Abstract
The genus Chlorella is a widely employed microalga for biodiesel, as it can be grown using photo/mixo/heterotrophic mode of cultivation. The present investigation was undertaken with the hypothesis that addition of different substrates (amino acids, carbon sources, vitamins) along with reducing agents may aid in diverting Acetyl CoA to malonyl CoA or fatty acid biosynthesis, under mixotrophic conditions in Chlorella sorokiniana. Preliminary investigations undertaken with two reducing agents individually (sodium thiosulphate and methyl viologen) along with selected substrates revealed the promise of sodium thiosulphate (1%) in enhancing lipid accumulation significantly. Further, the role of inclusion of twelve substrates and sodium thiosulphate revealed that supplementation with tryptophan (0.1%) recorded 57.28% enhancement in lipid productivity on 4th day. Highest values of lipid productivity of 33% were recorded on 8th day in 0.1% glucose supplemented medium containing sodium thiosulphate. Fatty Acid Methyl Ester (FAME) profiles generated revealed significant reduction in the content of Poly unsaturated fatty acids (PUFA) and enhanced Mono unsaturated fatty acids (MUFA) (especially oleic acid) in the treatments involving tryptophan, Vitamin B12, sodium pyruvate and glucose. This study reveals the promise of using sodium thiosulphate along with selected substrate for enriching the quality and quantity of lipids, which can be valuable for exploiting algae as a source of biodiesel.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
The last few decades have seen a growing interest in using microalgae, cyanobacteria and other photosynthetic bacteria as potential producers of renewable fuels, such as biodiesel, biohydrogen and biogas. Biodiesel production from microalgae is a relatively novel concept and these organisms offer the greatest photosynthetic efficiency, as a consequence of a minimum of internally competitive plant functions and limited nutrient requirements, besides exhibiting fast reproductive cycles. The yield of biodiesel from microalgae depends up on both the biomass concentration of the cultures and the oil content of individual cells (Becker 2004;Chisti 2008). The total content of lipids in microalgae may vary from about 1–85% of the dry weight (i.e. lipid productivity), with values higher than 40% being typically achieved under stress conditions (Chisti 2007).
Factors such as temperature, irradiance and, most markedly, nutrient availability have been shown to affect both lipid composition and lipid content in several algae (Takagi and Karseno 2006;Rao et al. 2007). To develop cost-effective algal oil production, researchers have experimented with photoheterotrophy / mixotrophy and heterotrophy for enhancing lipid productivity, especially with species of Chlorella (Ceron Garcia et al. 2005;Schenk et al. 2009). Recent studies have shown that the global flux distribution in oleaginous Chlorella protothecoides and Chlamydomonas reinhardtii remains stable under nitrogen limiting conditions and is controlled by the availability of carbon precursors (Xiong et al. 2010;Fan et al. 2012). Many microalgae can accumulate lipids due to excess photosynthesis and some species can accumulate high amount of lipids under heterotrophic or environmental stress, such as nutrient deficiency or salt stress (Jang et al. 2011).
The genus Chlorella has been a model organism in this context, especially in studies on modulating lipid accumulation, as several strains exhibit heterotrophy (Miao and Wu 2006;Xu et al. 2004;Liang et al. 2009;Ordog et al. 2012).Kay (1991) recorded that Chlorella sorokiniana w as a promising freshwater non-motile unicellular alga, accumulating high amounts of lipids and proteins. (Wan et al. 2011) analyzed the growth, lipid content and expression levels of three important genes involved in lipid biosynthesis pathway of Chlorella sorokiniana, as influenced by mixotrophy and found the organism most suited to mixotrophy, exhibiting 51% lipid content.
Our earlier studies revealed that certain microalgae, especially those belonging to the genus Chlorella exhibit enhanced growth and lipid accumulation under light and dark, in the presence of glucose. Among the set of Chlorella strains evaluated, Chlorella sorokiniana MIC-G5 highest lipid productivity in the presence of 2% glucose, both under mixotrophic and heterotrophic conditions (Ratha et al. 2012). This strain was therefore selected for further in depth analyses in the present investigation.
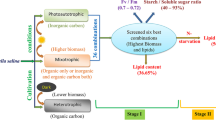
Researchers have recorded a high lipid content of 55% during heterotrophic growth of Chlorella protothecoides and developed efficient processes, combining bioengineering and transesterification for obtaining high quality diesel (Miao and Wu 2006). The point of concern is to identify stimuli which can enhance oil/lipid accumulation in micro algae without affecting their growth rate. The simultaneous operation of photosynthesis and respiration, in the presence of glucose and light is known to lead to more reactive oxygen species, than microalgae can themselves scavenge. The role of reducing agents such as sodium thiosulphate can be useful in this context, as observed in Chlorella sp. (Feng et al. 2005). (Mandal and Mallick 2009) reported enhanced lipid accumulation in a Scenedesmus strain, in the presence of sodium thiosulphate and glucose. However, other reducing agents have not been evaluated for their role in microalgal lipid accumulation and limited information on this aspect is available in published literature. The citrate synthase representing the pace-making enzyme in the first step of the Citric Acid Cycle (catalyzes the condensation of acetate from acetyl CoA with oxaloacetate to form citrate) is inhibited by high ratios of ATP:ADP, acetyl-CoA:CoA, and NADH:NAD, high concentrations of ATP, acetyl-CoA, and NADH. This is because such metabolic states reveal that the energy supply is high for the cell, hence, our experiments were focused towards addition of metabolic intermediates in the presence of a reducing agent for diverting acetyl CoA to malonyl CoA and thereby towards lipid biosynthesis (Additional file 1: Figure S1).
The objective of the present study was therefore directed towards identifying promising substrate-reducing agent combination which can lead to enhanced lipid quality and productivity in this promising strain of Chlorella sorokiniana under mixotrophic conditions.
