Abstract
Background
Various water clusters including hexamers, heptamers, octamers, decamers and 1D or 2D infinite water chains in a number of organic and inorganic-organic hybrid hosts, have been reported.
Results
{[Cu(pydc)(amp)].3H2O}n has been hydrothermally synthesized and characterized by elemental analysis and by IR spectroscopy. A wide range of hydrogen bonds (of the O-H...O, N-H...O and N-H...N type) are present in the crystal structure. Hydrogen bond interactions between the co-crystallized water molecules led to formation of six-membered rings with chair conformation.
Conclusion
In {[Cu(pydc)(amp)].3H2O}n, there are three uncoordinated water molecules. Thermal methods confirm number of co-crystallized water molecules in polymer. Hydrogen bond interactions between the co-crystallized water molecules led to the formation of a six-membered ring with the chair conformation. These rings are part of a 1D chain containing six-membered O6 rings, which are alternately made from (H2O)6 and [(H2O)4O2] rings. [(H2O)4O2] rings are also in chair conformation.

Similar content being viewed by others
Background
Water is nature's solvent and plays a fundamental role in biological and chemical processes. It is the most abundant and cheapest solvent available for the development of new reagents and the study of chemical processes [1]. Understanding of the behavior of water clusters are important and structural information of small water clusters is the first step towards the understanding of the behavior of bulk water [2]. An extensive investigation of small and medium sized water cluster (H2O)n (n = 2-100) structures have been reported in recent years [3, 4]. Various water clusters including pentamers [5, 6], hexamers [6–8], heptamers [9–11], octamers [6, 8, 12, 13], decamers [6, 14–16] and 1D or 2D infinite water chains [2, 17, 18] in a number of organic and inorganic-organic hybrid hosts, have been structurally characterized. Recently, we also reported a tetrameric water cluster rings in the crystal structure of a new proton transfer system derived from pyridine-2,6-dicarboxylic acid and 2-amino-4-methylpyridine [19]. Several different conformations for the water hexamer, such as all-boat [20], all chairs or an intermediate between chair and boat [3, 21] have been characterized by x-ray crystallography. The search for an understanding of small water cluster structures is important for an improvement of our knowledge about structures of liquid water or ice. The water hexamer is the building block of ice Ih [6] and it is known that cyclic hexameric water clusters are present in liquid water [22, 23].
Recently, Synthesis of supramolecular compounds has attached great interest due to their interesting topologies and potential applications as functional materials. The reasonable design of supramolecular structure necessarily depends on the concepts of self-assembly and utilizes non-covalent forces as varied as follows: (1) coordination bonds, (2) hydrogen bonding, including both strong hydrogen bonding (e.g., O-H···O) and weak hydrogen bonding (e.g., C-H···O and C-H···N), (3) electrostatic and charge-transfer attractions, and (4) aromatic π stacking interactions [24]. Hydrothermal synthesis has been successful for the preparation of some supramolecular compounds. In continuation of our earlier work on the synthesis of metal complexes with polycarboxylate ligands in the presence of 2-aminopyrimidine and under hydrothermal condition [25, 26], the reaction of Cu(NO3)2 ·3H2O with Pyridine-2,6-dicarboxylic acid and 2-aminopyrimidine was investigated. Crystal structure of complex moiety of the copper complex, {[Cu(pydc)(amp)].3H2O}n, (pydc = pyridine-2,6-dicarboxylate ion, amp = 2-aminopyrimidine), was reported by Altin et al. in 2004 [27]. Here we report the formation of hexameric water clusters with chair configuration in the crystal structure of above mentioned compound. Crystals of {[Cu(pydc)(amp)].3H2O}n were obtained from the reaction of pyridine-2,6-dicarboxylic acid, 2-aminopyrimidine and Cu(NO3)2 ·3H2O under hydrothermal condition.
