Abstract
Background
Small supernumerary marker chromosomes (sSMC) are extra structurally abnormal chromosomes that cannot be unambiguously identified with conventional chromosome banding techniques. These marker chromosomes may cause an abnormal phenotype or be harmless depending on different factors such as genetic content, chromosomal origin and level of mosaicism. When a sSMC is found during prenatal diagnosis, the main question is whether the sSMC contains euchromatin since in most cases this will lead to phenotypic abnormalities. We present the use of Multiplex Ligation Dependent probe Amplification (MLPA) for rapid distinction between non-euchromatic and euchromatic sSMC.
Results
29 well-defined sSMC found during prenatal diagnosis were retrospectively investigated with MLPA with the SALSA MLPA centromere kits P181 and P182 as well as with the SALSA MLPA telomere kits P036B and P070 (MRC Holland BV, Amsterdam, The Netherlands). All unique-sequence positive sSMC were correctly identified with MLPA, whereas the unique-sequence negative sSMC had normal MLPA results.
Conclusions
Although different techniques exist for identification of sSMC, we show that MLPA is a valuable adjunctive tool for rapidly distinguishing between unique-sequence positive and negative sSMC. In case of positive MLPA results, genetic microarray analysis or, if not available, targeted FISH can be applied for further identification and determination of the exact breakpoints, which is important for prediction of the fetal phenotype. In case of a negative MLPA result, which means that the sSMC most probably does not contain genes, the parents can already be reassured and parental karyotyping can be initiated to assess the heritability. In the mean time, FISH techniques are needed for determination of the chromosomal origin.
Similar content being viewed by others
Background
The finding of a sSMC presents a challenge in prenatal diagnosis particularly for prediction of the clinical consequences which will depend on its genetic content, familial occurrence, level of mosaicism and chromosomal origin [1–5] and parental origin of the sSMC related sister chromosomes [6]. According to the review of Liehr and Weise [7] sSMC are to be expected in 0.075% of all analysed prenatal cases. In case of fetal ultrasound abnormalities this frequency rises to 0.204%, which is 2.7x higher than in the general prenatal population.
Before the introduction of FISH for cytogenetics, identification studies involved the use of classical staining techniques such as GTG, QFQ, Ag-NOR, CBG and DA-DAPI [1]. Nowadays, different molecular cytogenetic techniques have been developed for identification of sSMC, such as FISH techniques like cenM- and subcenM-FISH [8, 9], multicolour banding (MCB)[10], microdissection followed by reverse FISH [11, 12], spectral karyotyping (SKY) [13], M-FISH [14] and genomic microarray analysis [15, 16]. These techniques are expensive and not available in all cytogenetic laboratories [17].
In this paper we present the use of Multiplex Ligation Dependent Probe Amplification (MLPA) (MRC Holland, Amsterdam, The Netherlands) as an alternative approach for identification of euchromatic sSMC. On the basis of 29 well characterised sSMC we show that MLPA can rapidly distinguish between unique sequence positive and negative sSMC, which is the most important task when finding a sSMC prenatally. However, other molecular cytogenetic techniques will remain necessary for determining the exact genetic content in case of a positive sSMC, whereas FISH techniques will still be indispensible for identification studies in case of an unique sequence negative sSMC.
Methods
Samples
We retrospectively tested the value of MLPA for sSMC identification on 29 well-defined sSMC found during prenatal diagnosis in amniotic fluid (AF)(n = 26) and chorionic villi (CV)(n = 3) (see table 1 and additional file 1). For routine cytogenetics GTG-banding was used in all cases according to standard techniques. Mostly, sSMC identification was done with FISH, sometimes after additional staining with DA-DAPI [18] (see additional file 1). In 23/29 cases the sSMC was present in all investigated cells. In 6/29 cases mosaicism was found in cultured CV or AF cells with the level of mosaicism varying between 30 and 89% (table 1).
