Abstract
Background
Lignin materials are abundant and among the most important potential sources for biofuel production. Development of an efficient lignin degradation process has considerable potential for the production of a variety of chemicals, including bioethanol. However, lignin degradation using current methods is inefficient. Given their immense environmental adaptability and biochemical versatility, bacterial could be used as a valuable tool for the rapid degradation of lignin. Kraft lignin (KL) is a polymer by-product of the pulp and paper industry resulting from alkaline sulfide treatment of lignocellulose, and it has been widely used for lignin-related studies.
Results
Beta-proteobacterium Cupriavidus basilensis B-8 isolated from erosive bamboo slips displayed substantial KL degradation capability. With initial concentrations of 0.5–6 g L-1, at least 31.3% KL could be degraded in 7 days. The maximum degradation rate was 44.4% at the initial concentration of 2 g L-1. The optimum pH and temperature for KL degradation were 7.0 and 30°C, respectively. Manganese peroxidase (MnP) and laccase (Lac) demonstrated their greatest level of activity, 1685.3 U L-1 and 815.6 U L-1, at the third and fourth days, respectively. Many small molecule intermediates were formed during the process of KL degradation, as determined using GC-MS analysis. In order to perform metabolic reconstruction of lignin degradation in this bacterium, a draft genome sequence for C. basilensis B-8 was generated. Genomic analysis focused on the catabolic potential of this bacterium against several lignin-derived compounds. These analyses together with sequence comparisons predicted the existence of three major metabolic pathways: β-ketoadipate, phenol degradation, and gentisate pathways.
Conclusion
These results confirmed the capability of C. basilensis B-8 to promote KL degradation. Whole genomic sequencing and systematic analysis of the C. basilensis B-8 genome identified degradation steps and intermediates from this bacterial-mediated KL degradation method. Our findings provide a theoretical basis for research into the mechanisms of lignin degradation as well as a practical basis for biofuel production using lignin materials.
Similar content being viewed by others
Background
The world has been confronting an energy crisis due to depletion of finite fossil fuel resources [1]. Furthermore, an ever-increasing level of greenhouse pollution from the combustion of fossil fuels in turn aggravates global warming and climate change [2]. This has led to a realization that modern society must turn to renewable forms of energy and chemical production. Hence, there is considerable interest in the utilization of plant biomass for the production of bioenergy and renewable chemicals [3].
Lignin is the most abundant aromatic compound on earth and is second only to cellulose in its contribution to living terrestrial biomass [4]. It is a complex aromatic heteropolymer comprised of phenylpropanoid aryl-C3 units linked via a variety of ether and carbon-carbon linkages, and it is recalcitrant to microbial degradation [5]. The biological degradation of lignin is not only one of the most important parts of the biospheric carbon and oxygen cycle [6], but it is also a central aspect for industrial use of cellulosic biomass, such as bioethanol production and manufacture of cellulose-base chemicals and materials [7].
Despite lignin’s natural recalcitrance, some of fungi are able to decompose lignin. The best-characterized degraders are white-rot fungi, in particular Phanerochaete chrysosporium[8]. In nature, phenol oxidases including lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase (Lac) are secreted by white-rot fungi and are assumed initially to attack lignin. These enzymes act through radical reactions [9]. Although fungi are the main contributors to lignin degradation, bacteria display versatile pathways to degrade aromatic substances, from simple phenols to highly complex lignins and related xenobiotic substances. Furthermore, some low molecular weight compounds (mostly aromatic carboxylic acids) formed from fungal lignin degradation may be further metabolized by bacteria [10]. Several bacteria strains such as Streptomyces viridosporus T7A [11], Nocardia, and Rhodococcus[12] have been reported to degrade lignin. The actual catabolic pathways of lignin derivatives and the responsible enzymes and genes have been investigated using molecular methods in a few bacterial strains, including Sphingomonas paucimobilis SYK-6 [9]. However, bacterial genomic analysis of lignin degradation pathways is still lacking. Comprehensive elucidation of the bacterial genes and enzyme systems for lignin degradation is important for understanding the process of the earth’s carbon cycle and for providing useful tools for the conversion of lignin into intermediate metabolites of industrial value [9]. The structure of natural lignin is very complex, and intact lignin is not commercially available. However, because of the similarities to natural lignin, kraft lignin (KL) has been widely used for lignin-related studies [13–17].
In our previous research, the novel beta-proteobacterium strain C. basilensis B-8 was isolated from steeping fluid of the erosive bamboo slips derived from Kingdom Wu during the Three-Kingdoms Dynasty of ancient China (A.D. 220–280). This bacteria was found to degrade KL and related aromatic compounds. However, its gene characteristics and the mechanisms by which it degrades KL were still unclear. Therefore, the objectives of this current study were to: (i) investigate the capability of this bacterial strain to degrade KL, (ii) identify the genes responsible for lignin degradation, (iii) identify the derivatives of this process, and (iv) reconstruct the metabolic pathways involved in degradation of lignin and generation of its derivatives.
