Abstract
Background
The aim was to confirm a previously defined prognostic index, combining a proliferation marker, histological grade, and estrogen receptor (ER) in different subsets of primary N0/N1 chemo-naïve breast cancer patients.
Methods/design
In the present study, including 1,854 patients, Ki67 was used in the index (KiGE), since it is the generally accepted proliferation marker in clinical routine. The low KiGE-group was defined as histological grade 1 patients and grade 2 patients which were ER-positive and had low Ki67 expression. All other patients made up the high KiGE-group. The KiGE-index separated patients into two groups with different prognosis. In multivariate analysis, KiGE was significantly associated with disease-free survival, when adjusted for age at diagnosis, tumor size and adjuvant endocrine treatment (hazard ratio: 3.5, 95% confidence interval: 2.6–4.7, P<0.0001).
Discussion
We have confirmed a prognostic index based on a proliferation marker (Ki67), histological grade, and ER for identification of a low-risk group of patients with N0/N1 primary breast cancer. For this low-risk group constituting 57% of the patients, with a five-year distant disease-free survival of 92%, adjuvant chemotherapy will have limited effect and may be avoided.
Similar content being viewed by others
Introduction
To predict clinical outcome and the effect of adjuvant systemic treatment in breast cancer, recommendations such as the St. Gallen breast cancer consensus guidelines (Goldhirsch et al. 2011) and the Adjuvant! Online tool (Ravdin et al. 2001) can be used. The development of array-based technologies and sequencing of the human genome (Perou et al. 2000; Sorlie et al. 2001; Paik et al. 2004; Sotiriou et al. 2006; Ivshina et al. 2006), provide additional information beyond the traditional criteria used to guide treatment decisions. This challenges the currently used factors, such as lymph node involvement, tumor size, age, histological grade, human epidermal growth factor receptor 2 (HER2), Ki67, and estrogen (ER) and progesterone receptor (PgR) status (Goldhirsch et al. 2011; Aebi et al. 2011; Harris et al. 2007). At the St. Gallen Consensus Meeting in 2011, Oncotype DX® was considered useful for predicting responsiveness to chemotherapy in an endocrine-responsive cohort, whereas other tests were considered not yet fully validated. Two of the most well-known gene-based assays, MammaPrint® and Oncotype DX® have, however, also been questioned with regard to their added prognostic value (Edén et al. 2004; Cuzick et al. 2011). The ongoing clinical trials, MINDACT and TAILORx, will hopefully provide more conclusive data (Rutgers et al. 2011; Zujewski & Kamin 2008).
High proliferation is a key feature in breast carcinogenesis and markers of proliferation have been shown to be associated to prognosis and to effect of adjuvant and palliative chemotherapy, to neoadjuvant endocrine therapy, and to prognosis after adjuvant endocrine treatment (Harris et al. 2007; Colozza et al. 2005; Beresford et al. 2006; De Azambuja et al. 2007; Goldhirsch et al. 2009; Urruticoechea et al. 2005; Hietanen et al. 1995; Amadori et al. 2008; Jones et al. 2009; Ellis et al. 2008; Viale et al. 2008). Ki67 is the marker of proliferation most widely used (Goldhirsch et al. 2011; De Azambuja et al. 2007; Urruticoechea et al. 2005; Dowsett et al. 2011; Luporsi et al. 2012), but the role of other markers, such as cyclin A (Bukholm et al. 2001; Michalides et al. 2002; Kuhling et al. 2003; Baldini et al. 2006; Ahlin et al. 2009; Strand et al. 2012) and phosphohistone H3 (Skaland et al. 2007), remains under debate. Furthermore, global gene expression analyses have shown that proliferation-associated genes seem to be among the most important for dividing patients into groups with different prognosis, especially in ER-positive and histological grade 2 breast cancers (Sotiriou et al. 2006; Ivshina et al. 2006; Teschendorff et al. 2007; Desmedt et al. 2008). In line with this, studies from our group, as well as others, have shown that single markers of proliferation (Ki67 and cyclin A) were of prognostic importance in ER-positive breast cancer and in the histological grade 2 (Strand et al. 2012; Klintman et al. 2010) and grade 1 (Aleskandarany et al. 2011) subgroups. We have previously evaluated the importance of a prognostic index based on the combination of cyclin A, histological grade, and ER (CAGE), in node-negative premenopausal breast cancer patients (Strand et al. 2012). The CAGE-index combined with HER2-status classified 53% of the women as low-risk patients with a five-year distant disease-free survival (DDFS) of 95%. For this low-risk group, adjuvant cytotoxic treatment will have limited efficacy and may be avoided. In the present study, we present data for Ki67. Importantly, avoiding unnecessary use of chemotherapy for low-risk patients with N0/N1 primary breast cancer is also the primary aim of the MINDACT and TAILORx trials (Rutgers et al. 2011; Zujewski & Kamin 2008).