FISH
Metaphase FISH was performed according to standard techniques. The probes that were used for identification were whole chromosome paints (wcp's)(Kreatech Diagnostics, Ankeveen, The Netherlands and Euro-Diagnostica AB, Nijmegen, The Netherlands), centromere probes (Abbott Molecular Inc., Des Plaines, USA; Resources for Molecular Cytogenetics, Bari, Italy (http://www.biologia.uniba.it/rmc/) and partially received from several investigators), subtelomere-probes [19], locus-specific probes (SNRPN from Cytocell Ltd, Cambridge, UK; LSI TEL AML1 from Abbott Molecular Inc., Des Plaines, USA and others like 102D10 (CES-probe), Y41 and Y11H11 (15q11), r521 (rDNA-probe) were kindly provided by several investigators) and subcentromere-BAC clones that were selected from the University of California Santa Cruz (UCSC) genome browser (http://genome.ucsc.edu) (see additional file 1).
FISH slides were analyzed using the Axioplan 2 Imaging microscope (Zeiss), and images were collected using Isis Software System (Metasystems).
Sample preparation for MLPA and SNP array
DNA was isolated from 4 ml of uncultured AF or from cultured CV or AF cells. AF cells were cultured by the in situ method and CV were cultured using trypsin-EDTA and collagenase treatment as described previously [20]. DNA-isolation from uncultured AF cells was done with the Chemagic Magnetic Separation Module I (Chemagen, Baesweiler, Germany). DNA isolation from cultured cells was performed using the QIAamp DNA Mini Kit from Qiagen (Hilden, Germany) or Puregene DNA Purification Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
MLPA-reaction and data analysis
-
4
SALSA MLPA kits were used: two centromere kits, P181 and P182, and two telomere-kits, P036B and P070 (http://www.mlpa.com/WebForms/WebFormMain.aspx). Between 20 and 70 ng of DNA was used in a MLPA reaction which was performed on a PCR thermocycler with heated lid (Biometra Thermal Cycler, Westburg, The Netherlands). MLPA reaction and data analysis were performed as described earlier [21]. In order to enable the detection of chromosomal mosaicism as was seen in 6/29 cases, we calculated own cut off values (median ± 2x SD) for the different probes on the different chromosomes for all four MLPA-kits on the basis of 95 (P181), 91 (P182), 104 (P036B) and 105 (P070) normal samples (see table 2).
SNP array, data analysis and interpretation
In two cases (cases 13 and 25) a SNP array (HumanCytoSNP-12, Illumina) was performed because of discrepancies between the results of GTG/FISH and MLPA. 200 ng of DNA isolated from cultured cells was used in both cases. DNA amplification, tagging and hybridisation were performed according to the manufacturer's protocol. Array slides were scanned on the iScan Reader (Illumina). Data analysis was performed using Genome Studio version 2010.1 (Illumina). The HapMap control set provided by the manufacturer was used as a control.
Results
Unique sequence positive sSMC
All unique sequence positive sSMC (Table 1) were correctly identified with MLPA with the centromere kits (cases 1 and 20), telomere kits (cases 6 and 13) or both (cases 3-5, 7, 11, 12, 14, 21-25) (see table 3) confirming GTG/FISH results. There were no false negative cases.
a. Non-mosaic cases
The relative probe signals in most non-mosaic cases (see table 1) correctly discriminated between 3 and 4 copies of the investigated probes, with relative probe signals > 1.3 and < 1.5 for a trisomy and > 1.6 for a tetrasomy (see table 3). In 3/23 non-mosaic cases a discrepancy was found.
Case 1: Although amplification of EPHA3 confirmed the results of FISH and DNA marker studies (sSMC=der(3)(:p12.2->cen:) in case 1, the relative probe signals of the 3p11.2 marker EPHA3 in both centromere-kits were not above 1.3 (1.178 in P181 and 1.226 in P182) as would be expected in a full blown case. However, they were clearly above the normal cut off value of 1.077 for both kits probably indicating loss of the sSMC in part of the cells and therefore mosaicism at the time that DNA for MLPA was isolated from the cell cultures.
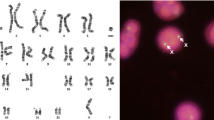
Case 13 showed a full blown neo(15) in cultured AF cells (8 cell clones investigated) (see Figure 1a), which is an analphoid sSMC with a neocentromere and consisting of two copies of the distal end of chromosome 15q. However, the relative probe signals of the 15q-subtelomere probes were only 1.45 and 1.428 in respectively P036B and P070, indicative of a trisomy but not a tetrasomy as expected (Figure 1b).