Result and discussion
Optimization of temperature and pH on KL degradation by C. basilensis B-8
According to the actual results, the temp optimum was 30°C and the pH optimum was between 7–8.5 (Figure 1), as presented in previous reports, e.g. the optimum pH of Aneurinibacillus aneurinilyticus is 7.6 [17] and the corresponding values for Comamonas sp. B-9 and Bacillus strain are 7 [18] and 7.6 [19], respectively. For Streptomyces strains, the optimal pH ranges from 7.8–8.5 [20].
Bacterial growth and KL degradation
The rate of C. basilensis B-8 growth was evaluated under seven different initial KL concentrations ranging from 0.5 g L-1 to 6 g L-1. C. basilensis B-8 grew well under initial concentrations from 1 g L-1 to 6 g L-1 (Figure 2a), indicating that bacterial growth would not be inhibited under the tested concentrations. However, the optical density (OD) value of the cultured sample increased with the increase in initial KL concentration. The rates of KL degradation under different initial concentrations all surpassed 31% on day 7 (Figure 2b), but there was no obvious correlation between the initial concentration and the KL degradation rate. The highest KL degradation rate of 44.4% was observed at an initial concentration of 2 g L-1, and the largest KL degradation capacity of 2.1 g L-1 was observed at the initial concentration of 6 g L-1.
Bacterial growth and KL degradation in different initial concentration of KL. ( a ) Bacterial growth in different initial concentration, ( b ) KL degradation rate at seventh day in different initial concentration, ( c ) Bacterial growth and COD reduction in 2 g · L-1 KL. Average values of three replicates are shown with the standard error of the mean as error bars.
The growth and KL degradation capacity of C. basilensis B-8 in nutrient medium with a KL concentration of 2 g L-1 were investigated in detail. The results are shown in Figure 2c. C. basilensis B-8 growth was substantially faster during the first 2 days and reached the maximum at day 4. KL degradation mainly occurred during the initial 2 days. Accordingly, the maximum KL degradation rate of 722.8 mg L-1 Day-1 was recorded during this period. From the third day, KL degradation was continuous, but the degradation rate was decreased. The chemical oxygen demand (COD) value reached 1455.4 mg L-1 from the initial 3276.4 mg L-1 at day 7. The growth and KL degradation observed in this experiment were different from those of Citrobacter strains, which must initially use glucose and peptone as carbon sources and subsequently utilize lignin as a co-metabolite [21]. Accordingly, KL could be the sole nutrition source of C. basilensis B-8, as it must be metabolized during the initial growth stage to provide carbon and energy for growth. A similar process of KL degradation was also reported for Comamonas sp. B-9 [18] and Streptomyces viridosporus[11], which also showed a great capacity for KL degradation. This predicts that bacteria that use lignin as their sole carbon source must metabolize it throughout the whole life cycle; therefore, the efficiency and total amount of lignin degradation would be relatively higher for these strains than those using lignin as co-metabolite.
Analysis of enzymes and genes related to KL degradation
Three major enzymes including LiP, MnP, and Lac, which use low-molecular-weight mediators to carry out lignin degradation, have been well characterized in microorganism [22]. The activity of these three enzymes from C. basilensis B-8 is shown in Figure 3. MnP activity increased significantly during the initial 3 days, with a maximum of 1685.3 U/L at day 3, followed by a slight decrease from day 4. Lac activity was maintained at a low level on day 1. A rapid increase was then observed from day 2, with a maximum of 815.6 U/L on day 4. These results indicated that MnP played a crucial role during the entire process of KL degradation by C. basilensis B-8, whereas Lac mainly functioned during the latter stages of the reaction. A similar conclusion was also proposed in previous reports [23, 24]. However, the mechanism of lignin micro-biodegradation is complicated; thus, a complete explanation requires further study. In addition, no obvious LiP activity was observed during the course of KL degradation, indicating that active LiP was not produced by C. basilensis B-8. Similar to this strain, some white-rot fungi and bacteria (i.e., Dichomitus squalens, Lentinula edodes[10], and Comamonas sp. B-9 [18]), which simultaneously produce MnP and Lac, were reported not to secret detectable levels of LiP. These organisms are also strong lignin degraders. Since LiP is responsible for the oxidation of non-phenolic syringyl and biphenyl model compounds (which exist in certain types of lignins, like hardwood) and subsequent ring cleavage [10], it is conceivable that the efficiency of hardwood degradation by C. basilensis B-8 and the other microorganisms mentioned above are relatively low.
No LiP activity was detected in this study, and it was not surprising that no gene encoding LiP was identified via genomic analysis of C. basilensis B-8 (see supplementary files). However, a genomic search for MnP genes in the genome of C. basilensis B-8 rendered only open reading frames (ORFs) with low amino acid (aa) identities of 27.0% and 30.0% with the MnP genes of the fungi Pleurotus ostreatus and Ganoderma australe, respectively. MnP gene retrieval using the NCBI public database showed that no MnP genes from bacteria were available; furthermore, very few reports detail the presence of MnP enzymes in bacterial systems [25].
Metabolite characterization via GC-MS
The low molecular weight compounds released from lignin due to KL degradation by C. basilensis B-8 were analyzed by GC-MS. The total ion chromatograph (TIC) patterns corresponding to the compounds extracted with ethyl acetate from the control (uninoculated medium sample) and degraded samples are shown in Figure 4a-c, and their peak identity is depicted in Table 1. In the TIC pattern of the control sample (Figure 4a), peaks at RT 8.1 and RT 10.6 were identified as acetic acid and phenol, respectively. The identification of these two important intermediate metabolites generated during the degradation of lignin by microorganisms [3, 26] may be attributed to the chemical oxidation of lignin due to aeration and agitation. Moreover, other lignin-related compounds were also indentified, suggesting partial degradation of KL during the industrial production process [27].