Results
Preliminary studies with this organism had shown that that Chlorella sorokiniana grown with glucose mixotrophically was most suitable for enhancing lipid productivity (Ratha et al. 2012). The present investigation was undertaken to evaluate further the role of different reducing agents and metabolic intermediates/substrates on lipid content and FAME profiles under mixotrophic conditions.
Effect on growth and lipid productivity
The effects of two different reducing agents (sodium thiosulphate and methyl viologen) along with six substrates (three carbon sources- sucrose, fructose and glucose; two amino acids- tryptophan and alanine) and sodium pyruvate on growth is presented in Additional file 2: Table S1. The growth was significantly enhanced upto 8th day in all treatments, with the highest values of 2.16 and 1.63 (Abs750) recorded in BBM supplemented with methyl viologen + fructose and sodium thiosulphate + tryptophan on the 8th day of cultivation respectively. The lipid accumulation was evaluated on 4th, 8th and 12th day (Figure 1) and the highest lipid content of 0.27 g L-1 was observed in the samples grown in sodium thiosulphate supplemented with glucose on 8th day of cultivation. In the methyl viologen treatments, the highest values of 0.248 g/L were recorded with fructose. Sodium thiosulphate increased the lipid productivity from 16% in its control to 39%, when glucose was added.
Lipid accumulation in Chlorella sorokiniana MIC-G5 in the presence of reducing agent (Sodium thiosulphate and methyl viologen). (A-C) sodium thiosulphate on 4, 8, 12 d and (D-E) methyl viologen on 4, 8, 12d respectively. A denotes type of treatments; A1 (BBM+sucrose), A2 (BBM+fructose), A3 (BBM+sodium pyruvate), A4 (BBM+tryptophan), A5 (BBM+alanine), A6 (BBM+glucose), A7 (BBM, Control). Mean n = 3 replicates.
Evaluation of sodium thiosulphate and different substrates
The effect of sodium thiosulphate with twelve substrates absorbance (Abs 750), chlorophyll and carotenoids contents are presented in Figure 2 and Additional file 2: Table S2. The highest chlorophyll content of 38.76 μg mL-1 and 4.8 μg mL-1 total carotenoids were recorded on 8th day in sodium thiosulphate supplemented with tryptophan. The lipid productivity (mg g-1) in BBM and BBM+ST gradually increased from 160–190 and 19-20% by the 12th day of incubation (Figure 3). The lipid productivity was enhanced in the presence of tryptophan on the 4th day (390 mg g-1). Highest values were observed in sodium thiosulphate + glucose supplemented culture, upto 8th day. In niacin supplemented culture, no growth was recorded on the 4th day, but by the 8th and 12th day, 18.33 and 27.33% lipids were recorded. The highest values were recorded in 8th day cultures, in terms of lipid productivity (% DCW) in glucose supplemented treatment (39% or 390 mg g-1), and followed by sodium pyruvate (38%) and Vitamin B12 (36%).
Growth of Chlorella sorokiniana MIC-G5 measured in terms of absorbance (750nm) on 4th, 8th, and 12thd of cultivation in BBM containing sodium thiosulphate (1%) and with different substrates (T); T denote type of substrates; T1 (sucrose), T2 (fructose), T3 (glycine), T4 (glycerol), T5 (sodium pyruvate), T6 (biotin), T7 (tryptophan), T8 (vitamin B12), T9 (leucine), T10 (niacin), T11 (alanine), T12 (glucose), T13 (BBM), T14 (sodium thiosulphate). Mean n = 3 replicates.
Lipid accumulation in different treatments (A) on 4th, (B) 8thand (C) 12thday of cultivation in BBM containing sodium thiosulphate (1%) and different substrates - T1 (sucrose ) / T2 (fructose) / T3 (glycine) / T4 (glycerol) / T5 (sodium pyruvate)/ T6 (biotin) / T7 (tryptophan) / T8 (vitamin B12) / T9 (leucine) / T10 (niacin) / T11 (alanine) / T12 (glucose) / T13 (BBM) / T14 (sodium thiosulphate) respectively. Mean n = 3 replicates.
Upscaling of selected promising substrates and effect on lipid productivity and quality
The promising substrates - comprising tryptophan, glucose, sodium pyruvate and Vitamin B12 were taken up further in 5 L flasks containing 2 L medium supplemented with sodium thiosulphate and the selected substrate.
On 4th day, the highest values in terms of turbidity (Abs750 1.26), chlorophyll (8.96 μg mL-1) and carotenoids (1.53 μg mL-1) were recorded in sodium thiosulphate + tryptophan treatment (Additional file 2: Table S3). The highest values of turbidity (Abs750 1.85), chlorophyll (16.6 μg mL-1) were obtained in medium supplemented with sodium thiosulphate alone, but highest lipid content of 0.238 g L-1 was recorded in medium supplemented with glucose and sodium thiosulphate on 8th day of cultivation. Cultures supplemented with sodium thiosulphate + vitamin B12 recorded highest total carotenoids (1.46 μg mL-1) d on 8th day of cultivation (Additional file 2: Table S4). In terms of lipid productivity (% DCW), medium supplemented with glucose and sodium thiosulphate recorded highest values of 33% and sodium pyruvate (31.33%) on the 8th day, followed by tryptophan (30.33%) on the 4th day (Figure 4).
Microscopic analyses
Light microscopic analyses showed that the cells appeared more robust when grown in BBM with sodium thiosulphate and tryptophan for 4th day and in Vitamin B12/ sodium pyruvate supplemented medium for 8th day (Figure 5 A,C,E,G). Nile red staining clearly illustrated the cell size enlargement and increased lipid content under the influence of tryptophan (Figure 5F) and thiosulphate (Figure 5H). The color of staining was enhanced, as observed in Figure 5 (F) and (H).
Light microscopic images (A, C, E and G) and Nile red stained photographs (B, D, F and H) of Chlorella sorokiniana MIC-G5, grown in BBM alone (A, B), or supplemented with sodium thiosulphate and Vitamin B 12 (C, D), or sodium thiosulphate and tryptophan (E, F) or sodium thiosulphate and sodium pyruvate (G, H).