Identification of the sSMC in case 13 with GTG and FISH (a), MLPA (b) and SNP array analysis (c). a. GTG and FISH results: left: partial karyotype of 2 cells showing a normal chromosome 15 on the left and the sSMC on the right. Middle: partial metaphase after FISH with WCP15. Three chromosomes, both normal chromosomes 15 and the sSMC (arrow), are stained, proving the chromosome 15 origin of the sSMC. Right: Partial metaphase after FISH with probe p80, located at 15q25-qter [37], showing 2 signals on both ends of the sSMC (arrow) indicative for a neo(15). b. MLPA results with the kits P036B (left) and P070 (right). The probes targeting ALDH1A3 and FLJ22604 (within red circle), respectively, both located at 15qtel, are clearly elevated but below 1.6 indicating 3 copies of both genes instead of four on the sSMC. c. HumanCytoSNP-12 result. Only chromosome 15 is depicted. The upper part shows the B-allele frequency (BAF) and the lower part shows the Log2 intensity along chromosome 15. Based on BAF (absence of a BAF of 0.5 at 15q26.3 while a meiotic origin is likely due to the presence of a BAF of 0.5 in a large part of the sSMC, indicating the presence of a third haplotype) and Log2 ratio (indicating 3 copies of the most distal end of 15q), the MLPA result of a trisomy at the distal q-arm at 15q26.3, is confirmed.
In order to elucidate this discrepancy, genomic microarray analysis was performed. Investigations with the HumanCytoSNP-12 indicate that this case is more complex than initially thought which may explain the MLPA results. Based on Log R ratio and B-allele frequency (BAF), we expect mosaicism of different abnormal cell lines containing different sSMC. However, BAF's of 0, 1, 0.333 and 0.667 and absence of a BAF of 0.5 at 15q26.3 (in contrast to the region 15q24.1-q26.2) indicate a trisomy at 15qtel which confirms the MLPA results (see Figure 1c).
In case 25 of an extra familial del(22)(q11.2) the results of MLPA with the 22q11 probes in the four kits were indicative for 4 copies of this chromosomal region: relative probe signals were 1.770 and 1.621 (P181), 1.760 and 1.869 (P182), 1.688 (P036B) and 1.806 (P070) (Figure 2b). This contradicts the FISH results with one signal on the sSMC for two different 22cen-probes, one for the rDNA-probe and one signal with the probe from the Cat Eye Syndrome (CES)-region (Figure 2a). HumanCytoSNP-12-analysis confirmed the sSMC to be at least a partial duplication of chromosome 22, indicated by a BAF of 0.5, resulting in 4 copies of the sequences detected by the proximal 22q-probes in the 4 MLPA kits (Figure 2c).
Identification of the familial sSMC in case 25: GTG and FISH (a), MLPA (b) and HumanCytoSNP-12 (c) results. a. Left. GTG partial metaphase showing the sSMC (arrow). 2nd picture: partial metaphase after FISH with the ribosomal DNA probe r521, showing one signal on the sSMC (arrow) and on the short arm of all acrocentric chromosomes. 3rd picture: partial metaphase after FISH with p190.22 (22cen) (green) and 22qtel probe (red) showing only one centromere signal and no 22qtel signal on the sSMC (arrow). Both normal chromosomes 22 are positive with 22cen as well as with 22qtel probe. Right: partial metaphase after FISH with a Cat Eye Syndrome critical region probe 102D10 showing one signal on the sSMC (arrow) and on both normal chromosomes 22. b. MLPA results with centromere kit P182 (left) en telomere kit P070 (right). The relative probe signals of the sequences targeting CECR1 and SLC25A18 in P182 and IL17R in P070 (in red circle) are clearly > 1.6 indicating 4 copies of these sequences on the sSMC. c. HumanCytoSNP-12 result. Only chromosome 22 is depicted. The upper part shows the B-allele frequency (BAF) and the lower part shows the Log R Ratio along chromosome 22. Based on the BAF (presence of a BAF of 0.5 in at least a part of the maternally inhereted sSMC, indicating the presence of a meiotic tetrasomy), the MLPA result of a partial tetrasomy 22q11, is confirmed.