The number of peaks in the TIC increased significantly after 3 and 6 days of incubation with C. basilensis B-8 as compared to the control. Many low molecular weight compounds such as 2, 3-dihydro-3, 5-dihydroxy-6-methyl-4-pyrone; 3, 5-dimethylbenzaldehyde; 2-methylnaphthalene; cinnamic acid; and gentisate were identified in the extract of the degraded sample (Figure 4b-c and Table 1), and these were not present in the extract of the control sample. The detected guaiacol-related compound and cinnamic acid could be easily related to the oxidation of the guaiacyl units from precursor coniferyl alcohol and ρ-hydroxyphenyl units generated from precursor ρ-coumary alcohol. These are considered to be basic moieties with syringyl units from precursor sinapyl alcohol that are components of the lignin structure [3]. Unfortunately, no syringyl-related compounds were identified in the sample. In addition to aromatic compounds, more acid-type compounds were identified than aldehyde and ketone-type compounds due to degradation of lignin. A similar study was also performed previously in Aneurinibacillus aneurinilyticus[17]. The low molecular weight compounds identified in the extracts of the inoculated sample favor the conclusion that KL was degraded by C. basilensis B-8.
The initial degradation of lignin into low molecular weight compounds by extracellular phenoloxidases
The extracellular oxidative enzymes LiP, MnP, and Lac are defined as phenoloxidases, which are responsible for the initial degradation of lignin and have been intensively studied in fungi. Although enzymology of bacterial lignin degradation has not been as thoroughly investigated as that of fungi, there are indications that bacteria use extracellular peroxidase for lignin degradation [11]. The activities of MnP and Lac from C. basilensis B-8 have been observed using colorimetric enzyme assays (Section “Analysis of enzymes related to KL degradation”). These observations indicted the existence of a novel MnP or its isozyme in C. basilensis B-8. The further study, purification, and characterization of these enzymes is currently under way.
High MnP activity has also been documented in a previous report investigating Citrobacter strains [21] that need glucose as an extra carbon source to produce hydrogen peroxide, which serves as a cosubstrate for the ligninolytic activity of MnPs via glucose oxidation. However, glucose was not involved in the lignin degradation by C. basilensis B-8; in addition, the genes encoding glyoxal oxidase and aryl alcohol oxidase that are responsible for hydrogen peroxide production in fungi were not found in C. basilensis B-8, suggesting hydrogen peroxide in C. basilensis B-8 is generated via other unknown mechanisms. The genomic search of C. basilensis B-8 for a Lac gene only indicated one ORF with low aa identity (32.0%) with the LAC gene from Thermus thermophilus HB27. Given that some dye-type peroxidases, which are active against KL and lignin model compounds, have been identified in several bacteria [3], it is reasonable to predict that the detected enzymes form C. basilensis B-8 may belong to this group.
Catabolism pathways for lignin components
Many low molecular weight compounds were produced from initial KL degradation. The enzymes that are involved in catabolic pathways for the degradation of lignin fragments have been identified and characterized in several bacterial [28–30]. Here, three important degradation pathways for lignin basic derivatives, including coumaric acid, ferulic acid, cinnamic acid, phenol, salicylate, and 3-hydroxybenzoate, were predicted basing on genomic analysis. Moreover, all of these compounds were shown to support the growth of C. basilensis B-8.
The β-ketoadipate central pathway for coumarate, ferulate, and cinnamate degradation
The central reactions of the β-ketoadipate pathway in C. basilensis B-8 are shown in Figure 5. The two branches of the β- ketoadipate pathway (i.e. the catechol branch encoded by cat genes and the protocatechuate branch encoded by pca genes) can convert catechol and protocatechuate into the Krebs cycle intermediates succinate and acetyl coenzyme A [31]. Biochemical studies and amino acid sequence data indicated that the enzymes of this pathway are highly conserved among phylogenetically diverse organisms that possess this pathway.
The cat and pca gene products of C. basilensis B-8 were significantly similar to proteins of known function from other bacteria, mainly Cupriavidus basilensis OR16 (Table 2), which has been reported earlier [32]. However, unlike many other bacteria whose cat and ben genes are usually organized in a single cluster, these genes in C. basilensis B-8 were organized in three clusters at different positions (see alignment result in supplementary files). The catA gene encoding catechol-1, 2-dioxygenase, which starts the catechol branch of the β- ketoadipate pathway, clustered together with the benABCD genes that encode the enzymes responcibe for converting benzoate into catechol. This operon was regulated by CatR, a member of the LysR family of regulatory proteins in P. pudita[33, 34]. There are two copies of the catC gene that shared 71.0% aa identity within the genome of C. basilensis B-8; one of these genes was located upstream of the catB gene and the other was located the downstream of the catD gene.
