Abstract
Invasive cell growth and migration is usually considered a specifically metazoan phenomenon. However, common features and mechanisms of cytoskeletal rearrangements, membrane trafficking and signalling processes contribute to cellular invasiveness in organisms as diverse as metazoans and plants – two eukaryotic realms genealogically connected only through the last common eukaryotic ancestor (LECA). By comparing current understanding of cell invasiveness in model cell types of both metazoan and plant origin (invadopodia of transformed metazoan cells, neurites, pollen tubes and root hairs), we document that invasive cell behavior in both lineages depends on similar mechanisms. While some superficially analogous processes may have arisen independently by convergent evolution (e.g. secretion of substrate- or tissue-macerating enzymes by both animal and plant cells), at the heart of cell invasion is an evolutionarily conserved machinery of cellular polarization and oriented cell mobilization, involving the actin cytoskeleton and the secretory pathway. Its central components - small GTPases (in particular RHO, but also ARF and Rab), their specialized effectors, actin and associated proteins, the exocyst complex essential for polarized secretion, or components of the phospholipid- and redox- based signalling circuits (inositol-phospholipid kinases/PIP2, NADPH oxidases) are aparently homologous among plants and metazoans, indicating that they were present already in LECA.
Reviewer: This article was reviewed by Arcady Mushegian, Valerian Dolja and Purificacion Lopez-Garcia.
Similar content being viewed by others
Introduction
Invasive cell growth and migration, including processes such as tumor or immune cell invasion into tissues or neurite outgrowth, is usually considered a topic of biomedically relevant metazoan biology. However, if we understand cellular invasiveness as a general ability to move and/or grow through a heterogenous (semi)solid environment, which may or may not be a living tissue, this phenomenon appears to be widespread among a huge variety of organisms. Recent research, revealed common features and mechanisms of cytoskeletal (mainly actin) rearrangements, membrane trafficking and (not only) GTPase-based signalling, contributing to cellular invasiveness in organisms as diverse as metazoans and plants. Here we summarize such common features shared by organisms connected only via the root of the eukaryotic tree the last common eukaryotic ancestor – LECA [1], aiming towards reconstructing a basic “toolbox” of common, and probably evolutionarily conserved, mechanisms involved in this cellular process.
Model invasive structures
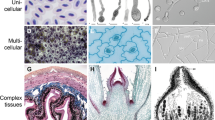
While cellular invasiveness is widespread among eukaryotes, we shall focus here only on invasive protrusions generated by model cell types from two major eukaryotic lineages – metazoans and plants. As strange as it might sound, plant cells can namely grow in an invasive manner, as documented in particular for root hairs and pollen tubes. Representative cells of our chosen models are shown in Figure 1.
Model invasive structures. Left: detailed image of an invadopodial structure from RsK4 sarcoma cells on dermis-based matrix, showing thin F-actin fibers (green) capped with phosphotyrosine signal (red). Middle: a polarised rat embryo hippocampal neuron after three days in culture, expressing cytoplasmic green fluorescent protein (green) and immunolabeled for neuron-specific beta-III-tubulin (red), which marks the axon. Arrow – cell body with dendrites, arrowhead – axon. Right: an in vitro cultured tobacco pollen tube labeled with fluorescent antibodies against a component of the Exocyst complex (red) and tubulin (green). The broad-spectrum signal in the pollen grain (located at the top) corresponds to autofluorescence. Tube length can reach several milimeters after a few hours in culture.
It has to be stressed that invasive cells are found not only in plants and metazoans, but especially fungi provide a plethora of alternative opisthokont models. The budding yeast Saccharomyces cerevisiae has served as a long time paradigmatic cell polarity model that helped to pinpoint the central position of RHO clade GTPases as polarity regulators. Much of the machinery responsible for yeast bud formation is shared also by species capable of true invasive hyphal growth (reviewed e.g. in [2–4]). At least one other eukaryotic supergroup - the chromalveolates - also contains organisms capable of invasive growth, but their characterization is lagging far behind studies in opisthokonts and plants. For instance, penetration of host tissues by Phytophtora sp. has been only recently characterized morphologically, and very little is known about the underlying molecular mechanisms [5, 6].
Our selection of models is, out of necessity, restricted by the need to maintain this text at a manageable size and within the scope of the authors’area of research, focused on multicellular animals and plants. We therefore chose two metazoan invasive cell types (transformed or tumor cells and neurons) to represent the opisthokonts, and root hairs and pollen tubes as examples of plant cell invasiveness.