Results and discussion
Crystal structure
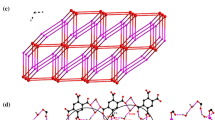
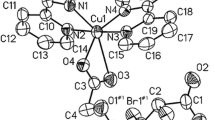
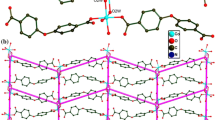
The crystal is built up of a complex Cu(pydc)(amp) and three co-crystallized water molecules (Scheme 1). The one-dimentional polymeric chains showing the connectivity and extending along the [010] direction are shown in Figure 1. Each CuII atom is coordinated by O,N,O-tridentate dipicolinate ligand (bound via pyridine N and two carboxylate O atoms) and one heterocyclic nitrogen atom of 2-aminopyrimidine ligand. Each metal ion is weakly connected to two neighboring ones, through two carboxylate bridging groups of dipicolinate and amino-nitrogen of NH2 group of 2-aminopyrimidine to form an infinite polymeric chain along the [010] direction. The crystal packing of complex is dominated by numerous hydrogen bonds of the O-H...O, N-H...O and N-H...N types (Figure 2).
The hydrogen bonding parameters are outlined in Table 1. As it was shown in Figure 3, uncoordinated water molecules are linked together and to carboxylate group of complex via hydrogen bonds. Hydrogen bond interactions between the co-crystallized water molecules (O-H···O hydrogen bonds, range from 2.74 - 2.79 Å) led to formation of a six-membered ring water cluster with chair conformation (Figure 4). These rings are part of a 1D chain (Figure 5) containing six-membered O6 rings, which are alternately made from (H2O)6 and [(H2O)4O2] rings (O-H···O hydrogen bonds range from 2.79-2.85 Å). Six- membered rings of [(H2O)4O2] are also in chair conformation (Figure 6) and the rings are linked as in cis-decalin. Hexameric water clusters with all-boat conformation are similar to ice Ih [20] and structures with all-chair conformation (all-cis) are different from ice Ih (all- trans) [3].
In addition, the crystal structure of complex is stabilized by some intermolecular C-H...O hydrogen bond interactions (C3-H3A...O2; D...A = 3.226(6) Å, < DHA = 167° and C11- H11A...O3W; D...A = 3.122(5) Å, < DHA = 131°).
IR Spectra
The FTIR spectrum of the compound (Figure 7) shows broad strong bands at the region 3275-3520 cm-1, which could be related to the existence of O-H···O hydrogen bonds between water molecules. It must have been coupled by other indicative peaks such as N-H and O-H stretching frequencies and the stretching frequencies due to the aromatic rings, which originally fall within this region [28–30].
Thermal Analyses
The thermogravimetric analysis curve (Figure 8) for compound shows that the weight loss from 200°C to 250°C, corresponds to the loss of three molecules of H2O (experimental value 15.20% and calculated value 14.30%).
The further exothermic decomposition began at 400°C and finished at 500°C indicating the complete removal of the organic part of the complex. The main product was CuO with a residual value of 20.0% (theoretical residual value, 21.1%).
Experimental
Materials and physical measurements
All purchased chemicals were of reagent grade and used without further purification. IR spectra were recorded using FTIR Spectra Bruker Tensor 27 spectrometer (KBr pellets, 4000-400 cm-1). TGA/DTA measurements were performed at heating rate of 10°C min-1 in the temperature range of 25-800°C, under nitrogen flow of 20 mL min-1 on instrument Shimadzu DTG-50H. Elemental analyses were performed using a Costech ECS 4010 CHNS analyzer.