The pca genes that are responsible for the protocatechuate branch of the β- ketoadipate pathway were dispersed throughout the genome of C. basilensis B-8 (Table 2), and this organization has not been previously reported for other bacteria. The pcaIJF genes encoding the enzymes required for the last two steps of the β- ketoadipate pathway clustered together. In some bacteria, the expression of the pcaIJF genes is induced by β- ketoadipate, which then activates IcIR family regulatory proteins PcaR/PcaQ. However, pcaR was found in another gene cluster and pcaQ was located in the vicinity of pcaH in C. basilensis B-8. It is not uncommon that transcriptional regulators control the expression of distal genes [29], though the exact mechanism of regulation for these genes requires further study. It was surprising that four copies of the pcaI and pcaJ genes were found in C. basilensis B-8 (Table 2), and the reason for this is still unknown. One striking aspect of the pca genes in C. basilensis B-8 is the presence of two copies of the pcaC and pcaD genes (which encode g-carboxymuconolactone decarboxylase and β-ketoadipate enol-lactone hydrolase, respectively) as well as a unique fused gene (pcaL) consisting of the pcaC and pcaD ORFs (Table 2). Sequence analysis of the pcaL gene revealed that the predicted C-terminal third was homologous to decarboxylases, whereas the N-terminal two thirds were homologous to enol-lactone hydrolases. Furthermore, DNA sequence data has revealed a remarkable feature of catD and pacD that encode the isozyme involved in the third from last step of the C. basilensis B-8 β-ketoadipate pathway (Figure 5). These two genes share only a 33% aa identity. Another striking aspect of the protocatechuate branch genes in C. basilensis B-8 is the presence of two 4-hydroxybenzoate 3-monooxygenase encoding genes, pobA and pobB. These two genes share 63.4% aa identity, indicating a distant evolutionary origin.
Ferulate and coumarate form a vast array of ether and ester bonds in lignin and suberin. In some bacteria, ferulate degradation follows a CoA-dependent non-β–oxidative pathway catalyzed by the feruloyl-CoA synthetase (HcaC) and enoyl-CoA hydratase/aldolase (HcaA) proteins, producing vanillin. Vanillin is further converted to protocatechuate via an aldehyde dehydrogenase (HcaB) and a demethylase (VanAB) [29]. The pathway for coumarate degradation into protocatechuate is similar to that of ferulate, which is conducted by PobA and/or PobB at the last step (Figure 5). Genes homologous to hcaABCDKXR have been identified in C. basilensis B-8. The hcaABCD genes are dispersed throughout the genome of C. basilensis B-8, whereas the hcaKXR genes are clustered together (Table 2). hcaD encoding an acyl-CoA dehydrogenase is not involved in the above biochemical pathway, but the protein could be responsible for a CoA-dependent β-oxidative pathway of ferulate degradation with HcaA and Aat (β-ketothiolase), as has been described in other organisms [28]. hcaR, hcaK, and hcaX encode a putative regulatory protein of the MarR family, a 3-hydroxyphenylpropionic acid transporter, and a putative porin of unknown function, respectively. Two van gene clusters were also identified in the genome of C. basilensis B-8 (Table 2), and one of them contained a transcriptional regulator of the GntR family (vanR). The hca gene cluster was not linked to the van cluster. A similar situation is also found in P. putida KT2440 and Acinetobacter sp. ADP1, and it has been suggested that this gene organization would facilitate the appearance of spontaneous van-deficient strains in natural Acinetobacter populations, which might allow the production of vanillate from ferulate as a chemical signal between plants and bacteria [35].
Cinnamate degradation via benzoate has been described in Cupriavidus necator JMP134 [29]. However, the enzymes that are responsible for the initial steps have not been characterized. Many similar intermediates including benzoate, produced during the process of cinnamate degradation by C. basilensis B-8 were observed on basis of our GC-MS analysis (date not shown). Accordingly, we could predict that cinnamate was also degraded through benzoate (Figure 5). The study of the enzymes involved in the first five steps of this pathway is currently in progress.
Phenol degradation pathways
Bacterial catabolic pathways for phenol and its derivatives have been studied extensively in C. necator JMP134 [29]. Genomic analysis of C. basilensis B-8 showed that all orthologous genes were present except phlX, which encodes a relatively hydrophobic protein. Phenol and its derivatives metabolized via the methylcatechol ortho ring-cleavage pathway (enzymes encoded by mml genes) and the catechol meta ring-cleavage pathway (enzymes encoded by phl genes) in C. basilensis B-8 are shown in Figure 5, and the involved enzymes are listed in Table 3. The mml genes organized in a single cluster. The mmlJIHGFRL is maintained in the mml clusters of C. basilensis B-8 (Table 3) and C. necator JMP134. Similar to C. necator JMP134, no putative gene encoding an isoenzyme of β-ketoadipate enollactone hydrolase was found in the mml gene cluster of C. basilensis B-8. This point supports the idea that 4-methyl-β-ketoadipate enollactone is not further metabolized through a classical β-ketoadipate pathway. The next step in the reaction process still requires further study.
Although the mml genes are clustered together, the phl genes were organized in two different clusters. phlGF encoding the 4-hydroxy-2-ketovalerate aldolase and the aldehyde dehydrogenase that catalyzes the final steps of the meta ring-cleavage pathway (Figure 5) are separated from the rest of the other meta ring-cleavage pathway genes. A similar arrangement is also present in C. necator JMP134, though one difference is that two copies of phlD (which encodes a 2-hydroxymuconic semialdehyde hydrolase) were not found in either of these two gene clusters (Table 3).