Invadopodia of transformed metazoan cells
Invadopodia (recently reviewed in [7–10] are stable actin-rich protrusions formed at the ventral surface of invasive tumor or transformed cells cultured on appropriate extracellular matrix (ECM) substrates such as gelatine, fibronectin, collagen, or laminin [11] and displaying focalized proteolytic activity towards the substrate [12, 13]. Molecular components involved include integrins, elements of signalling machineries, soluble and membrane-bound proteases (including matrix metalloproteases, or MMPs), and prominently actin and actin-associated proteins such as e.g. cortactin [14–18]. Focal degradation of the ECM at invadopodia involves tight integration of the membrane remodelling, trafficking and signalling machineries, similar to initial steps of tumor cell dissemination.
Microscopically invadopodia appear as roundish actin-rich structures at the ventral surface of cells, not confined to the cell periphery, containing cortactin (or dynamin 2, fascin and others) and/or phosphotyrosine, and associated with sites of substrate degradation, [19, 20]. Another feature is their extended half-life of up to 2 hours or more [19, 21] as compared to podosomes, related protrusive adhesions [22–24]. Recent studies using advanced 3D imaging methods have highlighted the complexity of actin regulation in invadopodia formation and elongation, further elucidated the specific role of some fundamental players (including microtubules in later stages of invadopodia elongation) and provided novel morphological insights [25–27].
We use invadopodia mainly as a model for understanding the roles of the actin remodelling system, the small GTPases, and finally lipids, which are recently entering the picture in big strides.
Vertebrate pyramidal neurons – a model of normal cell invasion
The main neuronal model of our review will be pyramidal neurons from the mammalian cerebral cortex or hippocampus, which can extend investigative protrusions to facilitate directed migration from their place of origin to their final destination in the forebrain, and subsequently migrate by climbing up scaffolds of specialised progenitor cells – the radial glia [28–31].
Several aspects of neuronal migration resemble forms of locomotion utilised by other metazoan cell types. These basic principles include chemotaxis [32], reorientation of the centrosome and Golgi apparatus to the base of the leading protrusion [33], and coordinated reorganisation of the F-actin and microtubule cytoskeletons enabling directed vesicle trafficking towards the leading edge [34–38]. Regulated attachment and dissociation takes place between migrating neurons and the radial glia on which they move, through integrin-rich adhesions and gap junctions [39, 40], followed by terminal dissociation from radial glia through specialised molecules such as Secreted protein acidic and rich in cysteine (SPARC)-like 1 and reelin, allowing cessation of migration once neurons have reached their final destination [41, 42]. Perturbation of these processes is associated with misdirected and/or arrested migration of pyramidal neurons [43–45].
Pyramidal neurons isolated from rodent embryos can undergo a sequential process of maturation in vitro on adhesive substrates such as laminin or poly-lysine [46–49], developing into mature cells with a single axon and multiple dendrites. This model system has been mainly used for the study of axon specification, though it may have some limitations [50, 51]. For instance, the role of centrosome positioning, or distinguishing signals that polarise the cell from those that promote neurite outgrowth remains controversial [45, 51–56]. Nevertheless, post-mitotic neurons are one of the best models for studying the coordinated interplay between the extracellular environment and internal signals in normal cell invasiveness.
Plant cell invasiveness: root hairs and pollen tubes
The two best studied invasive plant cell types are root hairs and pollen tubes, which elongate by tip growth and penetrate rather complex environments. Root hairs explore random micro-spaces between soil particles, while the growing pollen tube tip, guided by chemotaxis, invades highly organized live pistil tissues to deliver sperm cells to their two targets within the female gametophyte. While the chemotropic guidance is reminiscent of metazoan cell invasiveness, the molecules involved, such as pectins and cystein-rich lipid-transfer protein-like peptides [57], are very different, indicating evolutionary convergence rather than conservation. In another case of convergence with invasive metazoan cells, invasion of pollen tubes into intracellular spaces of the transmitting tract involves secretion of extracellular matrix-loosening enzymes [58]. For instance, xylanases released from pollen grains and expansins secreted by the growing tube help to drill a passage through the cell walls of the transmitting tract in maize [59]. Fortunately, both cell types can be grown in vitro and studied in the absence of the complex matrix that is being invaded in situ.
Plant cells share some features absent in metazoans, in particular the presence of a (semi-)rigid cell wall and a water solution-filled vacuole, which together produce outward pressure at the cytoplasmic membrane-cell wall interface, known as turgor and contributing, together with local changes in mechanical properties of the cell wall to cell expansion or its regulation [60, 61]. The plethora of plant-specific cell wall-modifying activities involved is beyond the scope of this review, albeit they can be understood as ultimate effectors of the conserved pathways discussed below.
Development of both root hairs and pollen tubes begins with specification of their outgrowth site (plant cells lack conventional centrosomes, which can contribute to the positioning of invasive projections e.g., in neurons). While pollen tubes emerge from pre-existing pores within the pollen grain exine, root hairs appear at the rootward end of trichoblasts, a subset of rhizodermis cells that are, in the best characterized model Arabidopsis thaliana, developmentally determined by a well-characterized transcriptional circuit [62, 63] and polarized by auxin gradient-based signalling [64]. Local relaxation of cell wall results in bulge formation followed by actual root hair outgrowth [65–68].