FAME profiles and their analyses
The quantitative analysis of the lipids generated in the form of FAME profiles, revealed that 97.1- 97.7% of the fatty acids belonged to C16-C18 type with USF/SFA ratio in the range of 1.1-2.1 in 4th day and 98.4-98.9% of C16-C18 and ratio between USF: SFA in the range 1.9-2.7 on 8th day of cultivation (Table 1). Highest amount of saturated fatty acids (47.9%) was recorded after growth in BBM + sodium thiosulphate on 4th day. The relative PUFA content ranged from 9.9 to 19 and 8.7 to 24.8 on 4th and 8th day (Tables 1 and 2) respectively. Palmitic acid (16:0) ranged from 25–43.6%, while the linoleic acid (18:2) ranged from 20.2-28.91. In general, the PUFA content reduced in treatments involving only addition of sodium thiosulphate from 60.2% to 42.4% on 4th day and 63.9% to 44.9% on 8th day respectively. The MUFA content increased from 8.0-9.7% and 6.5 to 23.4 % on 4th and 8th day respectively. Addition of tryptophan brought about a 50 and 75% enhancement over sodium thiosulphate supplemented flasks, and control (BBM) on the 4th day. An almost two folds increase in 16:1 and 18:1 fatty acids in sodium thiosulphate + tryptophan treatments, besides 60% enhancement in total lipids (Additional file 3: Figure S2). On 8th day, MUFA content, more than 50% increase was recorded in all the treatments.
Discussion
The cultivation of microalgae, which represent excellent sources of fatty acids, proteins and metabolites with diverse types of activity, are among the biotechnological processes that are receiving increasing attention from industries and researchers (Behrens and Kyle 1996;Courchesne et al. 2009). Microalgae exhibit a great variability in lipid content; oil content can reach up to 80%, and levels of 20-50% are quite common (Powell and Hill 2009). The fatty acids that are produced by microalgae can be extracted and converted into biodiesel (Brown and Zeiler 1993). However, variations are recorded due to different growing conditions and the methods of extraction of lipid and fatty acids, which has questioned the economic viability and feasibility of microalgae as sources of biodiesel. On the other hand, the ability of microalgae to adapt their metabolism to varying culture conditions provides opportunities to modify, control and thereby maximise the formation of targeted compounds with non-recombinant microalgae. Mixotrophy is one such potential method for high-density microalgae cultivation, as cultures display more efficient utilization of energy for biomass productivity (Lee et al. 1987;Liang et al. 2009).
In recent years, in-depth understanding of the many biosynthetic pathways that can be used for the production of biofuel feed stocks or high value bioproducts has emerged, and novel pathways for the production of specific bioenergy carriers are continuously being discovered in a variety of organisms (Liu et al. 2011b;Radakovits et al. 2010). It is considered feasible to generate highly efficient production of microalgal biomass, without the need for light in inexpensive, well-defined mineral medium, typically supplemented with glucose (Bumbak et al. 2011). Researchers have recorded cell densities of more than 100 g L−1 cell dry weight with Chlorella, Crypthecodinium and Galdieria species, while controlling the addition of organic sources of carbon and energy in fedbatch mode. C. sorokiniana is a non-motile, unicellular freshwater green microalga, which is known to accumulate large amounts of protein and lipid (Kay 1991). C. sorokiniana CCTCC M209220 exhibits a rapid growth rate and high oil content when cultured in mixotrophic condition, hence, considered as a promising candidate species for genetic manipulation and enhanced oil yield.
The critical role of Acetyl Co-A, in regulating not only the Kreb’s Cycle, but also as a precursor for fatty acid synthesis is known (Kim 1983;Brennan and Owende 2010). Therefore, inclusion of additives/carbon sources which can enhance acetyl CoA/malonyl CoA pool – which represents the central carbon donor for fatty acid synthesis, can be a possible strategy for enhancing lipid productivity. Analyses of global flux distribution in oleaginous Chlorella protothecoides revealed that in the presence of glucose, the glyoxalate shunt remains inactive; thereby leading to partitioning of carbon only through TCA (Xiong et al. 2010). Therefore, addition of certain metabolic intermediates/carbon sources etc., in a reducing environment (using sodium thiosulphate/methyl viologen) can help to divert metabolic intermediates to malonyl CoA, which represents the first step of fatty acid synthesis, instead of being used in Kreb’s Cycle. Reducing agents such as sodium thiosulphate are known to protect cells by scavenging reactive oxygen produced as a result of biodegradation of exogenous organic carbon and enhance the lipid pool (Feng et al. 2005). It is well known that an increase in reducing power in the cell can lead to an enhancement in the pool of NADH, and citrate synthase is not functional under such conditions (Feng et al. 2005;Mandal and Mallick, 2009). This can lead to diversion of Acetyl CoA to malonyl CoA, thereby increasing lipid pool. Methyl viologen, commonly known as paraquat is a widely employed broad spectrum herbicide, and its toxicity to animals and man is mediated by lipid peroxidation; however its role in lipid accumulation has not been investigated (Bus Aust and Gibsont 1976).
The present study was therefore directed towards understanding the effect of different substrates/metabolic intermediates and reducing agents sodium thiosulphate and methyl viologen) on enhancing lipid productivity of this promising Chlorella sp. In the present investigation, comparative growth kinetics and lipid productivity in the presence of two reducing agents- sodium thiosulphate and methyl viologen provided interesting results. Growth studies revealed that tryptophan was most productive in the presence of sodium thiosulphate, but with methyl viologen, fructose performed better. Lipid productivity was significantly higher in tryptophan supplemented cultures with both reducing agents. Sodium thiosulphate is known to play a dual role as a potent antioxidant and chelator of calcium and other toxic substances and is classified by the FDA as a direct food substance affirmed as generally recognized as safe. On the other hand, methyl viologen, undergoes redox cycling in vivo, being reduced by an electron donor such as NADPH, before being oxidized by an electron receptor such as dioxygen to produce superoxide, a major ROS (reactive oxygen species). It inhibits photosynthesis, besides being a groove-binding DNA ligand. In the present study, the low concentration used did not inhibit growth or lipid accumulation, but stringent monitoring may need to be employed for using methyl viologen, as compared to the safety of sodium thiosulphate utilization. Also, comparing the lipid accumulation in the presence of both reducing agents, sodium thiosulphate proved more effective, recording the highest values of 0.273 g/L on 8th day.