The gentisate pathway for catabolism of salicylate and 3-hydroxybenzoate
Salicylate is generated from benzoic acid hydroxylation or trans-cinnamic acid side chain β-oxidation in plants. The catabolism of salicylate into catechol by salicylate 1-hydroxylase, a flavoprotein monooxygenase, or by a three-component protein has been described in several bacteria [29]. Genetic analysis of the C. basilensis B-8 genome showed seven genes (data not shown) with high identity to those from Pseudomonas and Acinetobacter strains. An alternative route of salicylate degradation, via a gentisate intermediate, is initiated by a multicomponent oxygenase (salicylate-5-hydroxylase), as has been reported in Ralstonia sp. U2 [36]. The putative LysR-type transcriptional regulator encoding the hybR gene, large and small subunits of the oxygenase encoding hybB and hybC genes, as well as the ferredoxin encoding hybD gene were organized in a single gene cluster (Table 4), which shows a significant similarity with that from C. necator JMP134. Further study is required to develope our understanding of how salicylate is converted into catechol in C. basilensis B-8.
3-hydroxybenzoate is degraded through gentisate by 3-hydroxybenzoate-6-hydroxylase (3H6H) or through protocatechuate by 3-hydroxybenzoate-4-hydroxylase (3H4H) in Comamonas testosteroni[37] and Bacillus sp. [38]. No homologue of the 3H4H gene was found in the genome of C. basilensis B-8. However, a gene (mhbM) was identified with 82.0% aa identity with that of the 3H6H gene sequence from Burkholderia multivorans CGD1 (Table 4).
The gentisate pathway (Figure 5) is initiated by a gentisate-1, 2-dioxygenase (MhbD), which cleaves the aromatic ring to form maleylpyruvate. Maleylpyruvate could be further degraded by maleylpyruvate hydrolase or by maleylpyruvate isomerase, central metabolites of the Krebs cycle. Genomic analysis indicated the presence of an mhb gene cluster in the genome of C. basilensis B-8. The mhbR and mhbT genes were located upstream of the mhb gene cluster and encoded a LysR family regulator protein and 3-hydroxybenzoate transporter, respectively (Table 4). mhbI and mhbH are homologous to genes that encode maleylpyruvate isomerase and fumarylpyruvate hydrolase in C. necator JMP134 [29], respectively. Together, these observations suggested that gentisate is metabolized by a glutathione-dependent pathway in C. basilensis B-8, as it is in C. necator JMP134.
Conclusions
This study demonstrated that C. basilensis B-8 could be a useful tool for lignin utilization. The great capability for KL degradation by this strain was confirmed. The maximum degradation rate was 44.4% at the initial concentration of 2 g L-1 after 7 days of incubation. High activity of MnP and Lac as well as the presence of many intermediates was observed during the degradation progress. Comprehensive and systematic whole genomic analysis of bacterial lignin degradation pathways was also performed via sequencing and analysis of the C. basilensis B-8 genome, and many genes related to lignin degradation were identified. Three major pathways for lignin degradation were reconstructed via genomic analysis.
Methods
Bacterial strain and cultural conditions
A large number of bamboo slips of Kingdom Wu during the Three-Kingdoms Dynasty, were unearthed from Zoumalou, Hunan province, China, and stored in the Bamboo Slips Museum of Changsha, Hunan Province. The bamboo slips are rare cultural relic of Chinese history. After being unearthed, the bamboo slips were severely eroded by microorganisms. The surface layer of the eroded bamboo slips consisted of a translucent film material, and this was brittle and easily removed. It was likely that lignin and cellulose in the bamboo slips could be degraded by microorganisms present in the samples. The bamboo slips were kept in water to prevent air oxidation, and the bacterial strain C. basilensis B-8 was obtained from the steeping fluid of the eroding bamboo slips. Cells were grown in the Luria-Bertani broth medium and incubated at 30°C with shaking at 120 rpm to an OD600 (optical density at 600 nm) of the inoculums reached approximately 1.0. Then, 2 ml of culture was aseptically inoculated into three parallel culture flasks containing 100 ml sterile KL mineral salt medium (3 g KL, 2 g [NH4]2SO4, 1 g K2HPO4, 1 g KH2PO4, 0.2 g MgSO4, 0.1 g CaCl2, 0.05 g FeSO4, and 0.02 g MnSO4 in 1 L distilled water, pH 7.0). The flasks were incubated at 30°C with shaking at 120 rpm for 7 days. The temperature, pH, and the contents of KL were properly adjusted according to the experimental requirements. Uninoculated medium was used as a control. The KL (molecular weight of approximately 10 000) used in these experiments was purchased from Sigma Aldrich (St. Louis, MO, USA).