In both cell types, the growing invasive tip exhibits an apical cytoplasmic “clear zone”, devoid of obvious microscopically visible structures but containing numerous secretory vesicles, where surface expansion associated with vigorous membrane turnover takes place. Dynamic fine F-actin arrays participate in this process, while microtubules may contribute to controlling growth direction (see below).
The growth rate of both pollen tubes and root hairs often oscillates, accompanied by periodic changes in extracellular pH, reactive oxygen species (ROS) and cytoplasmic Ca2+ concentrations [69–71].
Root hairs and pollen tubes are not the only invasive plant cell types. Epidermal pavement cells of aboveground organs undergo localized expansion, coordinated among neighbors and resulting in the formation of puzzle-like interlocked lobes. Recent studies in Arabidopsis[72, 73] revealed that molecular mechanisms working in pavement cells interdigitation are related to those responsible for invasive growth of root hairs and pollen tubes.
Besides established models such as Arabidopsis, the moss Physcomitrella patens is gaining on importance due to ease of its genetic manipulations. Moss protonemata, branched chains of cells invading soil or growth medium in an almost mycelium-like fashion, can therefore serve as another interesting model system for the study of plant cell invasiveness. However, as the bulk of data on plant cell invasiveness comes from root hairs and pollen tubes, we focus mainly on these two models.
The great small GTPases
The Ras superfamily of small molecular weight GTPases controls fundamental cellular functions including those essential for invasive growth. Due to very slow spontaneous intrinsic GTP hydrolysis they act as binary molecular switches, converting between an active, guanosine triphosphate (GTP)-bound state, interacting with a number of effector proteins and thus promoting cellular responses, and an inactive, guanosine diphosphate (GDP)-bound state. Transitions between these states are catalyzed by GTPase-activating proteins (GAPs) stimulating “switch off” hydrolysis of GTP to GDP and by GDP/GTP exchange factor (GEFs) inducing “switch on” charging by fresh GTP [74–76].
Rac/Rho/Rop – the invasion leaders
Small GTPases of the RHO clade, including opisthokont Rho, Rac, and Cdc42 and plant Rop, participate in the control of cell polarity, motility and also invasive growth via their interaction with various effectors, including protein kinases, actin nucleators, secretory pathway regulators and phospholipases [77–79].
RHO GTPases promote cell invasiveness and motility through their ability to control plasma membrane protrusions and the turnover and integrity of adhesions [77]. In fibroblasts, Rac plays a central role in lamellipodia and membrane ruffling, Rho in stress fibre and focal adhesion formation and Cdc42 controls microspike and filopodia formation and is a master regulator of cell polarity [80–82]. Cdc42 appears to be the main RHO GTPase implicated in the formation of invadopodia, with roles spanning from the regulation of actin remodelling to the control of ECM degradation. Dominant-active mutants of Cdc42 or Rac enhanced both diffuse and dot-like(invadopodia-associated) fibronectin degradation [83] while Cdc42 downregulation suppressed invadopodia formation [21]. Cdc42, but not Rho A or Rac, was detected at invadopodia [19]. The interaction of the multi-domain polarity protein IQGAP1 with proteins of the secretion machinery regulating metalloproteinase activity at invadopodia is triggered by active Cdc42 and is essential for matrix degradation [84].
In neurons, Rac1 is essential for neurite outgrowth in vivo. An inactive, dominant Rac1 mutant inhibited axonal growth in Drosophila [85]. Progressive deletion of both Drosophila Rac genes (DRac1 and DRac2) as well as the highly related Mtl gene (Mtl), caused a stage-wise simplification of axon branching, defects in axon guidance and inhibition of axon growth [86–88]. Genetic analysis in Drosophila pinpointed the involvement of Rac1 upstream regulators, including Notch and especially the GEFs Trio, Vav and DOCK180, in the formation of neuronal protrusions [89–91]. Forebrain-specific deletion of mouse Rac1 revealed a specific need for Rac1 during axon guidance, rather than the initiation of neurite outgrowth [92, 93]. Functional overlap between Rac1, Rac2 and Rac3 in the developing mammalian cerebral cortex is confirmed by conditional, double deletion of Rac1 and Rac3 [94]. Rac1 loss from neuronal progenitors caused reduced elaboration of lamellipodia, impeding axon growth [95]. Rac1 is also essential for the plasma membrane localisation of the actin regulating protein WAVE, suggesting that in migrating cerebellar neurons lamellipodia are controlled by WAVE and the Arp2/3 complex. While deletion of Rac1 caused defects in the migration of interneurons [92] deletion of Cdc42 caused defects in the positioning of cortical progenitors, thus altering the fate of neurons that arise from them [96, 97].
