with the increase of sintering temperature may be attributed to the fact that at a high sintering temperature, partial reduction of Fe3+ to Fe2+ takes place, and these places act as donor center and are responsible for this decrease in activation energy. The conduction mechanism is due to hopping of electron of the types Fe2+ ↔ Fe3+. It can also be seen that samples that have low resistivity have low activation energies and vice versa[13].
Frequency-dependent dielectric constant for samples sintered at various temperatures has been determined from the experimental capacitance values through the following formula:
where κ is the dielectric constant, C is the measured capacitance, d is the thickness of the sample, є0 = 8.854 × 10−12 C2N−1 m−2, and A = пr2 is the area of the sample. The variations of dielectric constant as a function of frequency for samples NiFe2O4 are shown in Figure6. From Figure6, it is clear that the dielectric constant decreases rapidly with increasing frequency at lower frequency region and becomes asymptotic to lower values at high frequencies. The decrease of dielectric constant with increasing frequency is a normal dielectric behavior of spinel ferrites. The dielectric dispersion curve can be explained on the basis of Koops' two-layer model and Maxwell-Wagner polarization theory. To interpret the frequency response of dielectric constant in ferrite materials, Koops suggested a theory in which relatively good conducting grains and insulating grain boundary layers of ferrite material can be represented with the behavior of an inhomogeneous dielectric structure[14], as described by Maxwell[15]. Since an assembly of space charge carries in the inhomogeneous dielectric structure described requires finite time to line up its axes parallel to an alternating electric field, the dielectric constant naturally decreases if the frequency of the reversal field increased. This is in agreement with the observed dielectric dispersion.
The variation of dielectric constant with sintering temperature at frequency f = 100 kHz is shown in Figure7. From Figure7, it is observed that the dielectric constant (κ) increases with increasing sintering temperature. Dielectric constant in ferrites is contributed by several structural and microstructural factors. The space charge polarization resulting from electron displacement on application of electric field and the subsequent charge build up at the insulating grain boundary is a major contributor to the dielectric constant in ferrites. Therefore, the more the number of Fe2+ ions in the ferrite, the more the space charge polarization is expected. It is due to the ease of electron transfer between Fe3+ and Fe2+ ions and, consequently, higher dielectric constant. Now, with increasing sintering temperature, partial reduction of Fe3+ to Fe2+ takes place. Thus, the value of dielectric constant (κ) increases with increasing sintering temperature.
Conclusions
The influences of sintering temperature on the apparent density and transport properties of NiFe2O4 were investigated. Enhancement of apparent density has been observed with the increase in sintering temperature. A decrease in resistivity and activation energy with an increase in sintering temperature may be attributed to the microstructural factors such as grain size, porosity, grain boundary area, as well as conversion of trivalent Fe3+ ions to the divalent Fe2+ state. Increased value of dielectric constant has been observed because with an increase in sintering temperature, conversion of trivalent Fe3+ ions to the divalent Fe2+ ions took place, and thereby, dielectric constant increased. The variations in properties for this ferrite have many contributing factors, which include density, change in cation distribution, and relative stability of Fe+3/Fe2+ and/or Ni3+/Ni2+ ions.
Authors' information
SC is a professor in the Department of Physics, University of Dhaka; MAB was a MS research student of the same department. SMH is a principal scientific officer at Material Science Division, Atomic Energy Centre, Dhaka.
References
Berchmans LJ, Selvan RK, Selva Kumar PN, Augustin CO: Structural and electrical properties of Ni1-xMgxFe2O4 synthesized by citrate gel process. J. Magn. Magn. Mater. 2004, 279: 103. 10.1016/j.jmmm.2004.01.073
Shaik AM, Bellad SS, Chougule BK: Temperature and frequency-dependent dielectric properties of Zn substituted Li–Mg ferrites. J. Magn. Magn. Mater. 1999, 195: 384. 10.1016/S0304-8853(99)00138-9
Rao BP, Rao KH, Trinadha K, Caltunb OF: Dielectric behaviour of niobium doped Ni-Zn ferrites. J. Optoelectron. Adv. Mater. 2004, 6: 951.
Rahman IZ, Ahmed TT: Study of Ni-Zn-Cu based ferrite powders: processing and characterization. In: Proceedings of 2nd International Conference on Structure, Processing & Properties of Materials, SPPM2004, Dhaka, Bangladesh, 25–27 February 2004.
Ishino K, Narumiya Y: Development of magnetic ferrites: control and application of losses. American Ceram. Soc. Bull. 1987, 66: 1469.
Iftimie N, Rezlescu E, Popa PD, Rezlescu N: On the possibility of the use of a nickel ferrite as semiconducting glass ferrite. J. Optoelectron. Adv. Mater. 2005,7(2):911.
Xu C, Jun T, Miura N, Yamazo N: On the thermal stability of pure and doped SnO2 ultrafine particles. Chem. Lett. 1990, 3: 441.
Rezlescu N, Rezlescu E, Tudorache F, Popa PD: MgCu nanocrystalline ceramic with La3+ and Y3+ ionic substitutions used as humidity sensor. J. Optoelectron. Adv. Mater. 2004, 6: 695.
Doroftei C, Rezlescu N, Rezlescu E, Popa PD: Gas sensitivity of nanocrystalline nickel ferrite. J. Optoelectron. Adv. Mater. 2006,8(3):1016.
Gleiter H: Nanocrystalline materials. Prog. Mater. Sci. 1989,33(4):223. 10.1016/0079-6425(89)90001-7
Verma A, Chatterjee R: Effect of zinc concentration on the structural, electrical and magnetic properties of mixed Mn–Zn and Ni–Zn ferrites synthesized by the citrate precursor technique. J. Magn. Magn. Mater. 2006,306(2):313. 10.1016/j.jmmm.2006.03.033
Verma A, Thakur OP, Prakash C, Goel TC, Mendirafta RG: Temperature dependence of electrical properties of nickel–zinc ferrites processed by the citrate precursor technique. Mater. Sci. Eng. B 2005, 116: 1. 10.1016/j.mseb.2004.08.011
El-Shabasy M: DC electrical properties of Zn-Ni ferrites. J. Magn. Magn. Mater. 1997, 172: 188. 10.1016/S0304-8853(97)00014-0
Koops CG: On the dispersion of resistivity and dielectric constant of some semiconductors at audio frequencies. Phys. Rev. 1951, 83: 121. 10.1103/PhysRev.83.121
Maxwell JC: A Treatise on Electricity and Magnetism. Clarendon, Oxford; 1892.
Acknowledgment
One of the authors, Mahabub Alam Bhuiyan, is grateful to the Department of Physics, University of Dhaka and Materials Science Division, Atomic Energy Centre, Dhaka (AECD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MAB carried out preparation of the samples, measurement of DC resistivity, activation energy, and dielectric constant. SMH carried out SEM and XRD measurements. SC did the interpretation of the results and prepared the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Choudhury, S., Bhuiyan, M.A. & Hoque, S.M. Retracted: Effect of sintering temperature on apparent density and transport properties of NiFe2O4: synthesized from nanosize powder of NiO and Fe2O3. Int Nano Lett 2, 6 (2012). https://doi.org/10.1186/2228-5326-2-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-2-6