Abstract
Purpose
Several clinical trials have investigated the prognostic and predictive usefulness of molecular markers. With limited predictive value, molecular markers have mainly been used to identify prognostic subgroups in which the indication for chemotherapy is doubtful and the prognosis is favorable enough for chemotherapy to be avoided. However, limited information is available about which groups of patients may benefit from additional therapy. This study aimed to describe the prognostic effects of Ki-67 in several common subgroups of patients with early breast cancer.
Methods
This retrospective study analyzed a single-center cohort of 3140 patients with HER2−, hormone receptor-positive breast cancer. Five-year disease-free survival (DFS) rates were calculated for low (< 10%), intermediate (10–19%), and high (≥ 20%) Ki-67 expression levels, as assessed by immunohistochemistry, and for subgroups relative to age, body mass index, disease stage, tumor grade, and (neo-)adjuvant chemotherapy. It was also investigated whether Ki-67 had different effects on DFS in these subgroups.
Results
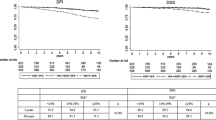
The 5-year DFS rates for patients with low, intermediate, and high levels of Ki-67 expression were 0.90, 0.89, and 0.77, respectively. Ki-67 was able to further differentiate patients with an intermediate prognosis into different prognostic groups relative to common clinical parameters. Patients with stage II breast cancer had 5-year DFS rates of 0.84, 0.88, and 0.79 for low, intermediate, and high levels of Ki-67 expression. Ki-67 had different prognostic effects in subgroups defined by age and tumor grade.
Conclusions
Ki-67 may help identify patients in intermediate prognostic groups with an unfavorable prognosis who may benefit from further therapy.



Similar content being viewed by others
References
Allegra CJ, Paik S, Colangelo LH, Parr AL, Kirsch I, Kim G, Klein P, Johnston PG, Wolmark N, Wieand HS (2003) Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes’ B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol 21:241–250. https://doi.org/10.1200/JCO.2003.05.044
Andre F, Arnedos M, Goubar A, Ghouadni A, Delaloge S (2015) Ki67—no evidence for its use in node-positive breast cancer. Nat Rev Clin Oncol 12:296–301. https://doi.org/10.1038/nrclinonc.2015.46
Beckmann MW, Brucker C, Hanf V, Rauh C, Bani MR, Knob S, Petsch S, Schick S, Fasching PA, Hartmann A, Lux MP, Haberle L (2011) Quality assured health care in certified breast centers and improvement of the prognosis of breast cancer patients. Onkologie 34:362–367. https://doi.org/10.1159/000329601
Boros M, Moncea D, Moldovan C, Podoleanu C, Georgescu R, Stolnicu S (2017) Intratumoral heterogeneity for Ki-67 index in invasive breast carcinoma: a study on 131 consecutive cases. Appl Immunohistochem Mol Morphol 25:338–340. https://doi.org/10.1097/PAI.0000000000000315
Caldarella A, Crocetti E, Paci E (2014) Ki67 in breast cancer: a useful prognostic marker? Ann Oncol 25:542. https://doi.org/10.1093/annonc/mdt561
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M, MINDACT Investigators (2016) 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717–729. https://doi.org/10.1056/NEJMoa1602253
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750. https://doi.org/10.1093/jnci/djp082
NCT02513394 (2015) PALbociclib CoLlaborative Adjuvant Study: a randomized Phase III Trial of palbociclib with standard adjuvant endocrine therapy versus standard adjuvant endocrine therapy alone for hormone receptor positive (HR+)/human epidermal growth factor receptor 2 (HER2)-negative early breast cancer (PALLAS). NIH U.S. National Library of Medicine. http://clinicaltrials.gov. Accessed 17 Nov 2018
NCT03155997 (2017) Endocrine therapy with or without abemaciclib (LY2835219) following surgery in participants with breast cancer (monarchE). NIH U.S. National Library of Medicine. http://clinicaltrials.gov. Accessed 17 Nov 2018