In contrast to the separation of functions between Rac and Cdc42 in metazoans, the sole plant RHO GTPase family of Rops regulates both invasive growth and cell fate [98–100]. Locally activated Rops leading the invasion in plant cells are well documented at very early pre-bulge stages of root hair initiation, as well as during later stages of pollen tube and root hair invasive growth [101–104]. Rop GTPases are activated mostly by a type of GEF proteins absent in Opisthokonts – the PRONE-GEFs [105] - whose activity is regulated by interactions with specific transmembrane Ser/Thr receptor kinases (RLKs; [105, 106]). This suggests crucial position of RLK signalling in the regulation of localized (invasive) plant cell growth and provides opportunity for achieving spatial and temporal specifity through choice between diverse members of the enormous plant RLK family [107].
During pollen polarization and germination, extracellular peptides regulate a specific RLKs/PRONE-GEF/Rop signalling module [106]. In root hairs other RLKs/PRONE-GEF/Rop modules may be controlled directly by the interaction with the cell wall macromolecules, their fragments or even by local auxin maxima [68, 108]. Also in the developing interdigitated lobed epidermal pavement cells Rop GTPases are crucial for reciprocal invasion of lobes into the neighbouring pavement cells, possibly instructed by local auxin maxima [72, 73].
While relatively little is known concerning the role of RHO GTPase regulatory proteins in model invasion cells structures, especially the GEFs arise as possible master regulators of downstream signaling from RHO in diverse systems [109] and might be important players in invasion and metastasis [110]. The limited evidence available points to the Cdc42-specific GEF Fgd1 [111] as a regulator of Cdc42 activity in invadopodia formation [112]. In plants, specific localization of Rop activity down-regulating GAPs to sub-apical region of the plasmalemma of tip-growing cells contributes, along with Rop GDP dissociation inhibitor (GDI), to the sharp restriction of active Rops to the expanding membrane domain [113].
Arfs and rabs – small GTPases involved in invasion
The ADP-ribosylation factor (ARF) class of small GTPases regulates endosomal membrane trafficking, exocytosis, and actin remodelling at the cell surface across eukaryots [114, 115]. In mammals ARF6, but not the other members, participates in acquisition of an invasive phenotype downstream of v-Src activation, by promoting traffic-mediated adherens junction disassembly and epithelial cell migration [116]. ARF6 is localized at invadopodia [117, 118], and its expression levels correlate with the invasive phenotype [117]. The mechanism of ARF6 action in invadopodia formation and activity, though not completely defined, appears to be dependent on ERK activation [118, 119].
In model neuron systems, ARF6, its GEF (ARNO) and GAPs regulate dendritic branching in a pathway downstream of Rac [120–123]. ARF GTPases, their regulators and their interaction with specific membrane lipids also contribute to invasive growth of plant root hairs [124–126]. AGD1, a class I ADP ribosylation factor GTPase-activating protein, was proven to be important for maintaining straight growth in Arabidopsis root hairs, since loss of function mutations in the AGD1 gene resulted in wavy root hair growth [125].
The Rab proteins comprise a large family of abundant small GTPases that regulate exocytic and endocytic intracellular trafficking by controlling membrane identity, vesicle formation, motility and fusion [127–129]. At least two members of this family, Rab8 [130–132] and Rab27b [132] have been directly implicated in invasive tumor cell migration. The findings are consistent with a function of these Rabs in invadopodia formation or function and underscore the direct relationship between the intracellular trafficking machinery and ECM degradation at invadopodia. In neurons, much of the research on Rabs has focused on their synaptic activity; some of them, in particular Rab8 (reviewed in [133–135]), are also crucial for neuronal morphogenesis, including invasiveness.
Plant Rab8 homologues, as well as Rab11 homologues (representing 26 out of total 56 Arabidopsis Rabs), control directional cell growth via regulation of exocytosis and recycling of PM proteins [129]. Localization and functions of plant Rab11 and Rab8 proteins is related to plant-specific function of the Trans Golgi Network (TGN) acting at the cross-roads of the exocytotic and endocytotic pathways as an early endosome [136]. Specific Rab11 and Rab8 paralogues and their known PIP kinase effectors (see below) participate in pollen tube and root hair growth in Arabidopsis or tobacco and localize, as expected, to the actively growing domains of the cell surface [137–139].
The cytoskeleton(s)
Since we intend to compare shared features of plant and metazoan cell invasiveness, and plant cells lack conventional intermediate filaments, we shall focus on the two cytoskeletal systems common to all our models – i.e. the actin microfilaments and, to a lesser extent, also the microtubular cytoskeleton, and their associated proteins.