Although microalgae grow with various carbonaceous compounds, glucose is considered the preferred carbon source, because of its ease of handling, availability and safety (Lee 2004;Perez-Garcia et al. 2011;Sun et al. 2008). Tryptophan, glycine and yeast extract have also been evaluated for their potential to enhance growth or product formation (Shen et al. 2010). Acetate and ethanol are considered possible alternatives but, because of their respective corrosive effects or high flammability, are only used when an exceptional productivity enhancement is achieved (de Swaaf et al. 2003). Although the cost of (pure) glucose is high for microalgal production, valorisation of the biomass as animal and fish feed supplements, after use of biomass as biodiesel may be promising from an industrial point of view (Chisti 20072008;Brennan and Owende 2010).
Further experiments were undertaken to evaluate the lipid productivity (expressed as % lipids, on DCW basis) - a product of biomass productivity and lipid content, using 12 different carbon sources/ metabolic intermediates, along with sodium thiosulphate. Supplementation with sodium thiosulphate (1%) only, enhanced the lipid productivity from 16.66 (BBM) to 19.66% on 4th day and 23.66% in 8th day cultures, which is equivalent to 18% and 42% increase over control respectively. On 4th day, highest lipid productivity of 39% was recorded with tryptophan supplementation. Highest lipid productivity of 39.33% in glucose, followed by 38.8% in sucrose, and 38.00% in sodium pyruvate, 35.66% in fructose and 36.0% in vitamin B12 was recorded on 8th day. But, the most significant enhancement in lipid productivity was observed in glucose, which was up to 39.33% on 8th day and in case of tryptophan up to 39.0% on the 4th day. The promising feature recorded was that the increase over control was highest in tryptophan in 4th day (57.26%) as compared to 8th day (10.93%). Dry cell weight or DCW (1086.6μg/ml) and lipid content (255.0 μg/ml) were recorded on 8th day, which represented a significant increase over 8th day control. In the case of glucose, maximum lipid productivity was observed in 8th day which represents an increase of 51.69% over control and 49.14% over 4th day values. In our earlier study, this strain exhibited 32.33% lipid productivity when grown mixotrophically with 2% glucose for 18 days; however, in the present study, an enhancement in lipid productivity to 39.33% was recorded by growing only for 8 days in medium supplemented with 0.1% glucose and 1% sodium thiosulphate.
Among the various carbon substrates, glucose, in particular, is used for the production of high-value compounds, where the processes need to be reproducible for prospective regulatory approval for pharmaceutical manufacture. The ability of a number of microalgal species to grow with organic carbon substrate has been demonstrated previously (Droop 1974). However, the number of current commercially important microalgae that are capable of growth on organic carbon substrates in the dark, and where experience of fedbatch cultivation has been gained, is very limited. The role of carbon substrates lead to different biomass/ substrate yields and also affect the formation of the targeted product through flux distribution is well investigated (Fan et al. 2012). The ability of microalgae to use organic carbon as an energy source is important because it can minimize the inhibitory effects of seasonal and diurnal light limitation on growth in outdoor cultures. A considerable number of algae, for example Chlamydomonas, Spirulina, Chlorella, Galdieria, Scenedesmus, and Micractinium, can grow mixotrophically and heterotrophically in the presence of organic matter such as carbohydrates and acetate (Brennan and Owende 2010;Lee 2004). The biochemical composition of microalgae under mixotrophy may not only improve biomass production, but also augment lipid content, which is very important for the production of biodiesel from microalgae (Xu et al. 2004), which was further illustrated in our study.
Majority of research work undertaken on mixotrophic cultivation of microalgae using limited amounts of glucose have shown it to be an effective method to obtain high microalgal biomass productivity and beneficial for increasing lipid and protein accumulation, especially for strains of Chlorella sorokiniana (Wan et al. 2011). Enhanced photosynthesis, on addition of glucose has been reported in several algae, including cyanobacteria (Wang et al. 2000). In the present study, all the substrates enhanced chlorophyll upto 8th day of growth, which is in agreement with observations on glucose-grown Chlorella vulgaris UAM101 cells, acetate-grown Chlamydomonas reinhardtii cells and fructose-grown Anabaena variabilis ATCC 29413 cells (Valiente et al. 1992;Courchesne et al. 2009). This may be because glucose promoted the donation of electrons to the plastoquinone pool from the respiratory substance, and the transforming of energy was promoted by photosynthetic system, which provided the energy needed by anabolism of cells caused by addition of glucose to the medium (Wang et al. 2000). But few reports revealed that glucose could reduce photochemical efficiency of photosystem II (PS II) and levels of the PS II reaction centre proteins (Liu et al. 2009).