b. Mosaic cases
In one out of four mosaic cases (case 3), MLPA correctly identified four copies of 9ptel (P070, P036B), 9p11 (p182) and 9p13.2 (P181) with relative probe signals > 1.6 (table 3). However, in three out of four mosaic cases (cases 4, 6 and 20), the relative probe signals of some probes were below the expected values (1.3 for a trisomy and 1.6 for a tetrasomy). In these cases, the level of mosaicism, which was determined in cultured AF cells, was unknown in uncultured AF cells from which the DNA for MLPA was isolated. Nevertheless, the sSMC in all three cases could be identified by making use of our own calculated cut off values (table 2) with relative probe signals of the involving probes above the normal cut off values in all kits.
Unique sequence negative sSMC
The unique sequence negative sSMC (cases 2, 8-10, 15-19, 26-30) all showed normal MLPA results with relative probe signals between the normal cut off values 0.7 and 1.3 confirming GTG/FISH results. There were no false positive cases.
Discussion
sSMC, when detected with conventional cytogenetic banding techniques, are still a problem as they often are too small or without a specific banding pattern to be considered for their chromosomal origin by traditional banding techniques. Therefore, molecular cytogenetic techniques are often needed for further characterisation. Since the phenotypic consequences of a sSMC will greatly depend on its genetic content and chromosomal origin, it is of particular clinical importance to rapidly distinguish unique sequence-negative from unique sequence-positive sSMC because the former are less likely to be associated with an abnormal fetal outcome. For a collection of all available reported sSMC cases, see the sSMC database [22].
Different papers describe the use of genomic array analysis for identification of sSMC [15, 16, 23, 24]. The big advantage of this technique is that the exact chromosomal content of the unique-sequence positive sSMC can be determined in one reaction, although targeted FISH is often used following an abnormal array result and is sometimes necessary to determine the structure of the sSMC [24]. However, genomic array analysis is labour intensive, time-consuming and expensive. The use of the MLPA technique for characterisation of some specific sSMC has already been described in postnatal cytogenetics [25–28]. In this paper, we show that MLPA with centromere and telomere kits may be a quick initial approach for sSMC characterisation in prenatal diagnosis when time is limited. From every AF sample that we receive in our laboratory 4 ml is used for direct DNA isolation. As soon as a sSMC is found, this DNA can be used for MLPA and results are available within less than 24 hours. In case of a positive result, targeted FISH and/or array on (un)cultured AF cells may be used for confirmation and further identification. However, as with array analysis, MLPA will give normal results in case of an unique sequence-negative sSMC or in case of low level mosaicism [16]. Therefore, other techniques such as cenM-FISH [8] or sequential targeted FISH [29], although labour-intensive and frequently time-consuming, will be necessary for determining the chromosomal origin. Since such a sSMC most probably does not contain euchromatin, in the meanwhile, the parents can be karyotyped after reassurance.
Despite the development of molecular techniques for sSMC identification, conventional staining techniques are still valuable. Since 35% of the marker chromosomes with a known chromosomal origin are derived from chromosome 15 [30], the first thing to do in case of a satellited sSMC is a DA-DAPI staining. If positive, targeted FISH with chromosome 15 specific probes can be applied for further identification. If negative, FISH with a 13/21 and 14/22 centromere probe in conjunction with subcentromere probes for these 4 chromosomes can quickly elucidate the chromosomal content of the sSMC. In case of a non-satellited sSMC, MLPA might be a rapid and rather non-expensive technique for distinguishing between an unique sequence-positive and -negative sSMC.