Actin is important for the invasion process
Mechanisms generating the forces behind membrane remodelling and protrusion at invadopodia still need to be completely defined; however, microfilaments clearly play a central part. Two main hypotheses have been proposed [140], one assuming that constant growth of a branched actin meshwork propels invadopodia into the underlying matrix, similar to lamellipodia protrusion, while the other suggests that the mechanical force required to overcome substrate stiffness is provided by actin bundles originating from the branched network, akin to filopodia formation. Both dendritic and bundled actin networks, assembled at the sites of contact with the ECM and than forming cores for invadopodia formation, may be relevant in various situations. Elongation of invadopodia apparently relies on the same machinery but requires participation of microtubules to extend invadopodia beyond 5 μm. Intermediate filaments may contribute at later stages [26, 140].
Microfilaments are also intimately involved in the initiation and extension of neuronal protrusions. Global application of an F-actin depolymerising drug, cytochalasin D, induced multiple axon-like neurites in unpolarised hippocampal neurons in vitro, and axonal characteristics were induced in a single unpolarised neurite after focal application of cytochalasin D. This approach has also provided a useful tool for dissecting actin-related signalling pathways. For instance, inhibition of RHO GTPases caused a similar phenotype as cytochalasin D, suggesting their instrumental role during axon development [50, 140]. Furthermore, perturbation of neuronal polarisation e.g. by mislocalization of the p21-activated kinase (Pak-1) can be rescued by cytochalasin D, suggesting signalling pathways downstream of small GTPases that may affect F-actin organisation and/or turnover during neuronal polarisation [141].
Actin dynamics is central also for plant cell invasive growth [142, 143]. However, its role in walled cells is likely to differ from that in soft-bodied animal cells or amoebae. Plant actin is important mainly for delivery and targeting of secretory vesicles to the growing plasmalemma domain, enabling at the same time the modification of cell wall properties and thus also changes in its mechanical properties allowing directed cell growth driven by the turgor pressure agains the yielding cell wall.
The very growing tips of pollen tubes (and less distinctly also root hairs or moss protonemata) are apparently free of long visible actin filaments. However, subapical F-actin structures (a “F-actin fringe” of very short and highly dynamic F-actin arrays) are important for secretory vesicle formation, delivery, recycling, and in particular spatial targeting [144–146]. Surprisingly, while complete actin depolymerisation by Latrunculin B or Cytochalasin D inhibits tip growth, mild doses of these inhibitors, destroying the fringe but not actin bundles, cause temporary tip swelling, i.e. enhanced surface expansion [147–149]. Stimulation of exocytosis after gentle actin depolymerization was observed also in a variety of metazoan cells [150–153]. Local balance between soluble G-actin, fine F-actin arrays and F-actin bundles may thus be crucial for maintaining cell expansion localized during invasive growth.
The role of actin nucleators
The “clasical” actin nucleation machinery based on the Arp2/3 complex, forming branched actin arrays, regulated and stabilized by cortactin, and its activator N-WASP [154, 155], is fundamental in the formation of invadopodia (as well as lamellipodia). A FRET-based study showed that N-WASP is active at the base of the invadopodial protrusions in a rat mammary carcinoma cell line [156]. In another transformed cell model, actin in actively degrading invadopodia formed dynamic structures with distinct “head” and “tail” sections, both containing Arp2/3 and N-WASP [19], resembling the actin “comets“associated with invading bacteria [157, 158].
Arp2/3-mediated actin nucleation participates also in the advancement of axon growth cones in developing neurites [159, 160]. Dendritic spines and their precursors in hippocampal neurons contain Arp2/3-nucleated arrays of fine actin filaments [161]. N-WASP – induced actin polymeration is also involved in neuronal growth factor (NGF)-induced differentiation of PC12 cells; intriguingly, some aspects of this process are inhibited by overexpression of the Exo70 subunit of the Exocyst complex, suggesting a strong link between the actin and membrane dynamics [162].
The Arp2/3 complex and its activators (SCAR/WAVE) play also a role in plant cell growth; although Arabidopsis loss-of-function mutants have mostly moderate phenotypic consequences affecting mainly trichomes, but also the shape and interdigitation of epidermal pavement cells (reviewed in [163]). Plant SCAR/WAVE- Arp2/3 module is activated by the Rop GEF SPIKE [164].
Major F-actin nucleators in land plants are, however, apparently the formins (FH2 proteins; [165–167]). Indeed, formins have entered the picture recently also in metazoan cells. The formins mDia1–3, cooperating with the Arp2/3 complex, are required for invadopodia assembly and function, [168], similar to the machinery acting in lamellipodia and filopodia [169]. In neurons, a different branch of the extensive formin family – the DAAM formins – participate in growth cone function [170].