The cell size and lipid content of Chlorella cells grown with different substrates were compared using light microscopy and Nile Red staining. Nile red, (9-diethylamino-5H-benzo[α]-phenoxazine-5-one) is a fluorescent hydrophobic dye, that fluoresces intensely, and in a range of colors, when in contact with organic solvents and hydrophobic lipids. Over the past two decades, there have been a number of papers reporting Nile red methods for the determination of intracellular lipids in different organisms. Most of these methods used relative fluorescence intensities to compare or estimate the intercellular lipid contents in these organisms, and they gave correlations between the fluorescence emission and cell number or lipid content (Chen et al. 2011). But in our investigation, light microscopic images with Nile red stain exhibited a good correlation of stain intensity with lipid content. It was found that the cell solidity and size changed after addition of substrates, tryptophan or sodium pyruvate in BBM with sodium thiosulphate. By using low-density cultures of Botryococcus braunii under both light and dark culture conditions, (Tanoi et al. 2011) found that both cell and colony size increased significantly, accompanied by increase in the size of oil granules in cells, when the cells were cultured with glucose. This finding was consistent with other Botryococcus strains, as recorded by (Zhang and Kojima1988) who recorded increase in colony size of B. braunii with high light intensity. Glucose seems to play a role in to bringing about changes in oil distribution inside the cells or by enhancing oil accumulation in cells and enlargement of the granule. However, the mechanism underlying the induction of large cell and granule size is unclear and the relationship between cell and granule size and oil content requires further investigation. But, based on our findings and available information, Nile red staining can be proposed as a simple, rapid, and inexpensive sentinel screen for lipids and identifying the potential biodiesel sources.
(Feng et al. 2005) were among the earliest researchers to illustrate the utility of appropriate concentrations of sodium thiosulphate and glucose in enhancing lipid accumulation in Chlorella sp. They observed an increase in polar lipids in the presence of sodium thiosulphate, which was however modified to similar values as in control on addition of both glucose and sodium thiosulphate. However, in depth analyses of individual classes of fatty acids was not undertaken. Phylogenetic relatives of Chlorella zofingiensis, such as Chlorella vulgaris and C. protothecoides were reported to accumulate high amounts of lipids when cultivated under heterotrophic conditions (Liu et al. 2011a;Hsieh and Wu 2009). However, knowledge on Chlorella species to accumulate lipids and fatty acids under different growth modes remains largely unknown.
(Petkov and Garcia 2007) found that the fatty acid composition of 14:0, 16:0; 16:1; 16:2, 16:3, 18:0, 18:1, 18:2 and 18:3 can be a useful marker for identifying Chlorella. In our investigation, Fatty acid Methyl ester (FAME) profiles were generated from upscaling experiment with selected promising samples. The analyses revealed highest qualities of saturated fatty acids (47.9%) in medium supplemented only with sodium thiosulphate on 4th day, with a concurrent 15-20% reduction in Poly unsaturated fatty acids (PUFA) and 9% enhanced Mono unsaturated fatty acids (MUFA) content. 16:1 and 18:1 were enhanced from 2.7 to 7.9% and 5.1 to 9.8% respectively, in BBM + sodium thiosulphate + tryptophan. However, relative PUFA values were two folds higher in this treatment as compared to control or BBM + sodium thiosulphate. FAME profiles showed interesting changes in the presence of sodium thiosulphate with/without substrates on the 8th day. PUFA content decreased from 63.9 to 44.9 in BBM+ST+Glucose, while MUFA content enhanced from 6.5 to 19.7 in BBM+ST+sodium pyruvate. Highest total lipids of 33% were recorded in BBM+ST+Glucose, which recorded four folds enhancement in MUFA, along with 33% decrease in PUFA. Our analyses are in consonance with earlier studies (D’ Oca et al. 2011;Liu et al. 2011a2011b) who also reported the predominance of 18:3 and 18:2 fatty acids in Chlorella species; additionally in our samples, 16:0 was found to be a major component. The absence of fatty acids with odd number of carbon atoms (related with bacteria) provides proof for an axenic culture being used for experiments and analyses (Petkov and Garcia, 2007).
Unlike photoautotrophic cells, heterotrophic cells are known to channel excessive carbon for the biosynthesis of storage lipids, e.g., neutral lipids, instead of converting carbon into membrane lipids for building photosynthetic apparatus, exhibiting a much higher amount of oleic acid (Liu et al. 2011a2011b). This in turn, helps to balance the oxidative stability and low-temperature properties and promotes the quality of biodiesel (Knothe 2009). In our investigation, a significant enhancement in oleic acid (18:1) was recorded on both 4th and 8th day, with a fivefold enhancement in BBM+ST+Glucose on 8th day.Wan et al.(2011) found that Chlorella sorokiniana was well suited for lipid production based on its high biomass production rate and lipid content reaching 51% during mixotrophy. Real-time PCR assays revealed increased expression levels of acc D (heteromeric acetyl-CoA carboxylase beta subunit), and reduced levels of rbc L (ribulose 1, 5- bisphosphate carboxylase/oxygenase large subunit). Similar to higher plants, microalgae synthesize fatty acids in the chloroplast using a single set of enzymes, of which acetyl-CoA carboxylase (ACCase) is a rate-limiting enzyme for fatty acid synthesis while stearoyl ACP desaturase plays an important role in determining the ratio of unsaturated to saturated fatty acids (Lane et al. 1974). Tryptone, glycine and yeast extract have also been evaluated for their potential to enhance growth or product formation (Shen et al. 2010). Amino acids are known to enter the TCA via several intermediates and their inclusion in medium can enhance the Acetyl CoA indirectly. Additionally, in the presence of a reducing agent such as sodium thiosulphate, as recorded in our investigation, a diversion towards malonyl CoA and fatty acid synthesis may have taken place. Furthermore, several microalgae that are grown in pure culture with mineral medium require supplementation with the vitamins (Droop 2007), and the enhancement observed with Vitamin B12 supplementation may be related to enhanced growth.
There is a need for the development of appropriate strategies for exploitation of the flexibility of biomass composition within its upper and lower limits as defined by different culture conditions and/or the altered supply of chemical elements in the culture medium. This as a means of enhancing biomass and/or product formation is one of the major challenges in the area of biofuels. Research efforts worldwide have indicated that this needs to be specific for each algal strain. Our investigation has clearly brought out the promise of using sodium thiosulphate along with selected metabolic intermediates/substrates-glucose, tryptophan, sodium pyruvate and vitamin B12 in modulating significant changes in lipid content and FAME profiles of Chlorella sorokiniana, especially, the reduction in PUFA and enhanced oleic acid content which further emphasize their significance for enhanced lipid accumulation and biodiesel production.