Synthesis of complex
Pyridine-2,6-dicarboxylic acid (0.167 g, 1 mmol) was dissolved in 10 ml deionized water containing 0.08 g (2 mmol) of NaOH and stirred for 30 min at room temperature. An aqueous solution of 0.241 g (1 mmol) of Cu(NO3)2.3H2O and 0.095 g (1 mmol) of 2-aminopyrimidine was added to pyridine-2,6-dicarboxylate acid solution. Reaction mixture was placed in a Parr-Teflon lined stainless steel vessel. It was sealed and heated at 130°C for 4 h. Blue crystals of the complex were obtained upon slow cooling (Yield 91%). IR (KBr) (, cm-1): 3275-3520 (b), 1669 (s), 1630 (s), 1594 (s), 1563(s), 1432 (s), 1385 (s), 1349 (s), 1199 (m), 1179 (m), 1084 (s), 914 (w), 775 (m), 680 (m), 487 (w). Anal. Calc. for C11H14CuN4O7 (M = 377.8): C, 49.93, H, 3.70, N, 14.82%. Found: C, 46.19, H, 3.58, N, 14.08%.
Crystallography
X-ray structure analysis was carried out on a Smart 1000 CCD area detector (Mo-Ka radiation, graphite monochromator, λ = 0.71073 Å, at 120(2) K). The crystal structure was solved by direct methods and refined by full-matrix least squares methods based on F2 values against all reflections including anisotropic displacement parameters for all non-H atoms. All hydrogen atoms were located from difference Fouier maps with exception of H3A, H4A, H5A, H9A, H10A and H11A, which were calculated from geometrical point of view. The H atoms were refined in isotropic approximation in riding model with the Uiso(H) parameters equal to 1.2 Ueq(C, N) and 1.5 Ueq(O), where U(C, N, O) are respectively the equivalent thermal parameters of the carbon, nitrogen and oxygen atoms to which corresponding H atoms are attached. Data collection, cell refinement and data reduction were performed with SMART (Bruker, 1998), SAINT Plus (Bruker, 1998) [31] respectively. Program used to solve structure: SHELXTL (Sheldrick, 2008) and program(s) used to refine structure: SHELXTL [32]. The molecular graphics were done with MERCURY (Version 2.3) [33]. The crystal parameters and data collection for compound are summarized in table 2.
Conclusion
In this paper, hydrothermally preparation, spectroscopic characterization, thermal properties and crystal structure of a copper complex, {[Cu(pydc)(amp)].3H2O}n, were presented. There are a wide range of intermolecular and intramolecular hydrogen bonds in this crystal structure which play important role in its stabilization. 1D chains containing six-membered O6 rings, which are alternately made from (H2O)6 and [(H2O)4O2] rings, are formed by hydrogen bond interactions.
Supplementary material
CCDC 772299 for complex contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html [or from the Cambridge Crystallographic Data Centre (CCDC), 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; email: http://deposit@ccdc.cam.ac.uk]. Structure factor table is available from the authors.
References
Luna-García R, Damián-Murillo BM, Barba V, Höpfl H, Beltrán HI, Zamudio-Rivera LS: Structural relationship between a host included chain of spirocyclic water hexamers and bulk water - the role of water clusters in self assembly and crystallization processes. Chem Commun. 2005, 5527-5529.
Ma B-Q, Sun H-L, Gao S: Formation of Two-Dimensional Supramolecular Icelike Layer Containing (H2O)12 Rings. Angew Chem Int Ed. 2004, 43: 1374-1376. 10.1002/anie.200353097.
Rodríguez-Cuamatzi P, Vargas-Díaz G, Höpfl H: Modification of 2D Water That Contains Hexameric Units in Chair and Boat Conformations-A Contribution to the Structural Elucidation of Bulk Water. Angew Chem Int Ed. 2004, 43: 3041-3044. 10.1002/anie.200453957.
Sreenivasulu B, Vitta JJ: Water Aggregates Hosted by the Metal Complexes of Reduced Schiff-base and Related Ligands. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry. 2008, 38: 118-124.
Ghosh SK, Bharadwaj PK: Supramolecularly assembled pentameric and octameric water clusters stabilized by a mixed complex of Ni(II). Inorganica Chimica Acta. 2006, 359: 1685-1689. 10.1016/j.ica.2005.12.071.