Although the study of plant formins is hampered by functional overlaps within the large gene family, some observations indicate their participation in invasive tip growth. Heterologous expression or overexpresion of several plant formins caused loss of cell polarity in pollen tubes and/or changes in actin dynamics, mainly extensive bundling [144, 171, 172]. Overexpression of another Arabidopsis formin (AtFH8) elicited root hair depolarization and branching [173] while its non-functional derivative inhibited root hair growth [174].
Yeast and metazoan mDia-related formins are well-described direct effectors of RHO GTPases [175], whereas plant formins lack the characteristic conserved GTPase binding domain (FH3/GBD) and cannot thus be localized to the membrane by their interaction with Rho GTPases. Nevertheless, some plant formins contain a transmembrane domain (Class I) or a presumably lipid-binding PTEN domain (Class II; [165–167]). In addition, non-seed plants possess a third class of formins with a putative GTPase-binding domain related to RhoGAPs [167]. In invasive protonemata of the moss Physcomitrella patens, inhibition of PTEN-domain formins via RNAi results in isodiametric cell expansion [176].
While common mechanisms of actin nucleation involving the Arp2/3 complex and formins suggest a conserved molecular apparatus of cell invasion, lineage-specific pathways and “non-traditional” actin nucleators [177] may be contributing as well. In particular, a novel actin nucleator, Cordon-bleu (Cobl) negatively regulates neurite branching via promoting actin bundling [178].
Myosins
Significant participation of the F-actin-associated motors, myosins, in the polarized/invasive cell growth was recently described in several models including root hairs and moss protonemal cells. It was found that elimination of the particular class XI myosins abolishes root hair growth or protonema elongation [179–183]. Furthermore, these myosins contribute to developmentally regulated organization of the F-actin bundles in the growing root hairs. In some combinatorial knockouts of myosin genes, the depolarization/branching defects (analogous to those seen when formin AtFH8 is up-regulated) were described [180]. Given that several plant myosins are expressed specifically in pollen [184], it seems likely that the myosins are indispensible for pollen tube growth as well. Although the roles of myosins in animal polar cell growth are less understood, myosin Va was found to pull ER intodendritic spines of the neurons [185]. Moreover, Myo10, an unconventional myosin with MyTH4-FERM domains, is required for the formation of invadopodia [26] and for the patterning of podosomes [186], invadopodia-like structures that are involved in integrin-dependent adhesion in cells such as osteoclasts.
To summarize, myosins emerge as important players in polar growth that contribute to the directed vesicle transport along the biosynthetic pathway [187, 188] and to organization of the tip growth presumably via bundling or transporting F-actin [179, 180].
Additional players and actin-microtubule crosstalk
Additional actin-binding proteins contribute to the formation of invadopodia. Cortactin probably regulates the availability of cofilin to sever filaments to create new barbed ends for actin polymerization [189]. The actin-bundling protein fascin is critical for invadopodia stability [25]. Proteins that modify F-actin turnover participate also in the development of polarised neuronal protrusions. For instance, genetically modified mice lacking the F-actin severing protein gelsolin exhibit reduced migration of neuronal progenitors [190]. Many actin-binding and -regulating proteins known from animals and yeast have homologs also in tip growing plant cells (reviewed in [191]).
While microtubules are abundant in invadopodia [192], little is known about their specific roles in invasion. The microtubular cytoskeleton may participate mainly in later stages of invadopodia extension or subsequent cell migration rather than the early steps of substrate invasion per se[26, 193].
Crosstalk between microfilaments and microtubules is apparently central to invasiveness of some cell types, including neurons (reviewed in [194–196]). Microtubules participate in extension of axonal branches, originally initiated mainly by actin-based mechanisms [197]. Axon-like neurites induced by cytochalasin D were enriched with dephosphorylated microtubule binding protein tau (Tau-1) and phosphorylated microtubule associated protein Map1b, known markers of polarisation [141]. Recent in vivo evidence from genetically modified mice suggests a specific role for the ubiquitously expressed plakin, microtubule-actin crosslinking factor 1 (MACF1), in migration of immature cortical pyramidal neurons [198, 199]. Studies on non-neuronal cells suggest a possible mechanism, since MACF1 regulates the dynamics of F-actin-microtubule-focal adhesion interactions during keratinocyte migration [200]. In primary fibroblasts lacking MACF1, extending microtubules failed to co-align with F-actin filaments at the plasma membrane [201]. MACF1 enhanced the co-localisation of microfilaments and microtubules in transfected cells [199]. A human mutation in a neuro-specific tubulin isoform causes a complex phenotype whose underlying cause appears to be a defect in axon guidance [202].