The centromere kits P181 and P182 are presented by MRC Holland as kits for identification of sSMC. However, we recommend using the telomere kits in addition to these centromere kits for sSMC identification for three reasons. Firstly, by using only the centromere kits, the neo(12) and neo(15) would both have been missed. This type of sSMC, first described by Blennow [31] is rare. Up till now about ~90 neocentric acentric marker chromosomes have been described in patients with idiopathic mental retardation but also in cancer cells [32, 33]. Secondly, the sSMC in cases 7 and 11 could be correctly identified as being unbalanced translocations by using centromere concomitant with telomere kits. Although rare, this type of sSMC, the so-called unique complex sSMC which are derived from more than one chromosome, may be underdiagnosed as suggested by Trifonov et al. [34], and MLPA with centromere and telomere kits may enhance their detection rate. And finally, since most sSMC are derived from the acrocentric chromosomes, the simultaneous use of centromere and telomere kits allows for more markers to be tested in the subcentromeric region since the telomere kits also contain probes in the proximal long arm of the acrocentric chromosomes instead of a specific short arm subtelomere probe which they lack.
In 5/6 mosaic cases with the level of mosaicism determined in cultured cells, DNA isolated from uncultured AF cells was used for MLPA hampering the interpretation of the results. For instance, normal MLPA results in cases 18 and 19 are most probably explained by absence of unique sequences on both sSMC. However, due to low-level mosaicism at least in the AF cell cultures and therefore probably also in the uncultured AF cells used for MLPA, a normal result caused by low-level mosaicism can never be excluded. It is known that discrepancies may exist concerning mosaicism level between cultured and uncultured AF cells with the level often being higher in uncultured cells since these are not subjected to selection, mostly in favour of normal cells as seen in cell cultures. However, the reverse has also been observed in some cases of tissue specific mosaicism if the contribution of the affected organ or organsystem to the total AF cell population is small [35], but also in cases of generalised mosaicism [36]. From this experience, we learned that it is important to make FISH-slides at the time of DNA isolation necessary for determination of the level of mosaicism in the DNA sample enabling a proper interpretation of molecular results.
In cases 13 and 25 we show that the use of molecular techniques such as MLPA and array analysis may show that some sSMC are much more complex than initially thought on the basis of conventional banding techniques and FISH. At the moment we are performing further FISH studies in order to elucidate the exact structure of the sSMC in both cases. Tsuchya et al. [24] already published the uncoverage of unexpected complexity in the form of complex rearrangements of some sSMC when they used array CGH. This will ultimately lead to a more accurate sSMC-phenotype correlation which is important for proper counselling of the prospective parents when the sSMC is found prenatally.
Conclusion
In this paper we show that MLPA with centromere (P181 and P182)-and telomere (P036B and P070)-kits allow for the rapid differentiation between unique sequence positive and negative sSMC. As compared to multicolour FISH techniques and microarray analysis, MLPA is a rather non-expensive and easy to perform technique in most clinical cytogenetic laboratories for the rapid elucidation of the harmfulness of a prenatally detected sSMC with results available within 24 hours.
References
Sachs ES, Van Hemel JO, den Hollander JC, Jahoda MGJ: Marker chromosomes in a series of 10 000 prenatal diagnoses. Cytogenetic and follow-up studies. Prenat Diagn 1987, 7: 81–89. 10.1002/pd.1970070204
Blennow E, Nielsen KB, Telenius H, Carter NP, Kristoffersson U, Holmberg E, Gillberg C, Nordenskjöld M: Fifty probands with extra structurally abnormal chromosomes characterized by fluorescence in situ hybridization. Am J Med Genet 1995,55(1):85–94. 10.1002/ajmg.1320550122
Müller-Navia J, Nebel A, Schleiermacher E: Complete and precise characterization of marker chromosomes by application of microdissection in prenatal diagnosis. Hum Genet 1995,96(6):661–667.
Brøndum-Nielsen K, Mikkelsen M: A 10-year survey, 1980–1990, of prenatally diagnosed small supernumerary marker chromosomes, identified by FISH analysis. Outcome and follow-up of 14 cases diagnosed in a series of 12,699 prenatal samples. Prenat Diagn 1995,15(7):615–619.