The aim of this study was to confirm a prognostic index based on the combination of proliferation (Ki67), histological grade, and ER (KiGE) in different subsets of chemo-naïve patients with N0/N1 primary breast cancer with special focus on five-year DDFS.
Materials and methods
Patients
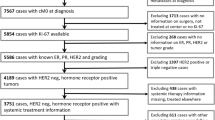
We included 1,854 women with primary breast cancer of which 1,522 originated from two randomized clinical studies (Patient materials I–II) and three cohorts (Patient materials III–V). The remaining 332 patients came from a case–control study (Patient material VI). Patients were excluded due to adjuvant chemotherapy and/or missing information on adjuvant therapy, Ki67, histological grade, or ER. Furthermore, for patients with more than three positive lymph nodes, adjuvant chemotherapy is recommended (as stated in the St. Gallen guidelines (Goldhirsch et al. 2011), hence these patients were also excluded. The endpoint for the 1,522 patients was defined as distant recurrence for 86% of the patients (Patient materials I–II and IV–V) and as any recurrence for the remaining 14% (Patient material III). Time to this endpoint will hereafter be referred to as event-free survival. Median follow-up for patients alive and event-free at last follow-up was 7.2 years (range: 1.1–17 years). Only the first five years of follow-up were used in the analyses.
Patient material I
SBII:2-pre (N=221, 68 distant recurrences). Premenopausal women with stage II breast cancer were enrolled, between 1986 and 1991, in a randomized trial with the aim to compare the effect of two years of tamoxifen (TAM) treatment versus no adjuvant systemic treatment. The original trial included 564 patients enrolled in the South and South-East Swedish Health Care Regions (Rydén et al. 2005).
Patient material II
SBII:2-post (N=166, 22 distant recurrences). Postmenopausal women with stage II breast cancer were enrolled, between 1983 and 1991, in a randomized trial launched by the Swedish Breast Cancer group of two versus five years of adjuvant TAM (Swedish Breast Cancer Cooperative Group 1996). The original trial included 1,107 patients from the South Swedish Health Care Region. Paraffin embedded tumor material has previously been collected from a subgroup of patients treated with TAM for two years, for comparison of a cytosol method and immunohistochemistry for analyses of ER and PgR (Chebil et al. 2003). In the present study, the paraffin embedded material was used for analyses of Ki67 and HER2, and for the re-evaluation of histological grade.
Patient material III
The Malmö cohort (N=217, 32 recurrences). The original cohort enrolled a consecutive series of 498 patients diagnosed with primary breast cancer at the Department of Pathology, Malmö University Hospital between 1988 and 1992. The purpose was to construct tissue microarrays for biomarker evaluation (Borgquist et al. 2008).
Patient material IV
The Bone marrow metastases cohort (N=379, 27 distant recurrences). The original study included 569 consecutive patients diagnosed with primary breast cancer in the South Swedish Health Care Region and included patients diagnosed between 1999 and 2003. The purpose was to study the prognostic value of the presence of cytokeratin positive cells in bone marrow aspirates from the sternum (Falck et al. 2012).
Patient material V
The Odense cohort (N=539, 86 distant recurrences). The original study enrolled a consecutive series of 841 patients with primary breast cancer referred to Odense University Hospital, Denmark. Patients were enrolled between 1980 and 1990 (Hansen et al. 2000). The purpose was to collect a population-based cohort for evaluation of prognostic factors.
All patients from these collections (Patient materials I–V) were pooled in a database from which the following subsets were extracted: Set 1: node-negative (N0), no adjuvant therapy, ≤50 years at diagnosis (N=169, 20 events), Set 2: N0, no adjuvant therapy, >50 years at diagnosis (N=488, 55 events), Set 3: node-positive (N1), no adjuvant therapy (N=167, 39 events), Set 4: N0, adjuvant endocrine therapy (N=291, 39 events), and Set 5: N1, adjuvant endocrine therapy (N=407, 82 events) (Table 1). The reason for the subdivision with regard to age between Sets 1 and 2 was to confirm the index from our original study (Strand et al. 2012) in a corresponding subgroup with regard to menopausal status, adjuvant therapy and lymph node status.