In invasive plant cells, microtubules are less central than actin. Rather than growth itself, they appear to control its direction (e.g. [143, 203, 204]), and only extremely high concentrations of the microtubule-stabilizing drug taxol inhibited tobacco pollen tube elongation [205]. Sub-apical spiral bundles of cortical microtubules may serve as a “structural memory” enabling to restore growth direction after obstacle encounter [143, 206]. Differential dependence of several pollen tube membrane proteins localization on actin and MTs cytoskeletons was recently described [207].
A family of plant-specific Ric (Rop interacting CRIB-domain) adaptor proteins was reported to mediate the communication between active Rop GTPases (see above) and both actin and microtubules [100, 208]. These proteins also act in co-ordinating morphogenesis of neighbouring lobed epidermal pavement cells, enabling thus “mutual invasion” of adjacent cells resulting in a “puzzle-like” structure of the epidermis [209].
Exocyst
The exocyst is essential for cell invasiveness
Exocytosis directly contributes to invasive cell behaviour. Probably the best studied relevant effector of specific RHO (but also Rab) GTPases is the vesicle tethering complex exocyst – a hetero-octameric protein complex originally described as a Rab/Sec4 effector in yeast, consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84 subunits, involved in the docking of exocytotic vesicles at the target cytoplasmic membrane before effective fusogenic t-v-SNARE complex formation [210, 211]. While the exocyst is, in general, evolutionarily conserved, some subunits seem to be lost in some eukaryotic lineages [212], and the land-marking Exo70 subunits have extremely diversified in land plants [213–215].
In cancer cells the exocyst is essential for invadopodia formation and function and for invasiveness [84, 216]. Knockdown of Exo70, Sec6, Sec8 or Sec10 decreased invadopodia number and/or reduced matrix degradation [84, 216].
The exocyst is also essential for neuron polarisation and subsequent protrusive outgrowth. Expression of dominant negative mutants of Exo70 in cortical neuronal progenitors of mouse embryos disrupted glial-guided migration of pyramidal neurons [35]. Perturbation of exocyst function in cortical neurons in vitro reduced neurite branching and disrupted normal polarisation [217, 218].
Plant exocyst is also implied in the invasive growth of pollen tubes and root hairs, as documented by phenotypes of Arabidopsis mutants [213, 214, 219, 220].
Mechanisms of exocyst function
The most obvious exocyst role in invasiveness is related to membrane trafficking, including, but not limited to, providing material for the expanding plasmalemma. In animal cells, exocyst participates also in the secretion of matrix-loosening enzymes [84, 216]. In plants, a plethora of cell wall-regulating activities – expansins, extensins, cell wall polysaccharide-modifying enzymes – are exocytosed at the invading/growing cell domain. Mere relaxation of cell wall can induce polarized cell expansion due to manifestation of turgor pressure at the localized area of the cell surface [65, 221]. Such local changes in cell wall properties usually tightly depend on localized exocytosis and membrane recycling.
Surprisingly, the exocyst also participates in cytoskeleton, especially actin remodelling, in particular via its association with the Arp2/3 actin nucleation complex. Mammalian Exo70 interacts with the Arpc1 subunit (Arc40 or p40) mediating the association of the whole exocyst to the Arp2/3 complex [222]. Exo70 and Arp2/3 complex co-localize at the leading edge of migrating cells, suggesting that Exo70 targets Arp2/3 to specific sites of plasma membrane [222]. Moreover, Exo70 stimulates Arp2/3 – mediated actin polymerization in vitro. Remarkably, the intensity of interaction between Exo70 and Arp2/3 correlated with invasive potential of cells, and expression of mutant Exo70 defective in interacting with Arp2/3 prevented invadopodia formation and matrix degradation [216]. Thus, association of Exo70 and Arp2/3 is essential for formation of invadopodia, and Exo70 may target Arp2/3 that subsequently mediates actin-based protrusions formation, required for cell migration.
Actin may not be the sole cytoskeletal efector of the exocyst, since both complete exocyst and its Exo70 subunits inhibit microtubule polymeriziation in vitro and Exo70 overexpression affects filopodia formation in rat kidney cells [223]. However, to date there are no microtubuli-related data directly related to cell invasiveness.
Exocyst – small GTPase interactions
Regulatory links between the exocyst and small GTPases are well established in many systems. In invadopodia, the exocyst interacts with IQGAP1, a Cdc42 and Rac effector, which regulates cell polarization during cell migration [224] and was implicated also in tumorigenesis and invasion [225]. The interaction between IQGAP1 and Sec3/Sec8 is regulated by Cdc42 or RhoA, and is important for invadopodia formation [84]. Consistently, Exo70 and Sec8 are enriched in invadopodia, where they co-localize with F-actin, MMPs and IQGAP1. The exocyst in invadopodia apparently directs targeted MMP secretion; indeed, knockdown of IQGAP1 reduced MMP secretion similar to effects of exocyst inhibition [84, 216]. Interaction of Exo70 with phosphatidylinositol-4,5-bisphosphate is necessary for MMP secretion [216].