Methods
The axenic culture of green alga Chlorella sorokiniana Shih. et Krauss MIC-G5 was obtained from the culture collection of the Division of Microbiology, IARI, New Delhi. The culture was routinely maintained through 2% inoculation into 150 ml Erlenmeyer flasks containing 40 ml Bold’s Basal Medium (BBM). A temperature at 25±2°C under a photoperiod of 16:8 h light and dark at light intensity of 33 μmol photon /m2/ s PAR (Photosynthetically Active Radiation) was used for growth. The culture was also grown in BBM supplemented with sodium thiosulphate (1000 ppm / 1% / 63 mM) and methyl viologen (0.01 ppm / 0.00001%), alone and supplemented with six selected substrates- sucrose (2%), fructose (2%), sodium pyruvate (0.1%), tryptophan (0.1%), alanine (0.1%), glucose (0.1%). One selected reducing agent was used further in BBM supplemented with 12 different substrates: sucrose (2%), fructose (2%), sodium pyruvate (0.1%), glycine (0.1%), glycerol (0.1%), biotin (0.1%), tryptophan (0.1%), leucine (0.1%), niacin (0.01%), alanine (0.1%), glucose (0.1%), Vitamin B12 (0.001%). The stock solutions of these compounds were prepared and filter sterilized using 0.22 μm pore size filter membrane, before addition into the autoclaved medium. Preliminary experiments were undertaken to decide the optimal concentration of the substrates used (Momocha 2012). The flasks were hand shaken two to three times daily to maintain proper mixing. Further, the promising combinations were upscaled in 5 L Haffkine flasks, containing 2 L medium and aeration (2 L/min) was provided for effective mixing under stationary conditions. The culture grown in BBM served as control.
Growth attributes, carotenoids and carbohydrates
The cell concentration was determined by measuring the changes of turbidity in the culture medium (Absorbance at 750 nm: Abs750) using a UV–VIS spectrophotometer (Perkin Elmer model Lambda) upto 12th day. Dry cell weight (DCW) was determined gravimetrically using a known amount of algal culture by centrifugation at 3000 g for 10 min. The algal pellet was washed twice with distilled water, and the harvested biomass was dried at 70°C in an oven until it reached a constant weight. To estimate chlorophyll, 10 ml of algal culture was centrifuged at 5000 g for 10 min and the pellet was treated with known volume of methanol and kept in a water bath for 30 min at 60°C. The absorbance of the pooled extracts was measured at 652 and 665 nm for chlorophyll (a + b) and at 470 nm for carotenoids. The concentrations were estimated using standard equations (Lichtenthaler 1987). Chlorophyll, carotenoids and carbohydrates were expressed (%), in terms of dry cell weight (DCW). All the experiments were carried out using triplicate samples.
Extraction and estimation of lipids
The total lipids were extracted from microalgal biomass using a modified method ofDittmer and Wells (1969). The lipids were extracted with mixture of chloroform and methanol (2:1, v/v), and then separated into chloroform and aqueous methanol layers by the addition of methanol and water to give a final solvent ratio of chloroform: methanol: water, 2:2:0.8. The organic layer containing the lipids was washed with 1% NaCl solution, collected and evaporated to dryness under vacuum. Activated charcoal was used to remove all pigments, before lipid content was determined gravimetrically. All the experiments were carried out in triplicate.
FAME analyses
The fatty acid composition of algal fatty acid methyl esters were determined by modification of the Association of Official Analytical Chemists (AOAC) Official Method 948.15 Fat (Crude) in Seafood, Acid Hydrolysis method, 1995 (Hungerford 1995). Fatty acid methyl esters of the oil were prepared by refluxing the dried sample at 70°C for 3 h in 2% sulphuric acid in methanol. The esters were extracted into ethyl acetate, washed free of acid and passed over anhydrous sodium sulphate. The ethyl acetate extracts were further concentrated using a rotary evaporator. The fatty acid composition was analyzed using an Agilent 6890 N series gas chromatography equipped with FID detector on a split injector. A fused silica capillary column (DB-225, 30 × 0.32 m i.d., J & W Scientifics, USA) was used with the injector and detector temperature maintained at 220°C and 255°C respectively. The oven temperature was programmed at 160°C for 2 min and finally increased to 230°C at 4°C/min. The carrier gas was nitrogen at a flow rate of 1.5 mL/min. The area percentages were recorded with a standard HP Chemstation Data System. Relative PUFA content is expressed as the ratio between the percentages of the different fatty acids: saturated (SATs), monounsaturated (MUFAs) and UFAs, using the formula (PUFA/SAT+MUFA). The unsaturation index was also determined by multiplying the percentage of each fatty acid by the number of double bonds present in the molecule.
Microscopic analyses
The cells were observed using Zeiss Model Axio Scope. A1, after incubation with different substrates. Nile red (9-(Diethylamino) -5H benzo [α] phenoxazin- 5-one) staining was used to detect intracellular lipid droplets (Greenspan et al. 1985).
Statistical analyses
The statistical analyses were performed using the software SPSS 10. One-way analysis of variance (ANOVA) was used to evaluate the differences among the treatments. In case, the ANOVA effects were significant, comparisons between the different means were made to quantify and evaluate the source of variation, and CD (Critical Differences) and SEM (Standard error of Means) values were calculated at 0.05 P level. SD (Standard deviation) values are depicted in the graphs as error bars. The superscripts in Tables denote the rankings based on Duncan’s Multiple Range test and different alphabets denote significantly different values, as analyzed using SPSS.
References
Becker W: Microalgae in human and animal nutrition. In Handbook of microalgal culture. Edited by: Richmond A. Blackwell, Oxford; 2004:312-351.