Patient material VI
The Uppsala study (N=166 cases and 166 controls, Table 2). The original study included 900 patients diagnosed with primary breast cancer in the Uppsala-Örebro region from 1993–2004. Exclusion criteria were tumor size >50 mm, lymph node metastases or adjuvant chemotherapy. Within this cohort, cases were defined as women who died from breast cancer. Eligible as controls were patients alive at the time of the corresponding case’s death (Ahlin et al. 2009).
Biomarker analysis and definition of KiGE
ER, PgR, Ki67, HER2, and histological grade were analyzed and evaluated as described elsewhere (Ahlin et al. 2009; Rydén et al. 2005; Chebil et al. 2003; Borgquist et al. 2008; Falck et al. 2012). If previously defined cut-points were available, they were used in the present study. Hence, cases above the median were considered Ki67 high in Patient material V. Furthermore, Ahlin et al. defined high Ki67 as cases above the seventh decile of the empirical Ki67 distribution (which corresponded to 20% positive cells) (Klintman et al. 2010) and therefore 20% was used for Patient materials I–IV and VI. For Patient materials I–IV and VI, Ki67 was evaluated on TMAs and for Patient material V on whole tissue sections. In order to confirm the combination of proliferation, histological grade, and ER, the previously applied index (CAGE) was used (Strand et al. 2012), but cyclin A was replaced by Ki67, thereby creating KiGE. The low KiGE-group was defined as histological grade 1 patients and grade 2 patients which were ER-positive and had low Ki67 expression. High KiGE consisted of all other patients.
Statistical methods
The Kaplan-Meier method was used to estimate event-free survival and the Cox proportional hazards model, stratified by patient material, was used for estimation of hazard ratios (HR:s). Proportional hazards assumptions were checked with Schoenfeld’s test (Schoenfeld 1983). To avoid severe problems with non-proportional hazards, the follow-up was restricted to the first five years after diagnosis.
In Patient material VI, conditional logistic regression analysis was used to estimate odds ratios (OR:s) and confidence intervals (CI:s), using the proportional hazards regression procedure in statistical analysis software (SAS).
Forest plots were used to visualize HR:s and 95% CI:s for the different subsets and the overall measure of effect which was estimated using a DerSimonian-Laird random-effects model.
All tests were two-sided. For evaluation of the primary aim, the effect of the KiGE-index, P-values <0.01 were considered significant. The statistical analysis software Stata 12.1, 2012 (StataCorp, College Station, TX) and SAS (SAS Institute, Inc.) were used for statistical calculations. Whenever applicable, the REMARK recommendations for reporting of tumor marker studies were followed (McShane et al. 2005). The study was approved by the ethics committee at Lund University (LU 240-01).
Results
KiGE evaluation
Univariate analyses
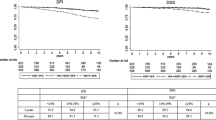
The HR:s for high KiGE versus low KiGE, when each set (Set 1–5) was analyzed separately, varied between 3.0 and 4.4, being statistically significant for all sets (Figures 1a–e and 2a). When including all patients in Set 1–5 (N=1,522, 235 events), a statistically significant association between the combination variable KiGE and event-free survival was found (HR: 3.9, 95% CI: 2.9–5.2, P<0.0001; Figure 1f).
Kaplan-Meier survival estimates of event-free survival, and hazard ratios(HR)with corresponding 95%confidence intervals(CI)for the different subsets(1a-e),stratified by patient material, and for all the patients(1f). From Patient materials I-V the following subsets were extracted: Set 1: node-negative (N0), no adjuvant therapy, ≤50 years at diagnosis (1a), Set 2: N0, no adjuvant therapy, >50 years at diagnosis (1b), Set 3: node-positive (N1), no adjuvant therapy (1c), Set 4: N0, adjuvant endocrine therapy (1d), and Set 5: N1, adjuvant endocrine therapy (1e). Event-free survival corresponds to distant disease-free survival for Patient materials I-II and IV-V, and to recurrence-free survival for Patient material III.