Assessment of aromatic compounds as sole carbon and energy sources for C. basilensis B-8
The aromatic compounds including 4-hydroxycinnamate (coumarate) ; ferulate; cinnamate; benzoate; 3, 4- dihydroxybenzoate (protocatechuate); vanillate; phenol; 4-methylcatechol; 2-methylphenol; salicylate; 3-hydroxybenzoate; ethyl salicylate; and 3-hydroxbenzylalcohol were added into each flask, which contain 50 ml of mineral salt medium described above, at a concentration of 100 mg L-1. The strain that was grown for about 12 h in 200 mL of LB medium was harvested by centrifugation at 10 000 rpm for 10 min, washed three times with sterilized deionized water, and resuspended in 50 ml of sterilized deionized water. Samples (1 ml) were added into each flask with shaking at 150 rpm. A flask without any aromatic compounds was used as a control.
Bacterial growth and COD measurements
The rate of C. basilensis B-8 growth was determined by measuring the OD600 of cultured samples using a spectrophotometer (U-4100; Hitachi, Tokyo, Japan). The control and cultured samples (liquid samples) were centrifuged at 12 000 g for 10 min to remove biomass. The appropriate volume of the supernatant was introduced into digestion solution (100–1000 mg · L-1) containing potassium dichromate, sulfuric acid, and mercuric sulfate. The mixture was then incubated for 15 min at 165°C ± 2°C in a COD reactor (Model 45600; Hach Company, Loveland, CO, USA). The COD concentration was measured colorimetrically using a spectrophotometer.
Enzyme assays
A total of 1 ml of the control or cultured samples were centrifuged at 12 000 rpm for 5 min to remove suspended solids. Cell-free supernatant was used as the enzyme source to determine the activity of laccase, lignin peroxidase, and manganese peroxidase. Laccase activity was determined by monitoring the oxidation of ABTS at 420 nm (ε420 = 36000 mol-1 cm-1) [39]. Lignin peroxidase activity was determined by monitoring the peroxide-dependent oxidation of 2 mM veratryl alcohol to veratraldehyde at 310 nm (ε310 = 9 300 mol-1 cm-1) [40]. Manganese peroxidase activity was determined by monitoring the oxidation of 2, 6-DMP to coerulignone at 469 nm (ε469 = 49600 mol-1 cm-1) [41].
GC-MS analysis
The control and cultured samples containing 3 g L-1 KL were periodically withdrawn and centrifuged at 12 000 rpm for 10 min. Supernatants were acidified to pH 2.0 with 6 mmoL L-1 HCL and then extracted with an equal volume of ethyl acetate. Three portion of extracts were collected, dewatered over anhydrous Na2SO4, filtered though filter paper, and evaporated at 40°C under vacuum on a rotary vacuum evaporator. Then, 0.1 ml dioxane and 0.01 ml pyridine were added in the samples followed by silylation with 0.05 ml TMS. The mixture was heated at 60°C for 15 min with periodic shaking to dissolve the residues. GC-MS analysis of organic extracts was conducted using the method reported previously [18]. The identification of low molecular weight compounds as TMS derivatives derived from bacterial degradation was performed by comparing their mass spectra with that of the National Institute of Standards and Technology (NIST) library available in the instrument and also by comparing the retention time (RT) with those of available authentic compounds.
Sequencing and analysis of the C. basilensis B-8 genome
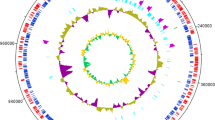
The genomic DNA of C. basilensis B-8 was extracted and purified using a TIANamp Bacteria DNA Kit (Tiangen Biotech, Beijing, China) and then used for sequencing. A total of 495 Mb of clean data from an insert size library (500 bp) was generated for the strain using a high-throughput HiSeq2000 paired-end strategy (Illumina, San Diego, CA, USA). Contigs were assembled and aligned using SOAPdenovo 1.05 software developed by the Beijing Genomics Institute. A total of 8448 coding sequences (CDSs) were predicted using Glimmer3.0, rRNAmmer, tRNAscan, and Rfam software [42–45]. Before the manual annotation of the predicted genes (Additional file 1), automatic annotation was computed using different tool results as follows: similarity searches were performed against different databases including KEGG, Swiss-Prot, TrEMBL, and NR using BLASTall v.2.2.21 software. The annotation results are available in Additional file 2. The aa sequences of the ORFs were compared with those present in finished and unfinished microbial genome databases using the TBLASTN algorithm at the NCBI server (http://www.ncbi.nlm.nih.gov/blast/blast.cgi). Nucleotide and protein sequence similarity searches were also performed using BLAST programs at the BLAST server of NCBI. Pairwise and multiple protein sequence alignments were made with the ALIGN and CLUSTALW programs, respectively, available at the INFOBIOGEN server (http://www.infobiogen.fr/services/menuserv.html). A number of genes responsible for degradation of lignin and its derivatives were identified. The aa sequence of the products from the genes responsible for lignin-related aromatic compound degradation from other reported bacterial strains were compared with the translated genome of C. basilensis B-8. By this approach, we were able to identify the predicted genes responsible for the lignin degradation pathways. The Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession AKXR00000000. The version described in this paper is the first version, AKXR01000000.
Abbreviations
- KL:
-
Kraft lignin
- MnP:
-
Manganese peroxidase
- Lac:
-
Laccase
- LiP:
-
Lignin peroxidase
- OD:
-
Optical density
- COD:
-
Chemical oxygen demand
- TIC:
-
Total ion chromatograph
- ORFs:
-
Open reading frames
- aa:
-
Amino acid
- CDSs:
-
Coding sequences
- 3H6H:
-
3-hydroxybenzoate-6-hydroxylase
- 3H4H:
-
3-hydroxybenzoate-4-hydroxylase
- OD600:
-
Optical density at 600 nm.