Hsu LY, Yu MT, Richkind KE, Van Dyke DL, Crandall BF, Saxe DF, Khodr GS, Mennuti M, Stetten G, Miller WA, Priest JH: Incidence and significance of chromosome mosaicism involving an autosomal structural abnormality diagnosed prenatally through amniocentesis: a collaborative study. Prenat Diagn 1996,16(1):1–28. 10.1002/(SICI)1097-0223(199601)16:1<1::AID-PD816>3.0.CO;2-W
Kotzot D: Supernumerary marker chromosomes (SMC) and uniparental disomy (UPD): coincidence or consequence? J Med Genet 2002,39(10):775–778. 10.1136/jmg.39.10.775
Liehr T, Weise A: Frequency of small supernumerary marker chromosomes in prenatal, newborn, developmentally retarded and infertility diagnostics. Int J Mol Med 2007,19(5):719–731.
Nietzel A, Rocchi M, Starke H, Heller A, Fiedler W, Wlodarska I, Loncarevic IF, Beensen V, Claussen U, Liehr T: A new multicolor-FISH approach for the characterization of marker chromosomes: centromere-specific multicolor-FISH (cenM-FISH). Hum Genet 2001,108(3):199–204. 10.1007/s004390100459
Starke H, Nietzel A, Weise A, Heller A, Mrasek K, Belitz B, Kelbova C, Volleth M, Albrecht B, Mitulla B, Trappe R, Bartels I, Adolph S, Dufke A, Singer S, Stumm M, Wegner RD, Seidel J, Schmidt A, Kuechler A, Schreyer I, Claussen U, von Eggeling F, Liehr : Small supernumerary marker chromosomes (SMC's): genotype-phenotype correlation and classification. Hum Genet 2003,114(1):51–67. 10.1007/s00439-003-1016-3
Liehr T, Starke H, Heller A, Kosyakova N, Mrasek K, Gross M, Karst C, Steinhaeuser U, Hunstig F, Fickelscher I, Kuechler A, Trifonov V, Romanenko SA, Weise A: Multicolor fluorescence in situ hybridization (FISH) applied to FISH-banding. Cytogenet Genome Res 2006,114(3–4):240–244. 10.1159/000094207
Engelen JJM, Albrechts JCM, Hamers AJH, Geraedts JPM: A simple and efficiënt method for microdissection and microFISH. J Med Genet 1998, 35: 265–268. 10.1136/jmg.35.4.265
de Pater JM, Kroes HY, Verschuren M, van Oppen AC, Albrechts JC, Engelen JJ: Mosaic trisomy (8)(p22 --> pter) in a fetus caused by a supernumerary marker chromosome without alphoid sequences. Prenat Diagn 2005,25(2):151–155. 10.1002/pd.1097
Schröck E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA, Ning Y, Ledbetter DH, Bar-Am I, Soenksen D, Garini Y, Ried T: Multicolor spectral karyotyping of human chromosomes. Science 1996, 273: 494–497.
Speicher MR, Gwyn Ballard S, Ward DC: Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nat Genet 1996,12(4):368–375. 10.1038/ng0496-368
Ballif BC, Hornor SA, Sulpizio SG, Lloyd RM, Minier SL, Rorem EA, Theisen A, Bejjani BA, Shaffer LG: Development of a high-density pericentromeric region BAC clone set for the detection and characterization of small supernumerary marker chromosomes by array CGH. Genet Med 2007,9(3):150–162. 10.1097/GIM.0b013e3180312087
Baldwin EL, Lee JY, Blake DM, Bunke BP, Alexander CR, Kogan AL, Ledbetter DH, Martin CL: Enhanced detection of clinically relevant genomic imbalances using a targeted plus whole genome oligonucleotide microarray. Genet Med 2008,10(6):415–429. 10.1097/GIM.0b013e318177015c
Karaman B, Aytan M, Yilmaz K, Toksoy G, Onal EP, Ghanbari A, Engur A, Kayserili H, Yuksel-Apak M, Basaran S: The identification of small supernumerary marker chromosomes; the experiences of 15,792 fetal karyotyping from Turkey. Eur J Med Genet 2006,49(3):207–14. 10.1016/j.ejmg.2005.06.002