Forest plots for the different subsets, showing hazard ratios (HR:s) with corresponding 95% confidence intervals (CI:s) for KiGE in univariate analysis (a) and in multivariate analysis (b), adjusted for age at diagnosis and tumor size. The diamonds and the vertical dashed lines represent the overall measures of effect. The areas of the grey squares are proportional to each subset’s weight in the meta-analysis.
In the case–control study (Patient material VI), there was a statistically significant association between the combination variable KiGE and breast cancer death (OR: 2.7, 95% CI: 1.7–4.3, P<0.0001).
Multivariate analyses
The HR:s for KiGE for each of the five subsets, when adjusted for age at diagnosis and tumor size, were similar compared to those without adjustment, as illustrated by the forest plot (Figure 2b). When all subsets (Set 1–5) were included, KiGE was significantly associated with event-free survival, after adjustment for age at diagnosis, tumor size, and adjuvant endocrine treatment (HR: 3.5, 95% CI: 2.6–4.7, P<0.0001). Including HER2 in the multivariate analysis (N=830, 133 events), KiGE remained significantly associated with event-free survival (HR: 4.0, 95% CI: 2.7–6.0, P<0.0001).
In the case–control study, KiGE was significantly associated with breast cancer death, after adjustment for age at diagnosis, tumor size, and adjuvant endocrine treatment (OR: 3.2, 95% CI: 1.8–5.5, P<0.0001).
Subset analyses of Ki67 in patients not treated with adjuvant tamoxifen (Set 1–3)
Ki67 in ER-positive versus ER-negative cases
In order to confirm results from previous investigations (Teschendorff et al. 2007; Desmedt et al. 2008; Klintman et al. 2010) showing that the prognostic importance of Ki67 is dependent on ER-status, subgroup analyses were performed, stratified by ER-status. In univariate analyses, Ki67 was a significant prognostic factor in the ER-positive subgroup (HR: 3.3, 95% CI: 2.0–5.5, P<0.0001; N=652, 76 events), but not in the ER-negative subgroup (HR: 1.0, 95% CI: 0.49–2.1, P=0.96; N=172, 38 events). The difference in prognostic importance of Ki67 between ER-positive and ER-negative cases was further analyzed in a Cox model allowing for interaction between the two factors. The interaction effect, corresponding to the ratio of the HR:s for Ki67 in the ER-positive and ER-negative subgroups, was 2.7 (95% CI: 1.2–6.1, P=0.02).
Ki67 in histological grade subgroups
Previous studies have demonstrated that the prognostic importance of Ki67 is mainly attributed to the histological grade 2 subgroup (Klintman et al. 2010; Aleskandarany et al. 2011). Similar trends were seen in the present study. In the histological grade 2 tumors (N=429, 51 events), the HR for high versus low Ki67 was 1.8 (95% CI: 1.0–3.4, P=0.05). The five-year event-free survival figures were 82% (95% CI: 73–88%) for high Ki67 and 90% (95% CI: 86–93%) for low Ki67, respectively. There were too few events (N=196, 5 events) to draw any conclusions on the impact of Ki67 in the histological grade 1 subgroup. The five-year event-free survival in the histological grade 1 subgroup was 97% (95% CI: 93–99%), independent of Ki67. Ki67 was not a significant prognostic factor in the histological grade 3 subgroup (HR: 1.0, 95% CI: 0.56–1.9, P=0.91; N=199, 58 events), with five-year DDFS of 73% (95% CI: 64–79%) and 65% (95% CI: 52–76%) for high and low Ki67 groups, respectively.
Five-year DDFS for high and low KiGE (Patient materials I–II and IV–V)
Use of the KiGE-index in the N0/N1-subgroup with information on distant recurrences (N=1,305, 203 distant recurrences) identified a low-risk group constituting 57% of the patients, with a five-year DDFS of 92% (95% CI: 89–93%). The DDFS for the remaining 43% of the patients was 73% (95% CI: 69–77%). The association between the KiGE-index and DDFS was statistically significant (HR: 3.4, 95% CI: 2.5–4.6, P<0.0001; Figure 3).