Behrens PW, Kyle DJ: Microalgae as a source of fatty acids. J Food Lipids 1996, 3: 259-272. 10.1111/j.1745-4522.1996.tb00073.x
Brennan L, Owende P: Biofuels from microalgae – a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energy Rev 2010, 14: 557-577. 10.1016/j.rser.2009.10.009
Brown ML, Zeiler KG: Aquatic biomass and carbon dioxide trapping. Energy Conv Managmt 1993, 34: 1005-10013. 10.1016/0196-8904(93)90048-F
Bumbak F, Cook S, Zachleder V, Hauser S, Kovar K: Best practices in heterotrophic high-cell-density microalgal processes: achievements, potential and possible limitations. Appl Microbiol Biotechnol 2011, 91: 31-46. 10.1007/s00253-011-3311-6
Bus Aust SD, Gibsont JE: Paraquat Toxicity: Proposed Mechanism of Action Involving Lipid Peroxidation. Environ Health Perspect 1976, 16: 139-146.
Ceron Garcia MC, Sanchez Miron A, Fernandez Sevilla JM, Molina Grima E, Garcia Camacho F: Mixotrophic growth of the microalgae Phaeodactylum tricornutum , influence of different nitrogen and organic carbon source on productivity and biomass composition. Process Biochem 2005, 40: 3125-3131. 10.1016/j.procbio.2005.03.006
Chen W, Sommerfeld M, Hu Q: Microwave-assisted Nile red method for in vivo quantification of neutral lipids in microalgae. Bioresour Technol 2011, 102: 135-141. 10.1016/j.biortech.2010.06.076
Chisti Y: Biodiesel from microalgae beats bioethanol. Trends Biotechnol 2008, 26: 126-131. 10.1016/j.tibtech.2007.12.002
Chisti Y: Biodiesel from microalgae. Biotechnol Adv 2007, 25: 294-306. 10.1016/j.biotechadv.2007.02.001
Courchesne NMD, Parisien A, Wang B, Lan CQ: Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. Biotechnol J 2009, 141: 31-41. 10.1016/j.jbiotec.2009.02.018
de Swaaf ME, Pronk JT, Sijtsma L: Fed-batch cultivation of the docosahexaenoic-acid-producing marine alga Crypthecodinium cohnii on ethanol. Appl Microbiol Biotechnol 2003, 61: 40-43.
D’ Oca MGM, Vegas CV, Lemoes JS, Miyasaki EK, Moron-Villarreyes JA, Primel EG, Abreu PC: Production of FAMEs from several microalgal lipidic extracts and direct transesterification of Chlorella pyrenoidosa . Biormass Bioenergy 2011, 35: 1533-1538. 10.1016/j.biombioe.2010.12.047
Dittmer JC, Wells MA: Quantitative and qualitative analysis of lipid and lipid components. Meth Enzymol 1969, 14: 482-530.
Droop MR Stewart WDP. In Heterotrophy of carbon. Algal physiology and biochemistry, Blackwell Oxford; 1974:530-559.
Droop MR: Vitamins, phytoplankton and bacteria: symbiosis or scavenging? J Plankton Res 2007, 29: 107-113. 10.1093/plankt/fbm009
Fan J, Yan C, Andre C, Shanklin J, Schwender J, Xu C: Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii . Plant Cell Physiol 2012, 53: 1380-1390. 10.1093/pcp/pcs082
Feng FY, Yang W, Jiang GZ, Xu YN, Kuang TY: Enhancement of fatty acid production of Chlorella sp. (Chlorophyceae) by addition of glucose and sodium thiosulphate to culture medium. Process Biochem 2005, 40: 1315-1318. 10.1016/j.procbio.2004.06.011
Greenspan P, Mayer EP, Fowler SD: Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 1985, 100: 965-973. 10.1083/jcb.100.3.965
Hsieh CH, Wu WT: Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 2009, 100: 3921-3926. 10.1016/j.biortech.2009.03.019
Hungerford JM: Fish and other marine products. In Official methods of analysis of AOAC international. 16th edition. Edited by: Cunniff P. AOAC International, Arlington, VA; 1995:1-30.
Jang YS, Park JM, Choi S, Choi YJ, Seung DY, Cho JH, Lee SY: Engineering of microorganisms for the production of biofuels and perspectives based on systems metabolic engineering approaches. Biotechnol Adv 2011, 71: 1-6.
Kay RA: Microalgae as food and supplement. Crit Rev Food Sci Nutr 1991, 30: 555-573. 10.1080/10408399109527556
Kim KH: Regulation of acetyl-CoA carboxylase. Curr Top Cell Regul 1983, 22: 143-176.
Knothe G: Improving biodiesel fuel properties by modifying fatty ester composition. Ener Environ Sci 2009, 2: 759-766. 10.1039/b903941d
Lane MD, Moss J, Polakis SE: Acetyl-CoA carboxylase. Curr Top Cell Regul 1974, 8: 138-195.
Lee HY, Erickson LE, Yang SS: Kinetics and bioenergetics of light-limited photoautotrophic growth of Spirulina platensis . Biotechnol Bioeng 1987, 29: 832-843. 10.1002/bit.260290705
Lee YK: Algal nutrition: heterotrophic carbon nutrition. In Handbook of microalgal culture: Biotechnology and Applied Phycology. Edited by: Richmond A. Blackwell, Oxford; 2004:116-124.
Liang Y, Sarkany N, Cui Y: Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 2009, 31: 1043-1049. 10.1007/s10529-009-9975-7
Lichtenthaler HK: Chlorophylls and carotenoids: pigments of photosynthetic biomembranes, In: Packer L, Douce R, (eds). Meth Enzymol 1987, 148: 350-382.
Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F: Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis : Assessment of algal oils for biodiesel production. Bioresour Technol 2011, 102: 106-110. 10.1016/j.biortech.2010.06.017
Liu X, Duan S, Li A, Xu N, Cai Z, Hu Z: Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum . J Appl Phycol 2009, 21: 239-246. 10.1007/s10811-008-9355-z
Liu X, Sheng J, Curtiss R: Fatty acid production in genetically modified cyanobacteria. Proc Natl Acad Sci 2011, 108: 6899-6904. 10.1073/pnas.1103014108
Mandal S, Mallick N: Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 2009, 84: 281-291. 10.1007/s00253-009-1935-6
Miao X, Wu Q: High quality biodiesel production from heterotrophic microalgal oil. Bioresour Technol 2006, 97: 841-846. 10.1016/j.biortech.2005.04.008
Ördög V, Stirk WA, Bálint P, Staden van J, Lovász C: Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J Appl Phycol 2012, 24: 907-914. 10.1007/s10811-011-9711-2
Perez-Garcia O, Escalante FM, De-Bashan LE, Bashan Y: Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 2011, 45: 11-36. 10.1016/j.watres.2010.08.037
Petkov G, Garcia G: Which are the fatty acids of the green alga Chlorella . Biochem Syst Ecol 2007, 35: 281-285. 10.1016/j.bse.2006.10.017
Powell EE, Hill GA: Economic assessment of an integrated bioethanol biodiesel-microbial fuel cell facility utilizing yeast and photosynthetic algae. Chem Eng Res Des 2009, 87: 1340-1348. 10.1016/j.cherd.2009.06.018
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC: Genetic engineering of algae for enhanced biofuel production. Eukary Cell 2010, 9: 486-501. 10.1128/EC.00364-09
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA: Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 2007, 98: 560-564. 10.1016/j.biortech.2006.02.007
Ratha SK, Babu S, Renuka N, Prasanna R, Prasad RBN, Saxena AK: Exploring nutritional modes of cultivation for enhancing lipid accumulation in microalgae. J Basic Microbiol 2012. 10.10021/jobm.20120001
Schenk P, Thomas-Hall S, Stephens E, Marx U, Mussgnug J, Posten C: Second generation biofuels: high-efficiency microalgae for biodiesel production. Bio Energy Res 2009, 1: 20-43.
Shen Y, Yuan W, Pei Z, Mao E: Heterotrophic culture of Chlorella protothecoides in various nitrogen sources for lipid production. Appl Biochem Biotechnol 2010, 160: 1674-1684. 10.1007/s12010-009-8659-z
Sun N, Wang Y, Li YT, Huang JC, Chen F: Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process Biochem 2008, 43: 1288-1292. 10.1016/j.procbio.2008.07.014
Takagi M, Karseno YT: Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 2006, 101: 223-226. 10.1263/jbb.101.223
Tanoi T, Kawachi M, Watanabe MM: Effects of carbon source on growth and morphology of Botryococcus braunii . J Appl Phycol 2011, 23: 25-33. 10.1007/s10811-010-9528-4
Valiente EF, Nieva M, Avendano MC, Maeso ES: Uptake and utilization of fructose by Anabaena variabilis ATCC 29413 effect on respiration and photosynthesis. Plant Cell Physiol 1992, 33: 307-313.
Wan M, Liu P, Xia J, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie Z, Qiu G: The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana . Appl Microbiol Biotechnol 2011, 91: 835-844. 10.1007/s00253-011-3399-8
Wang YH, Ye JY, Mi HL, Li YG, Zhang CL: Relationship between the growth of Synechococcus sp. PCC6803 on medium with glucose and the photosynthetic energy transformation. Acta Bot Sinica 2000, 42: 1122-1125.
Xiong W, Liu L, Yang C, Wu Q: 13 C tracer and Gas chromatography–mass spectrometry analyses reveal metabolic flux distribution in the oleaginous microalga Chlorella protothecoides . Plant Physiol 2010, 154: 1001-1011. 10.1104/pp.110.158956
Xu F, Cong W, Cai ZL, Ouyang F: Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J Appl Phycol 2004, 16: 499-50. 10.1007/s10811-004-5520-1
Zhang K, Kojima E: Effect of light intensity on colony size of microalga Botryococcus braunii in bubble column photobioreactors. J Ferment Bioeng 1988, 86: 573-576.
Acknowledgments
The authors are thankful to the Post Graduate School and Director, Indian Agricultural Research Institute, New Delhi, India for providing for fellowship towards M.Sc. program of the senior author. The authors are also grateful to the Division of Microbiology, Indian Agricultural Research Institute (IARI), New Delhi and Indian Council of Agricultural Research for providing the facilities and financial support, to undertake the investigations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
MN and SKR undertook the experimentation and analyses of data; RP formulated the experiments, supervised the research work and wrote the manuscript; AKS conceived the idea and provided critical suggestions; DWD provided useful suggestions; Chandragiri Sarika and Rachapudi Badari Narayana Prasad undertook the preparation of FAMEs of the samples and their analyses. All the authors have approved the submission of the manuscript.
Electronic supplementary material
40064_2012_14_MOESM2_ESM.doc
Additional file 2: Table S1. Comparative growth kinetics of Chlorella sorokiniana MIC-G5 in grown in sodium thiosulphate/methyl viologen supplemented with along with substrates. Table S2. Chlorophyll and carotenoids of Chlorella sorokiniana MIC-G5 grown in BBM containing sodium thiosulphate and different substrates. Table S3. Growth, chlorophyll and carotenoids of Chlorella sorokiniana MIC-G5 grown in Haffkine flasks with different substrates on 4th day of cultivation. Table S4. Growth, chlorophyll and carotenoids of Chlorella sorokiniana MIC-G5 grown in under Haffkine flasks with different substrates on 8th day of cultivation. (DOC 61 KB)
40064_2012_14_MOESM3_ESM.pdf
Additional file 3: Figure S2. Chromatograph depicting FAME profile of Chlorella sorokiniana grown in BBM containing sodium thiosulphate (1%) and tryptophan. (PDF 950 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ngangkham, M., Ratha, S.K., Prasanna, R. et al. Biochemical modulation of growth, lipid quality and productivity in mixotrophic cultures of Chlorella sorokiniana. SpringerPlus 1, 33 (2012). https://doi.org/10.1186/2193-1801-1-33
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-1-33