References
Khan SA, Hussain MZ, Prasad S, Banerjee UC, Rashmi : Prospects of biodiesel production from microalgae in India. Renew Sust Energ Rev 2009,13(9):2361-2372. 10.1016/j.rser.2009.04.005
Balat M, Balat M: Political, economic and environmental impacts of biomass-based hydrogen. Int J Hydrogen Energ 2009, 34: 3589-3603. 10.1016/j.ijhydene.2009.02.067
Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R: Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep 2011,28(12):1883-1896. 10.1039/c1np00042j
Hammel KE: Fungal degradation of lignin. In Driven by Nature: Plant Litter Quality and Decomposition. Edited by: Cadish G, Giller KE. Wallingford, UK: CAB International; 1997:33-45.
Bugg TDH, Ahmad M, Hardiman EM, Singh R: The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 2010, 22: 1-7.
Crawford DL: Microbial degradation of lignin. Enzyme Microb Technol 1980, 2: 11-22. 10.1016/0141-0229(80)90003-4
Ruiz-Duenas FJ, Martinez AT: Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb Biotechnol 2009, 2: 164-177. 10.1111/j.1751-7915.2008.00078.x
Ahmad M, Taylor CR, Pink D, Burton K, Eastwood D, Bending GR, Bugg TDH: Development of novel assays for lignin degradation: comparative analysis of bacterial and fungal lignin degraders. Mol Biosyst 2010, 6: 815-821. 10.1039/b908966g
Masai E, Katayama Y, Fukuda M: Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotech Bioch 2007, 71: 1-15. 10.1271/bbb.60437
Tuor U, Winterhalter K, Fiechter A: Enzymes of white-rot fungi involved in lignin degradation and ecological determinants of wood decay. J Biotechnol 1995, 41: 1-17. 10.1016/0168-1656(95)00042-O
Ramachandra M, Crawford DL, Hertel G: Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus . Appl Environ Microbiol 1988, 54: 3057-3063.
Zimmermann W: Degradation of lignin by bacteria. J Biotechnol 1990, 13: 119-130. 10.1016/0168-1656(90)90098-V
Leisola M, Brown C, Laurila M, Fiechter A: Polysaccharide synthesis by Phanerochaete chrysosporium during degradation of kraft lignin. Eur J Appl Microbiol Biotechnol 1982, 15: 180-184. 10.1007/BF00511245
Ball AS, Betts WB, McCarthy AJ: Degradation of lignin-related compounds by actinomycetes. Appl Environ Microbiol 1989, 55: 1642-1644.
Bourbonnais R, Paice MG, Reid ID, Lanthier P, Yaguchi M: Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2'-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol 1995,61(5):1876-1880.
Lund M, Ragauskas AJ: Enzymatic modification of kraft lignin through oxidative coupling with water-soluble phenols. Appl Microbiol Biotechnol 2001,55(6):699-703. 10.1007/s002530000561
Raj A, Chandra R, Reddy MMK, Purohit HJ, Kapley A: Biodegradation of kraft lignin by a newly isolated bacterial strain, Aneurinibacillus aneurinilyticus from the sludge of a pulp paper mill. World J Microb Biotechnol 2007, 23: 793-799. 10.1007/s11274-006-9299-x
Chen YH, Chai LY, Zhu YH, Yang ZH, Zheng Y, Zhang H: Biodegradation of kraft lignin by a bacterial strain Comamonas sp. B-9 isolated from eroded bamboo slips. J Appl Microbiol 2012,112(5):900-906. 10.1111/j.1365-2672.2012.05275.x
Raj A, Reddy MMK, Chandra R, Purohit HJ, Kapley A: Biodegradation of Kraft-lignin by Bacillus sp. isolated from sludge of pulp and paper mill. Biodegradation 2007, 18: 783-792. 10.1007/s10532-007-9107-9
Giroux H, Vidal P, Bouchard J, Lamy F: Degradation of Kraft Indulin Lignin by Streptomyces viridosporus and Streptomyces badius . Appl Environ Microbiol 1988,54(12):3064-3070.
Chandra R, Abhishek A: Bacterial decolorization of black liquor in axenic and mixedcondition and characterization of metabolites. Biodegradation 2011, 22: 603-611. 10.1007/s10532-010-9433-1
Perez J, Dorado JM, Rubia TD, Martinez J: Biodegradation and biological treatment of cellulose, hemicellulose and lignin: An overview. Int Microbiol 2002, 5: 53-63. 10.1007/s10123-002-0062-3
Hofrichter M: Review: Lignin conversion by manganese peroxidase (MnP). Enzyme Microb Technol 2002, 30: 454-466. 10.1016/S0141-0229(01)00528-2
Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuzewska A, Hofrichter M, Wesenberg D, Rogalski J: Fungal laccase: properties and activity on lignin. J Basic Microbiol 2001, 41: 185-227. 10.1002/1521-4028(200107)41:3/4<185::AID-JOBM185>3.0.CO;2-T
Dick GJ, Torpey JW, Beveridge TJ, Tebo BA: Direct identification of a bacterial manganese (II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl Environ Microb 2008,74(5):1527-1534. 10.1128/AEM.01240-07
Hernes PJ, Benner R: Photochemical and microbial degradation of dissolved lignin phenols: implications for the fate of terrigenous dissolved organic matter in marine environments. J Geophys Res 2003, 108: 1-9.