NCT03701334 (2018) A trial to evaluate efficacy and safety of ribociclib with endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer (NATALEE). NIH U.S. National Library of Medicine. http://clinicaltrials.gov. Accessed 17 Nov 2018
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 29:4273–4278. https://doi.org/10.1200/JCO.2010.31.2835
Denkert C, Loibl S, Muller BM, Eidtmann H, Schmitt WD, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, Fehm T, Barinoff J, Jackisch C, Prinzler J, Rudiger T, Erbstosser E, Blohmer JU, Budczies J, Mehta KM, von Minckwitz G (2013) Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol 24:2786–2793. https://doi.org/10.1093/annonc/mdt350
Dieras V, Rugo HS, Schnell P, Gelmon K, Cristofanilli M, Loi S, Colleoni M, Lu DR, Mori A, Gauthier E, Huang Bartlett C, Slamon DJ, Turner NC, Finn RS (2018) Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2− advanced breast cancer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djy109
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF, International Ki-67 in Breast Cancer Working Group (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 103:1656–1664. https://doi.org/10.1093/jnci/djr393
Early Breast Cancer Trialists’ Collaborative Group, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444. https://doi.org/10.1016/S0140-6736(11)61625-5
Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, Rauh C, Schulz-Wendtland R, Bani MR, Schrauder M, Kahmann L, Lux MP, Strehl JD, Hartmann A, Dimmler A, Beckmann MW, Wachter DL (2011) Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 11:486. https://doi.org/10.1186/1471-2407-11-486
Fasching PA, Loibl S, Hu C, Hart SN, Shimelis H, Moore R, Schem C, Tesch H, Untch M, Hilfrich J, Rezai M, Gerber B, Costa SD, Blohmer JU, Fehm T, Huober J, Liedtke C, Weinshilboum RM, Wang L, Ingle JN, Muller V, Nekljudova V, Weber KE, Rack B, Rubner M, von Minckwitz G, Couch FJ (2018) BRCA1/2 mutations and bevacizumab in the neoadjuvant treatment of breast cancer: response and prognosis results in patients with triple-negative breast cancer from the GeparQuinto Study. J Clin Oncol 36:2281–2287. https://doi.org/10.1200/JCO.2017.77.2285
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16:25–35. https://doi.org/10.1016/S1470-2045(14)71159-3
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11:R77. https://doi.org/10.1186/bcr2419
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925–1936. https://doi.org/10.1056/NEJMoa1607303
Focke CM, Decker T, van Diest PJ (2016) Intratumoral heterogeneity of Ki67 expression in early breast cancers exceeds variability between individual tumours. Histopathology 69:849–861. https://doi.org/10.1111/his.13007
Gass P, Lux MP, Rauh C, Hein A, Bani MR, Fiessler C, Hartmann A, Haberle L, Pretscher J, Erber R, Wachter DL, Schulz-Wendtland R, Beckmann MW, Fasching PA, Wunderle M (2018) Prediction of pathological complete response and prognosis in patients with neoadjuvant treatment for triple-negative breast cancer. BMC Cancer 18:1051. https://doi.org/10.1186/s12885-018-4925-1
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel m (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223. https://doi.org/10.1093/annonc/mdt303
Himuro T, Horimoto Y, Arakawa A, Tanabe M, Saito M (2016) Ki67 heterogeneity in estrogen receptor-positive breast cancers: which tumor type has the most heterogeneity? Int J Surg Pathol 24:103–107. https://doi.org/10.1177/1066896915605179
Jang MH, Kim HJ, Chung YR, Lee Y, Park SY (2017) A comparison of Ki-67 counting methods in luminal breast cancer: the average method vs. the hot spot method. PLoS ONE 12:e0172031. https://doi.org/10.1371/journal.pone.0172031
Khor LY, Bae K, Paulus R, Al-Saleem T, Hammond ME, Grignon DJ, Che M, Venkatesan V, Byhardt RW, Rotman M, Hanks GE, Sandler HM, Pollack A (2009) MDM2 and Ki-67 predict for distant metastasis and mortality in men treated with radiotherapy and androgen deprivation for prostate cancer: RTOG 92-02. J Clin Oncol 27:3177–3184. https://doi.org/10.1200/JCO.2008.19.8267