Yang J, Ma J-F, Liu Y-Y, Ma J-C, Jia H-Q, Hu N-H: Two New CuII Coordination Polymers: Studies of Topological Networks and Water Clusters. Eur J Inorg Chem. 2006, 1208-1215.
Moorthy JN, Natarajan R, Venugopalan P: Characterization of Planar Cyclic Ring Form of Water Hexamer in an Organic Supramolecular Complex. An Unusual Self-Assembly of Bimesityl-3,-3'-Dicarboxylic Acid. Angew Chem Int Ed. 2002, 41: 3417-3420. 10.1002/1521-3773(20020916)41:18<3417::AID-ANIE3417>3.0.CO;2-B.
Doedens RJ, Yphannes E, Khan MI: Novel water clusters in the crystalline state: structures of a symmetrical, cyclic hexamer and an 'opened-cube' octamers. Chem Commun. 2002, 62-63.
Mir MH, Wang L, Wong MW, Vittal JJ: Water helicate (H2O)7, hosted by a diamondoid metal-organic framework. Chem Commun. 2009, 4539-4541.
Mir MH, Vittal JJ: Single-Crystal to Single-Crystal Transformation of Cyclic Water Heptamer to Another (H2O)7 Cluster Containing Cyclic Pentamer. Crystal Growth & Design. 2008, 8: 1478-1480. 10.1021/cg800244g.
Hedayetullah Mir M, Vittal JJ: Phase Transition Accompanied by Transformation of an Elusive Discrete Cyclic Water Heptamer to a Bicyclic (H2O)7 Cluster Angew. Chem Int Ed. 2007, 46: 5925-5928. 10.1002/anie.200701779.
Atwood JL, Barbour LJ, Ness TJ, Raston CL, Raston PL: A Well-Resolved Ice-like (H2O)8 Cluster in an Organic Supramolecular Complex. J Am Chem Soc. 2001, 123: 7192-7193. 10.1021/ja015757k.
Blanton WB, Gordon-Wylie SW, Clark GR, Jordan KD, Wood JT, Geiser U, Collins TJ: Synthesis and crystallographic characterization of an octameric water complex (H2O)8. J Am Chem Soc. 1999, 121: 3551-3552. 10.1021/ja982100z.
Jin Y, Che YX, Zheng JM: Supramolecularly assembled decameric water cluster stabilized by dichromate anions in complex of Ni(II). Inorg Chem Comm. 2007, 10: 514-516. 10.1016/j.inoche.2007.01.018.
Barbour LJ, Orr GW, Atwood JL: Characterization of a well resolved supramolecular ice-like (H2O)10 cluster in the solid state. Nature. 1998, 393: 671-673. 10.1038/31441.
Barbour LJ, Orr GW, Atwood JL: An intermolecular (H2O)10 cluster in a solid-state supramolecular complex. Chem Commun. 2000, 859-860.
Sreenivasulu B, Vittal JJ: Encapsulation of Hydrogen- Bonded Water Molecules in a Staircase Coordination Polymer. Angew Chem Int Ed. 2004, 43: 5769-5772. 10.1002/anie.200460516.
Leong WL, Vittal JJ: One-Dimensional Coordination Polymers: Complexity and Diversity in Structures, Properties, and Applications. Chem Reviews. 2011, 111: 688-764. 10.1021/cr100160e.
Sharif MA, Tabatabaee M, Adinehloo M, Aghabozorg H: 2-Amino-4-methylpyridinium 6-carboxypyridine-2-carboxylate sesquihydrate. Acta crytallogr. 2010, E66: o3232-
Park K-M, Kuroda R, Iwamoto T: A Two-Dimensional Ice with the Topography of Edge-Sharing Hexagons Intercalated between CdNi(CN)4 Layers. Angew Chem Int Ed. 1993, 32: 884-886. 10.1002/anie.199308841.
Lunelli B, Soave R, Destro R: Structure and stability of bis(dimethylamino)squaraine and its hydrates: A study using XRD, IR spectroscopy, and thermodynamic measurements. Phys Chem Chem Phys. 1999, 1: 1469-1477.