Hernandez M, Rodriguez J, Perez MI, Ball AS, Arias ME: 13C NMR cross polarization and magic angle spinning (CPMAS) and gas chromatography/mass spectroscopy analysis of the products from a soda pulp mill effluent decolourised with two Streptomyces strains. Appl Microbiol Biot 1997, 74: 272-278.
Jose IJ, Baltasar M, Jose LG, Eduardo D: Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ Microbiol 2002,4(12):824-841. 10.1046/j.1462-2920.2002.00370.x
Perez-Pantoja D, De la Iglesia R, Pieper DH, Gonzalez B: Metabolic reconstruction of aromatic compounds degradation from the genome of the amazing pollutant-degrading bacterium Cupriavidus necator JMP134. FEMS Microbiol Rev 2008, 32: 736-794. 10.1111/j.1574-6976.2008.00122.x
Diaz E, Ferrandez A, Prieto MA, Garcia JL: Biodegradation of aromatic compounds by Escherichia coli . Microbiol Mol Biol Rev 2001,65(4):523-569. 10.1128/MMBR.65.4.523-569.2001
Harwood CS, Parales RE: The beta-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol 1996, 50: 553-590. 10.1146/annurev.micro.50.1.553
Cserháti M, Kriszt B, Szoboszlay S, Tóth Á, Szabó I, Táncsics A, Nagy I, Horváth B, Nagy I, Kukolya J: De novo genome project of Cupriavidus basilensis OR16. J Bacteriol 2012,194(8):2109-2110. 10.1128/JB.06752-11
Rothmel RK, Aldrich TL, Houghton JE, Coco WM, Ornston LN, Chakrabarty AM: Nucleotide sequencing and characterization of Pseudomonas putida catR : a positive regulator of the catBC operon is a member of the LysR family. J Bacteriol 1990,172(2):922-931.
Parsek MR, Shinabarger DL, Rothmel RK, Chakrabarty AM: Roles of CatR and cis, cis-Muconate in Activation of the catBC Operon, Which Is Involved in Benzoate Degradation in Pseudomonas putida. J Bacteriol 1992,174(23):7798-7806.
Segura A, Bunz PV, D’Argenio DA, Ornston LN: Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J Bacteriol 1999, 181: 3494-3504.
Zhou NY, Al-Dulayymi J, Baird MS, Williams PA: Salicylate-5-hydroxylase from Ralstonia sp. strain U2: a monooxygenase with close relationships to and shared electron transport proteins with naphthalene dioxygenase. J Bacteriol 2002, 184: 1547-1555. 10.1128/JB.184.6.1547-1555.2002
Hiromoto T, Fujiwara S, Hosokawa K, Yamaguchi H: Crystal structure of 3-hydroxybenzoate hydroxylase from Comamonas testosteroni has a large tunnel for substrate and oxygen access to the active site. J Mol Biol 2006, 364: 878-896. 10.1016/j.jmb.2006.09.031
Mashetty SB, Manohar S, Karegoudar TB: Degradation of 3-hydroxybenzoic acid by a Bacillus species. Indian J Biochem Biophys 1996, 33: 145-148.
Nakagawa Y, Sakamoto Y, Kikuchi S, Sato T, Yano A: A chimeric laccase with hybrid properties of the parental Lentinula edodes laccases. Microbiol Res 2010, 165: 392-401. 10.1016/j.micres.2009.08.006
Orth AB, Royse DJ, Tien M: Ubiquity of lignin-degrading peroxidases among various wood-degrading fungi. Appl Environ Microb 1993, 59: 4017-4023.
Kapich AN, Prior BA, Botha A, Galkin S, Lundell T, Hatakka A: Effect of lignocellulose-containing substrates on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enzyme Microb Tech 2004, 34: 187-195. 10.1016/j.enzmictec.2003.10.004
Delcher AL, Bratke KA, Powers EC, Salzberg SL: Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23: 673-679. 10.1093/bioinformatics/btm009
Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A: Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res 2005, 33: 121-124.
Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW: RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 2007, 35: 3100-3108. 10.1093/nar/gkm160
Schattner P, Brooks AN, Lowe TM: The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 2005, 33: 686-689. 10.1093/nar/gki366
Acknowledgements
This work was funded by the National Funds for Distinguished Young Scientists of China (50925417) and the National Natural Science Foundation of China (51074191).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YS designed the study, performed the experiments, analyzed the results, and wrote the manuscript. LYC co-designed the study. TCJ and YZH revised the manuscript. HZ and YZ carried out the GC-MS experiments. RHC and YHC contributed to writing of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shi, Y., Chai, L., Tang, C. et al. Characterization and genomic analysis of kraft lignin biodegradation by the beta-proteobacterium Cupriavidus basilensis B-8. Biotechnol Biofuels 6, 1 (2013). https://doi.org/10.1186/1754-6834-6-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1754-6834-6-1