Loehberg CR, Almstedt K, Jud SM, Haeberle L, Fasching PA, Hack CC, Lux MP, Thiel FC, Schrauder MG, Brunner M, Bayer CM, Hein A, Heusinger K, Heimrich J, Bani MR, Renner SP, Hartmann A, Beckmann MW, Wachter DL (2013) Prognostic relevance of Ki-67 in the primary tumor for survival after a diagnosis of distant metastasis. Breast Cancer Res Treat 138:899–908. https://doi.org/10.1007/s10549-013-2460-y
Margulis V, Lotan Y, Karakiewicz PI, Fradet Y, Ashfaq R, Capitanio U, Montorsi F, Bastian PJ, Nielsen ME, Muller SC, Rigaud J, Heukamp LC, Netto G, Lerner SP, Sagalowsky AI, Shariat SF (2009) Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst 101:114–119. https://doi.org/10.1093/jnci/djn451
Ohno S, Chow LW, Sato N, Masuda N, Sasano H, Takahashi F, Bando H, Iwata H, Morimoto T, Kamigaki S, Nakayama T, Nakamura S, Kuroi K, Aogi K, Kashiwaba M, Yamashita H, Hisamatsu K, Ito Y, Yamamoto Y, Ueno T, Fakhrejahani E, Yoshida N, Toi M (2013) Randomized trial of preoperative docetaxel with or without capecitabine after 4 cycles of 5-fluorouracil–epirubicin–cyclophosphamide (FEC) in early-stage breast cancer: exploratory analyses identify Ki67 as a predictive biomarker for response to neoadjuvant chemotherapy. Breast Cancer Res Treat 142:69–80. https://doi.org/10.1007/s10549-013-2691-y
Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF (2009) Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 27:1323–1333. https://doi.org/10.1200/JCO.2007.14.8197
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Pivot X, Vidam G, Wang Y, Rodriguez Lorenc K, Miller M, Taran T, Jerusalem G (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36:2465–2472. https://doi.org/10.1200/JCO.2018.78.9909
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35:2875–2884. https://doi.org/10.1200/JCO.2017.73.7585
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360:790–800. https://doi.org/10.1056/NEJMra0801289
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98:262–272. https://doi.org/10.1093/jnci/djj052
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379:111–121. https://doi.org/10.1056/NEJMoa1804710
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005–2014. https://doi.org/10.1056/NEJMoa1510764
Sueta A, Yamamoto Y, Hayashi M, Yamamoto S, Inao T, Ibusuki M, Murakami K, Iwase H (2014) Clinical significance of pretherapeutic Ki67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer: is it equally useful across tumor subtypes? Surgery 155:927–935. https://doi.org/10.1016/j.surg.2014.01.009
Taran FA, Schneeweiss A, Lux MP, Janni W, Hartkopf AD, Nabieva N, Overkamp F, Kolberg HC, Hadji P, Tesch H, Wockel A, Ettl J, Luftner D, Wallwiener M, Muller V, Beckmann MW, Belleville E, Wallwiener D, Brucker SY, Fasching PA, Fehm TN, Schutz F (2018) Update breast cancer 2018 (Part 1)—primary breast cancer and biomarkers. Geburtshilfe Frauenheilkd 78:237–245. https://doi.org/10.1055/s-0044-101613
Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, Campos-Gomez S, Villanueva Vazquez R, Jung KH, Babu KG, Wheatley-Price P, De Laurentiis M, Im YH, Kuemmel S, El-Saghir N, Liu MC, Carlson G, Hughes G, Diaz-Padilla I, Germa C, Hirawat S, Lu YS (2018) Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 19:904–915. https://doi.org/10.1016/S1470-2045(18)30292-4
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Andre F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1810527
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Voros A, Csorgo E, Kovari B, Lazar P, Kelemen G, Rusz O, Nyari T, Cserni G (2015) Different methods of pretreatment Ki-67 labeling index evaluation in core biopsies of breast cancer patients treated with neoadjuvant chemotherapy and their relation to response to therapy. Pathol Oncol Res 21:147–155. https://doi.org/10.1007/s12253-014-9800-z
Warth A, Cortis J, Soltermann A, Meister M, Budczies J, Stenzinger A, Goeppert B, Thomas M, Herth FJ, Schirmacher P, Schnabel PA, Hoffmann H, Dienemann H, Muley T, Weichert W (2014) Tumour cell proliferation (Ki-67) in non-small cell lung cancer: a critical reappraisal of its prognostic role. Br J Cancer 111:1222–1229. https://doi.org/10.1038/bjc.2014.402