Schweitzer D, Ambros P, Andrle M: Modification of DAPI banding on human chromosomes by prestainig with a DNA binding oligopeptide, distamycin A. Exp Cell Res 1978, 111: 327–332. 10.1016/0014-4827(78)90177-5
Knight SJ, Lese CM, Precht KS, Kuc J, Ning Y, Lucas S, Regan R, Brenan M, Nicod A, Lawrie NM, Cardy DL, Nguyen H, Hudson TJ, Riethman HC, Ledbetter DH, Flint J: An optimized set of human telomere clones for studying telomere integrity and architecture. Am J Hum Genet 2000, 67: 320–332. 10.1086/302998
Smidt-Jensen S, Christensen B, Lind AM: Chorionic villus culture for prenatal diagnosis of chromosome defects: reduction of the long-term cultivation time. Prenat Diagn 1989,9(5):309–319. 10.1002/pd.1970090503
Van Opstal D, Boter M, de Jong D, van den Berg C, Brüggenwirth HT, Wildschut HIJ, de Klein A, Galjaard R-JH: Rapid aneuploidy detection with multiplex ligation-dependent probe amplification: a prospective study of 4000 amniotic fluid samples. Eur J Hum Genet 2009, 17: 112–121. 10.1038/ejhg.2008.161
Liehr T: Small supernumerary marker chromosomes database. [http://www.med.uni-jena.de/fish/sSMC/00START.htm]
Vermeesch JR, Melotte C, Salden I, Riegel M, Trifnov V, Polityko A, Rumyantseva N, Naumchik I, Starke H, Matthijs G, Schinzel A, Fryns JP, Liehr T: Tetrasomy 12pter-12p13.31 in a girl with partial Pallister-Killian syndrome phenotype. Eur J Med Genet 2005,48(3):319–327. 10.1016/j.ejmg.2005.04.018
Tsuchiya KD, Opheim KE, Hannibal MC, Hing AV, Glass IA, Raff ML, Norwood T, Torchia BA: Unexpected structural complexity of supernumerary marker chromosomes characterized by microarray comparative genomic hybridization. Mol Cytogenet 2008, 1: 7. 10.1186/1755-8166-1-7
Medne L, Zackai EH, Emanuel BS: Emanuel Syndrome. In GeneReviews [Internet]. Edited by: Pagon RA, Bird TC, Dolan CR, Stephens K. Seattle (WA): University of Washington, Seattle; 1993. [updated 2010 May 11]
Pacanaro AN, Christofolini DM, Kulikowski LD, Belangero SI, da Silva Bellucco FT, Varela MC, Koiffmann CP, Yoshimoto M, Squire JA, Schiavon AV, Heck B, Melaragno MI: A rare case of trisomy 15pter-q21.2 due to a de novo marker chromosome. Am J Med Genet A 2010,152A(3):753–758. 10.1002/ajmg.a.33308
Hoppman-Chaney NL, Dawson DB, Nguyen L, Sengupta S, Reynolds K, McPherson E, Velagaleti G: Partial hexasomy for the Prader-Willi-Angelman syndrome critical region due to a maternally inherited large supernumerary marker chromosome. Am J Med Genet A 2010,152A(8):2034–2038. 10.1002/ajmg.a.33483
Kleefstra T, de Leeuw N, Wolf R, Nillesen WM, Schobers G, Mieloo H, Willemsen M, Perrotta CS, Poddighe PJ, Feenstra I, Draaisma J, van Ravenswaaij-Arts CM: Phenotypic spectrum of 20 novel patients with molecularly defined supernumerary marker chromosomes 15 and a review of the literature. Am J Med Genet A 2010,152A(9):2221–2229. 10.1002/ajmg.a.33529
Cotter PD, Drexler K, Corley AL, Covert SM, Moland JS, Govberg IJ, Norton ME: Prenatal diagnosis of minute supernumerary marker chromosomes. Gynecol Obstet Invest 2005,60(1):27–38. 10.1159/000083482
Crolla JA, Youings SA, Ennis S, Jacobs PA: Supernumerary marker chromosomes in man: parental origin, mosaicism and maternal age revisited. Eur J Hum Genet 2005,13(2):154–160. 10.1038/sj.ejhg.5201311
Blennow E, Telenius H, de Vos D, Larsson C, Henriksson P, Johansson O, Carter NP, Nordenskjöld M: Tetrasomy 15q: two marker chromosomes with no detectable alpha-satellite DNA. Am J Hum Genet 1994,54(5):877–883.