In the N0-subgroup (N=793, 97 distant recurrences), the KiGE-index identified a low-risk group constituting 57% of the patients with a five-year DDFS of 93% (95% CI: 90–95%; HR: 3.2, 95% CI: 2.0–4.9, P<0.0001). Equally, in the N1-subgroup (N=512, 106 distant recurrences), the KiGE-index identified a low-risk group of similar size (56%) with a five-year DDFS of 89% (95% CI: 85–92%; HR: 3.7, 95% CI: 2.4–5.7, P<0.0001).
Discussion
This confirmation study for N0/N1 chemo-naïve breast cancer patients, confirms the prognostic value of a previously defined index combining proliferation (previously cyclin A, in the present study Ki67), histological grade, and ER. Importantly, the KiGE-index separated chemo-naïve patients into groups with different risk, independent of menopausal status, lymph node status, and whether endocrine adjuvant treatment was given or not. The robustness of the index is strengthened by the fact that the evaluation of Ki67, histological grade, and ER was performed in different studies by different persons using different cut-points, that studies from three Swedish health care regions and one Danish region were included, and by the fact that different study designs were used (randomized, cohort and case-control studies). Furthermore, when analyzing Patient materials I–V separately (not the five sets), KiGE remained a significant prognostic factor (HR:s varied between 2.1 and 9.0) in all but one patient material (Patient material II, P=0.09; data not shown). We were also able to confirm the previous finding that the prognostic value of Ki67 is limited to ER-positive breast cancer and is most pronounced for the histological grade 2 subgroup. The latter findings are furthermore in line with gene expression analyses (Sotiriou et al. 2006; Ivshina et al. 2006; Teschendorff et al. 2007; Desmedt et al. 2008). However, a recent publication (Munzone et al. 2012) showed that within the group of patients with node-negative triple-negative breast cancer, Ki67 was associated with different prognosis when using a higher cut-point (35%). The KiGE-index is similar to the index proposed at the St. Gallen consensus meeting in 2011 (Goldhirsch et al. 2011), with Ki67 separating clinicopathologically classified ‘Luminal’ ER-positive breast cancer into ‘Luminal A’ and ‘Luminal B’ subgroups with different prognoses and thereby influence on the choice of therapy. Chemotherapy, with or without anti-HER2 therapy, is suggested for the ‘Luminal B’ subgroup, but not for the ‘Luminal A’ subgroup (Goldhirsch et al. 2011). According to the St. Gallen guidelines, not taking histological grade into consideration, patients with ER-positive low proliferating (and HER2 normal) tumors will be classified as having ‘Luminal A’ breast cancer. According to the KiGE-index, with the inclusion of histological grade, some of these patients will instead be considered as having worse prognosis. In this large patient material, patients with ER-positive, low Ki67, and histological 3 breast cancer (N=42) have a poor prognosis, with a five-year DDFS of 64% (95% CI: 47–76%). This subgroup constituted 6% (42/652) of the ER-positive (chemo-naïve) patients in our study (Patient materials I–V). In a recent publication by the International Ki67 in Breast Cancer Working Group (Dowsett et al. 2011), certain important drawbacks for Ki67 analyses were highlighted, including number of cancer cells being scored and cut-point used. Furthermore, the distribution of Ki67 values makes it difficult to define a cut-point. The inherent drawbacks with the evaluation of Ki67 may at least partly be overcome by also considering histological grade, as suggested in the present study. Our study was, probably due to low power, unable to demonstrate any prognostic importance of Ki67 in histological grade 1 breast cancer. Aleskandarany et al. however, demonstrated that patients with high Ki67 had a significantly worse prognosis than those with low Ki67, in a large study, including 494 histological grade 1 breast cancers (Aleskandarany et al. 2011). Therefore, we do not exclude the possibility that Ki67 has prognostic value in the histological grade 1 subgroup.