Wiesner FG, Magener A, Fasching PA, Wesse J, Bani MR, Rauh C, Jud S, Schrauder M, Loehberg CR, Beckmann MW, Hartmann A, Lux MP (2009) Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients. Breast 18:135–141. https://doi.org/10.1016/j.breast.2009.02.009
Wockel A, Festl J, Stuber T, Brust K, Stangl S, Heuschmann PU, Albert US, Budach W, Follmann M, Janni W, Kopp I, Kreienberg R, Kuhn T, Langer T, Nothacker M, Scharl A, Schreer I, Link H, Engel J, Fehm T, Weis J, Welt A, Steckelberg A, Feyer P, Konig K, Hahne A, Kreipe HH, Knoefel WT, Denkinger M, Brucker S, Luftner D, Kubisch C, Gerlach C, Lebeau A, Siedentopf F, Petersen C, Bartsch HH, Schulz-Wendtland R, Hahn M, Hanf V, Muller-Schimpfle M, Henscher U, Roncarati R, Katalinic A, Heitmann C, Honegger C, Paradies K, Bjelic-Radisic V, Degenhardt F, Wenz F, Rick O, Holzel D, Zaiss M, Kemper G, Budach V, Denkert C, Gerber B, Tesch H, Hirsmuller S, Sinn HP, Dunst J, Munstedt K, Bick U, Fallenberg E, Tholen R, Hung R, Baumann F, Beckmann MW, Blohmer J, Fasching PA, Lux MP, Harbeck N, Hadji P, Hauner H, Heywang-Kobrunner S, Huober J, Hubner J, Jackisch C, Loibl S, Luck HJ, von Minckwitz G, Mobus V, Muller V, Nothlings U, Schmidt M, Schmutzler R, Schneeweiss A, Schutz F, Stickeler E, Thomssen C, Untch M, Wesselmann S, Bucker A, Krockenberger M (2018) Interdisciplinary screening, diagnosis, therapy and follow-up of breast cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017)—Part 1 with recommendations for the screening, diagnosis and therapy of breast cancer. Geburtshilfe Frauenheilkd 78:927–948. https://doi.org/10.1055/a-0646-4522
Wockel A, Lux MP, Janni W, Hartkopf AD, Nabieva N, Taran FA, Overkamp F, Hadji P, Tesch H, Ettl J, Luftner D, Muller V, Welslau M, Belleville E, Brucker SY, Schutz F, Fasching PA, Fehm T, Schneeweiss A, Kolberg HC (2018) Update breast cancer 2018 (Part 3)—genomics, individualized medicine and immune therapies—in the middle of a new era: prevention and treatment strategies for early breast cancer. Geburtshilfe Frauenheilkd 78:1110–1118. https://doi.org/10.1055/s-0043-111601
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11:174–183. https://doi.org/10.1016/S1470-2045(09)70262-1
Zabaglo L, Salter J, Anderson H, Quinn E, Hills M, Detre S, A’Hern R, Dowsett M (2010) Comparative validation of the SP6 antibody to Ki67 in breast cancer. J Clin Pathol 63:800–804. https://doi.org/10.1136/jcp.2010.077578
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AH has received honoraria from Roche, Janssen, Merck Sharp Dome, AstraZeneca, Novartis, Cepheid, Abbvie and Boehringer-Ingelheim. His institution performs research with Grants from Biontech, Janssen, Cepheid, Roche and Qiagen. DJS has received honoraria from Novartis, Pfizer and Lilly and owns stock in Pfizer, Amgen and Seattle Genetics. His institution performs research with Grants from Novartis. PAF has received honoraria from Novartis, Amgen, Pfizer, Roche, Teva, Celgene, Eisai, Daiichi-Sankyo and PUMA. His institution performs research with Grants from Biontech and Novartis. PG has received honoraria from Novartis, travel support from Novartis, Roche and PharmaMar. MPL has received honoraria from Novartis, AstraZeneca, Roche, Pfizer, Lilly Eisai, Genomic Health and Tesaro. The remaining authors declare that they have no conflict of interest.
Ethical approval
Approval for this retrospective study was obtained from the Ethics Committee of the Faculty of Medicine at Friedrich Alexander University of Erlangen-Nuremberg. All procedures were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from each individual participant included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fasching, P.A., Gass, P., Häberle, L. et al. Prognostic effect of Ki-67 in common clinical subgroups of patients with HER2-negative, hormone receptor-positive early breast cancer. Breast Cancer Res Treat 175, 617–625 (2019). https://doi.org/10.1007/s10549-019-05198-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05198-9