Ludwing R: Water: From Clusters to the Bulk. Angew Chem Int Ed. 2001, 40: 1808-1827. 10.1002/1521-3773(20010518)40:10<1808::AID-ANIE1808>3.0.CO;2-1.
Silverstein KAT, Haymet ADJ, Dill KA: A simple model of water and thr hydrophobic effect. J Am Chem Soc. 1998, 120: 3166-3175. 10.1021/ja973029k.
Tabatabaee M, Sharif MA, Vakili F, Saheli S: Hydrothermal synthesis and structural studies of a new coordination polymer of lanthanum(III) with benzene-1,2,4,5-tetracarboxylic acid and 4,4'-bipyridine. J Rare Earth. 2009, 27: 356-361. 10.1016/S1002-0721(08)60250-1.
Tabatabaee M, Abbasi F, Kukovec B-M, Nasirizadeh M: Preparation and structural, spectroscopic, thermal, and electrochemical characterizations of iron(III) compounds containing dipicolinate and 2-aminopyrimidine or acridine. j Coor Chem. 2011, 64: 1718-1728. 10.1080/00958972.2011.580843.
Tabatabaee M: (2-Aminopyrimidine-κN1)diaqua- (pyridine-2, 6-dicarboxylato-κ3-O2, N, O6)-nickel(II) monohydrate. Acta Crystallogr. 2010, E66: m647-m648.
Altin E, Kirchmaier R, Lentz A: Crystal structure of (2-aminopyrimidine)-(pyridine-2,6-dicarboxylato)-copper(II) trihydrate, Cu(C4H5N3)(C7H3NO4) · 3H2O. Z Kristallogr NCS. 2004, 219: 35-36.
Aghabozorg H, Sadr-khanlou E, Shokrollahi A, Ghaedi M, Shamsipur M: Synthesis, Characterization, Crystal Structures, and Solution Studies of Ni(II), Cu(II) and Zn(II) Complexes Obtained from Pyridine-2,6-dicarboxylic Acid and 2,9-Dimethyl-1,10-Phenanthroline. J Iran Chem Soc. 2009, 6: 55-70.
Aghajani Z, Aghabozorg H, Sadr-khanlou E, Shokrollahi A, Derki S, Shamsipur M: Chromium(III) and Calcium(II) Complexes Obtained from Dipicolinic Acid: Synthesis, Characterization, X-Ray Crystal Structure and Solution Studies. J Iran Chem Soc. 2009, 6: 373-385.
Tabatabaee M, Kukovec B-M, Kazeroonizadeh M: A unique example of a co-crystal of [Ag(atr)2][Cr(dipic)2] (dipic = dipicolinate; atr = 3-amino-H-1,2,4-triazole) and dinuclear [Cr(H2O)(dipic)(μ-OH)]2, with different coordination environment of Cr(III) ions. Polyhedron. 2011, 30: 1114-1119. 10.1016/j.poly.2011.01.010.
Bruker : SADABS, AMART and SAINT-Plus. 1998, Bruker AXS INC., Madison, Wisconsin, USA
Sheldrick GM: A short history of SHELX. Acta Crystallogr A. 2008, 64: 112-122. 10.1107/S0108767307043930.
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, Streek J, Wood PA: Mercury CSD 2.0 - new features for the visualization and investigation of crystal structures. J Appl Crystallogr. 2008, 41: 466-470. 10.1107/S0021889807067908.
Acknowledgements
The author is grateful to Dr. Hopfl for his valuable discussion. She also acknowledges Islamic Azad University, Yazd Branch, for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tabatabaee, M. Supramolecular assembled of hexameric water clusters into a 1D chain containing (H2O)6 and [(H2O)4O2] stabilized by hydrogen bonding in a copper complex. Chemistry Central Journal 6, 5 (2012). https://doi.org/10.1186/1752-153X-6-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-153X-6-5