Identifying a subgroup of patients not in need of adjuvant chemotherapy, was found to be the top priority on a list of the most urgent research areas in breast cancer in a recent web-consultant study (Dowsett et al. 2007). The Early Breast Cancer Trialists´ Collaborative Group (Peto et al. 2012) demonstrated that the effect of chemotherapy was independent of age, node status, tumor size, differentiation, ER-status, and tamoxifen use. However, information on quantitative immunohistochemistry of proliferation was not included. Ki67 combined with ER-status and histological grade may be helpful in this respect. Breast cancer patients with histological grade 1 tumors or patients with ER-positive and histological grade 2 tumors with low Ki67 expression constitute 57% of the patients with N0/N1 cancers in this study, with a five-year DDFS of 92%. Adjuvant chemotherapy would have limited added value for this group. The identification of a low-risk group not in need of adjuvant chemotherapy is also the primary aim of two ongoing clinical trials (MINDACT and TAILORx) (Rutgers et al. 2011; Zujewski & Kamin 2008) evaluating the gene profiles MammaPrint® and Oncotype DX®. The MINDACT trial (Rutgers et al. 2011) is a prospective, randomized trial using the 70-gene signature (MammaPrint®) together with the common clinical-pathological criteria for selecting patients for adjuvant chemotherapy. It is being tested in patients with N0/N1 breast cancer. MINDACT has a null hypothesis of a five-year distant metastasis-free survival of 92%, which will be tested for the group of patients who have a low-risk gene prognosis signature and high clinical-pathological criteria, and who were randomized to use the 70-gene signature and thus receive no chemotherapy. In a pilot phase based on the first 800 patients from this trial, the low-risk group constituted 65% (Rutgers et al. 2011). This figure is in line with 57% being the percentage of low-risk patients in our study, who furthermore had the same five-year DDFS as the null hypothesis in the MINDACT trial (92%). Risk group stratification may be further modified by also considering other established prognostic factors, e.g. lymph node status, tumor size, age, and HER2-status. The incidence of recurrences in the low-risk group could thereby be further decreased, but with the consequence of a smaller low-risk group.
In conclusion, we have confirmed a prognostic index based on proliferation (Ki67), histological grade, and ER for identifying a low-risk group of patients with N0/N1 breast cancer. For this large low-risk group, with a five-year DDFS of 92%, adjuvant chemotherapy will have limited effect and may thus be avoided.
References
Aebi S, Davidson T, Gruber G, Cardoso F: Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Offic J Eur Soc Med Oncol/ESMO 2011, 22(Suppl 6):vi12-24.
Ahlin C, Zhou W, Holmqvist M, Holmberg L, Nilsson C, Jirstrom K, Blomqvist C, Amini RM, Fjallskog ML: Cyclin A is a proliferative marker with good prognostic value in node-negative breast cancer. Canc Epidemiol Biomark Prev Publ Am Assoc Canc Res Cosponsored Am Soc Prev Oncol 2009, 18(9):2501-2506.
Aleskandarany MA, Rakha EA, Macmillan RD, Powe DG, Ellis IO, Green AR: MIB1/Ki-67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups. Breast Cancer Res Treat 2011, 127(3):591-599. 10.1007/s10549-010-1028-3
Amadori D, Nanni O, Volpi A, Casadei Giunchi D, Marangolo M, Livi L, Ravaioli A, Rossi AP, Gambi A, Luzi Fedeli S, Perroni D, Scarpi E, Becciolini A, Silvestrini R: Phase III randomized multicenter study on the effects of adjuvant CMF in patients with node-negative, rapidly proliferating breast cancer: twelve-year results and retrospective subgroup analysis. Breast Cancer Res Treat 2008, 108(2):259-264. 10.1007/s10549-007-9593-9
Baldini E, Camerini A, Sgambato A, Prochilo T, Capodanno A, Pasqualetti F, Orlandini C, Resta L, Bevilacqua G, Collecchi P: Cyclin A and E2F1 overexpression correlate with reduced disease-free survival in node-negative breast cancer patients. Anticancer Res 2006, 26(6B):4415-4421.
Beresford MJ, Wilson GD, Makris A: Measuring proliferation in breast cancer: practicalities and applications. Breast Cancer Res 2006, 8(6):216. 10.1186/bcr1618
Borgquist S, Holm C, Stendahl M, Anagnostaki L, Landberg G, Jirstrom K: Oestrogen receptors alpha and beta show different associations to clinicopathological parameters and their co-expression might predict a better response to endocrine treatment in breast cancer. J Clin Pathol 2008, 61(2):197-203.