Murmann AE, Conrad DF, Mashek H, Curtis CA, Nicolae RI, Ober C, Schwartz S: Inverted duplications on acentric markers: mechanism of formation. Hum Mol Genet 2009,18(12):2241–2256. 10.1093/hmg/ddp160
Liehr T, Utine GE, Trautmann U, Rauch A, Kuechler A, Pietrzak J, Bocian E, Kosyakova N, Mrasek K, Boduroglu K, Weise A, Aktas D: Neocentric small supernumerary marker chromosomes (sSMC)--three more cases and review of the literature. Cytogenet Genome Res 2007,118(1):31–37. 10.1159/000106438
Trifonov V, Fluri S, Binkert F, Nandini A, Anderson J, Rodriguez L, Gross M, Kosyakova N, Mkrtchyan H, Ewers E, Reich D, Weise A, Liehr T: Complex rearranged small supernumerary marker chromosomes (sSMC), three new cases; evidence for an underestimated entity? Mol Cytogenet 2008, 1: 6. 10.1186/1755-8166-1-6
Van Opstal D, van den Berg C, Galjaard RJ, Los FJ: Follow-up investigations in uncultured amniotic fluid cells after uncertain cytogenetic results. Prenat Diagn 2001,21(2):75–80. 10.1002/1097-0223(200102)21:2<75::AID-PD990>3.0.CO;2-B
Veenma DCM, Beurskens LWJE, Douben J, Eusen B, Noomen P, Govaerts L, Grijseels EW, Lequin MH, de Krijger RR, Tibboel D, de Klein A, Van Opstal D: Comparable low-level mosaicism in affected and non affected tissue of a complex CDH patient. PLoS ONE 2010,5(12):e15348. 10.1371/journal.pone.0015348
Heisterkamp N, Groffen J, Stephenson JR, Spurr NK, Goodfellow PN, Solomon E, Carritt B, Bodmer WF: Chromosomal localization of human cellular homologues of two viral oncogenes. Nature 1982,299(5885):747–749. 10.1038/299747a0
Cockwell AE, Jacobs PA, Crolla JA: Distribution of the D15Z1 copy number polymorphism. Eur J Hum Genet 2007, 15: 441–445. 10.1038/sj.ejhg.5201780
Srebniak M, Noomen P, dos Santos P, Halley D, van de Graaf R, Govaerts L, Wouters C, Galjaard RJ, Van Opstal D: An incomplete trisomy 3 rescue resulting in a marker chromosome and UPD(3)--difficulties in interpretation. Prenat Diagn 2008,28(10):967–970. 10.1002/pd.2077
Van Opstal D, Eussen HJ, Van Hemel JO, Sachs ES: Application of fluorescent in situ hybridization for 'de novo' anomalies in prenatal diagnosis. Prenat Diagn 1993,13(9):825–832. 10.1002/pd.1970130906
Acknowledgements
The authors wish to thank Dr. H. Beverloo for performing SKY in case 19, the colleagues from the postnatal cytogenetic laboratory for performing parental studies and T. de Vries Lentsch for his contribution to both figures. Bert Eussen is acknowledged for the development of many FISH probes used for identification of sSMC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DVO coordinated the study and wrote the paper. MB performed the MLPA and microarray analyses. PN performed cytogenetic and FISH analyses. MIS, DVO, GH and R-JHG were responsible for the final (molecular) cytogenetic diagnoses and reports. GH and DVO studied the literature. R-JHG coördinated the genetic counselling of the parents. All authors read and approved the manuscript.
Electronic supplementary material
13039_2010_81_MOESM1_ESM.DOC
Additional file 1: Supplemental Table. 29 prenatal cases with a sSMC: indication, sSMC identification with conventional staining and FISH techniques, positive or negative for euchromatin and final karyotype. (DOC 94 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Van Opstal, D., Boter, M., Noomen, P. et al. Multiplex ligation dependent probe amplification (MLPA) for rapid distinction between unique sequence positive and negative marker chromosomes in prenatal diagnosis. Mol Cytogenet 4, 2 (2011). https://doi.org/10.1186/1755-8166-4-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1755-8166-4-2