Bukholm IR, Bukholm G, Nesland JM: Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int J Canc J Int Canc 2001, 93(2):283-287. 10.1002/ijc.1311
Chebil G, Bendahl PO, Idvall I, Ferno M: Comparison of immunohistochemical and biochemical assay of steroid receptors in primary breast cancer–clinical associations and reasons for discrepancies. Acta Oncol 2003, 42(7):719-725. 10.1080/02841860310004724
Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ: Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol 2005, 16(11):1723-1739. 10.1093/annonc/mdi352
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF: Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011, 29(32):4273-4278. 10.1200/JCO.2010.31.2835
De Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M: Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007, 96(10):1504-1513. 10.1038/sj.bjc.6603756
Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, Delorenzi M, Piccart M, Sotiriou C: Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 2008, 14(16):5158-5165. 10.1158/1078-0432.CCR-07-4756
Dowsett M, Goldhirsch A, Hayes DF, Senn HJ, Wood W, Viale G: International Web-based consultation on priorities for translational breast cancer research. Breast Cancer Res 2007, 9(6):81. 10.1186/bcr1798
Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF: Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011, 103(22):1656-1664. 10.1093/jnci/djr393
Eden P, Ritz C, Rose C, Ferno M, Peterson C: "Good Old" clinical markers have similar power in breast cancer prognosis as microarray gene expression profilers. Eur J Cancer 2004, 40(12):1837-1841. 10.1016/j.ejca.2004.02.025
Ellis MJ, Tao Y, Luo J, A'Hern R, Evans DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith I, Eiermann W, Dowsett M: Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008, 100(19):1380-1388. 10.1093/jnci/djn309
Falck AK, Bendahl PO, Ingvar C, Isola J, Jonsson PE, Lindblom P, Lovgren K, Rennstam K, Ferno M, Rydén L: Analysis of and prognostic information from disseminated tumour cells in bone marrow in primary breast cancer: a prospective observational study. BMC Cancer 2012, 12(1):403. 10.1186/1471-2407-12-403
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ: Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 2009, 20(8):1319-1329. 10.1093/annonc/mdp322
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ: Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011, 22(8):1736-1747. 10.1093/annonc/mdr304
Hansen S, Grabau DA, Sorensen FB, Bak M, Vach W, Rose C: The prognostic value of angiogenesis by Chalkley counting in a confirmatory study design on 836 breast cancer patients. Clin Canc Res Offic J Am Assoc Canc Res 2000, 6(1):139-146.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr: American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007, 25(33):5287-5312. 10.1200/JCO.2007.14.2364
Hietanen P, Blomqvist C, Wasenius VM, Niskanen E, Franssila K, Nordling S: Do DNA ploidy and S-phase fraction in primary tumour predict the response to chemotherapy in metastatic breast cancer? Br J Cancer 1995, 71(5):1029-1032. 10.1038/bjc.1995.198
Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, Wong JE, Liu ET, Bergh J, Kuznetsov VA, Miller LD: Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res 2006, 66(21):10292-10301. 10.1158/0008-5472.CAN-05-4414
Jones RL, Salter J, A'Hern R, Nerurkar A, Parton M, Reis-Filho JS, Smith IE, Dowsett M: The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2009, 116(1):53-68. 10.1007/s10549-008-0081-7
Klintman M, Bendahl PO, Grabau D, Lovgren K, Malmstrom P, Ferno M: The prognostic value of Ki67 is dependent on estrogen receptor status and histological grade in premenopausal patients with node-negative breast cancer. Mod Pathol 2010, 23(2):251-259. 10.1038/modpathol.2009.167
Kuhling H, Alm P, Olsson H, Ferno M, Baldetorp B, Parwaresch R, Rudolph P: Expression of cyclins E, A, and B, and prognosis in lymph node-negative breast cancer. J Pathol 2003, 199(4):424-431. 10.1002/path.1322
Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, Egele C, Poulet B, Clough KB, Crouet H, Fourquet A, Lefranc JP, Mathelin C, Rouyer N, Serin D, Spielmann M, Haugh M, Chenard MP, Brain E, de Cremoux P, Bellocq JP: Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 2012, 132(3):895-915. 10.1007/s10549-011-1837-z
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM: Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005, 97(16):1180-1184. 10.1093/jnci/dji237
Michalides R, van Tinteren H, Balkenende A, Vermorken JB, Benraadt J, Huldij J, van Diest P: Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br J Cancer 2002, 86(3):402-408. 10.1038/sj.bjc.6600072
Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nole F, Mastropasqua M, Rotmensz N, Colleoni M, Esposito A, Adamoli L, Luini A, Goldhirsch A, Viale G: Prognostic value of Ki-67 labeling index in patients with node-negative, triple-negative breast cancer. Breast Cancer Res Treat 2012, 134(1):277-282. 10.1007/s10549-012-2040-6
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N: A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Eng J Med 2004, 351(27):2817-2826. 10.1056/NEJMoa041588
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D: Molecular portraits of human breast tumours. Nature 2000, 406(6797):747-752. 10.1038/35021093
Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K: Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379(9814):432-444.
Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, Parker HL: Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 2001, 19(4):980-991.
Rutgers E, Piccart-Gebhart MJ, Bogaerts J, Delaloge S, Veer LV, Rubio IT, Viale G, Thompson AM, Passalacqua R, Nitz U, Vindevoghel A, Pierga JY, Ravdin PM, Werutsky G, Cardoso F: The EORTC 10041/BIG 03-04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer 2011, 47(18):2742-2749. 10.1016/j.ejca.2011.09.016
Rydén L, Jonsson PE, Chebil G, Dufmats M, Ferno M, Jirstrom K, Kallstrom AC, Landberg G, Stal O, Thorstenson S, Nordenskjold B: Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: a randomised, controlled trial with long-term follow-up. Eur J Cancer 2005, 41(2):256-264. 10.1016/j.ejca.2004.06.030
Schoenfeld DA: Sample-size formula for the proportional-hazards regression model. Biometrics 1983, 39(2):499-503. 10.2307/2531021
Skaland I, Janssen EA, Gudlaugsson E, Klos J, Kjellevold KH, Soiland H, Baak JP: Phosphohistone H3 expression has much stronger prognostic value than classical prognosticators in invasive lymph node-negative breast cancer patients less than 55 years of age. Mod Pathol 2007, 20(12):1307-1315. 10.1038/modpathol.3800972
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL: Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001, 98(19):10869-10874. 10.1073/pnas.191367098
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M: Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 2006, 98(4):262-272. 10.1093/jnci/djj052
Strand C, Ahlin C, Bendahl PO, Fjallskog ML, Hedenfalk I, Malmstrom P, Ferno M: Combination of the proliferation marker cyclin A, histological grade, and estrogen receptor status in a new variable with high prognostic impact in breast cancer. Breast Cancer Res Treat 2012, 131(1):33-40. 10.1007/s10549-011-1386-5
Swedish Breast Cancer Cooperative Group: Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst 1996, 88(21):1543-1549.
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C: An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol 2007, 8(8):157. 10.1186/gb-2007-8-8-r157
Urruticoechea A, Smith IE, Dowsett M: Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005, 23(28):7212-7220. 10.1200/JCO.2005.07.501
Viale G, Regan MM, Mastropasqua MG, Maffini F, Maiorano E, Colleoni M, Price KN, Golouh R, Perin T, Brown RW, Kovacs A, Pillay K, Ohlschlegel C, Gusterson BA, Castiglione-Gertsch M, Gelber RD, Goldhirsch A, Coates AS: Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst 2008, 100(3):207-212. 10.1093/jnci/djm289
Zujewski JA, Kamin L: Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol 2008, 4(5):603-610. 10.2217/14796694.4.5.603
Acknowledgments
We are indebted to participating departments of the South and South-East Swedish Breast Cancer Groups, of the Uppsala-Örebro region, and of the Odense region for providing samples and clinical follow-up. We thank Kristina Lövgren for technical skills in creating the TMA blocks and Marit Holmqvist for statistical analysis of the case–control study.
Funding
This work was supported by funding from the Swedish Cancer Society, the Swedish Research Council, the Gunnar Nilsson Cancer Foundation, the Mrs. Berta Kamprad Foundation, the Anna and Edwin Bergers foundation, the Swedish Breast Cancer Association (BRO), the Skåne University Hospital Research Foundation, the Skåne County Council’s Research and Development Foundation, and Governmental Funding of Clinical Research within the National Health Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors have no conflicts of interest to declare.
Authors’ contributions
CS, MK, POB, PM and MF were responsible for concept and design of the study. Financial and administrative support was provided by MF. MB, SB, GC, AKF, MLF, DG, KJ, MK, PM, HO, LR and OS provided study material and information of patients. CS, DG, IH, PM, POB and MF were responsible of data analysis and interpretation. CS, MF and POB were responsible for the manuscript writing. All authors read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Strand, C., Bak, M., Borgquist, S. et al. The combination of Ki67, histological grade and estrogen receptor status identifies a low-risk group among 1,854 chemo-naïve women with N0/N1 primary breast cancer. SpringerPlus 2, 111 (2013). https://doi.org/10.1186/2193-1801-2-111
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-2-111