No direct link between the exocyst and RHO GTPases was reported to date in neurons. However, the small GTPase Ral-A controls the ability of the neuronal exocyst proteins to associate with molecular regulators of polarity and protrusion [217]. Another small GTPase, TC10, interacts with Exo70 and triggers translocation of the exocyst to the plasma membrane during polarisation and neurite elongation; interestingly, the Exo70-TC101 complex can locally antagonize actin rearrangements induced by Cdc42 [162, 226]. The absence of TC10 or Exo70 resulted in reduced elongation of neurites and the absence of axons, associated with the loss of polarised membrane insertion of the insulin growth factor -1 (IGF-1) receptor, a known regulator of axon specification [227].
In plants, the first link discovered between the exocyst and Rop GTPases is an indirect one through the Sec3 subunit interacting with a plant-specific adaptor protein Icr1, likely to participate in pollen germination and tube growth [228, 229].
While there are no reports directly related to cell invasiveness, Rab GTPases are well-established exocyst effectors in both metazoans and fungi [230].
Lipid signaling
Invasive structures are membrane microdomains with specific lipid composition
Steroid- and sphingolipid-enriched membrane microdomains, often referred to as lipid rafts, play an important part in cellular invasive structures.
Invadopodia biogenesis and integrity rely on tightly controlled levels of plasma membrane cholesterol. Lipid raft perturbation e.g. by direct manipulation of cholesterol levels or by inhibition of glycosphingolipid synthesis impaired invadopodia formation and function [231, 232]. Furthermore, caveolin 1 is a key regulator of plasma membrane cholesterol homeostasis required for invadopodia formation and ECM degradation, through a tyrosine-phosphorylation-dependent mechanism [231].
Similarly, a number of studies have shown the requirement for sphingolipid synthesis during outgrowth and morphological restructuring of neurites in cultured pyramidal neurons [233, 234]. Interestingly, whereas during brain development cholesterol is autonomously generated by neurons, postnatally the majority is provided by supporting astrocytes, which release cholesterol-rich lipoproteins [235]. Thus, the role of astrocytes in supporting neurite outgrowth, synaptogenesis and regeneration in the brain is likely to be tightly linked with the regulated secretion of lipids [236].
In plants, sterols, which are characteristic for membrane lipid rafts, accumulate specifically at the point of bulging and at the tip during the active phase of Arabidopsis root hair elongation [237], and at growing pollen tube tips of spruce Picea meyeri, where their disruption by filipin inhibited growth [238]. Studies on Arabidopsis mutants defective in sterol biosythesis showed the crucial importance of sterol homeostasis for establishment and maintenance of plant cell polarity – especially for the endocytotic recycling-dependent dynamic polarization [239].
Function of lipid rafts in invasive structures
In invadopodia, lipid rafts could participate in targeting signaling and proteolytic activities to degradation foci. Several invadopodia components can be regulated by, and preferentially sorted to, raft domains, including N-WASP, dynamin 2, MT1-MMP, integrins and ARF6 [240–244].
Lipid rafts also serve as scaffolds for membrane signalling molecules in neurons [245]. A correlation between cholesterol and the levels of raft-associated Fyn, a non-receptor tyrosine kinase known to affect neurite outgrowth, suggests a lipid-dependent signalling pathway controlling neurite growth [246, 247]. Optimal levels of cholesterol may be essential for correct outgrowth of axons and dendrites in pyramidal neurons, since experimentally imposed changes in cholesterol levels attenuated outgrowth. Lower levels of cholesterol in cultured cortical neurons may account for faster neurite outgrowth when compared to hippocampal neurons [246]. Furthermore, exposure of neurons to cholesterol-reducing drugs, such as pravastatin, increased the rate of neurite outgrowth and branching through the inhibition of Rho isoprenylation and thus activity [248].
Many neural cell adhesion and transmembrane molecules involved in signal transduction during neurite outgrowth and pathfinding contain immunoglobulin-like (Ig) motifs and localise to lipid rafts [249, 250]. Their recruitment to lipid rafts is dependent on the cytoskeleton, particularly F-actin [251]. The involvement of microtubules was demonstrated in cultured hippocampal neurons, where recombinant antibody-induced clustering of cholesterol and ganglioside GM1 on the cell surface promoted axon outgrowth via stabilisation of microtubules [252].
Membrane lipid raft fractions from plant cells are enriched in, among others, Rop GTPases, GPI-anchored proteins and NOX proteins [253, 254], suggesting a role for lateral membrane compartmentation also in plant cell invasiveness. Indeed, sterol-rich microdomains were shown to be important for ROS-based signalling in Picea meyeri pollen tubes [238] Activated Rops were shown to be anchored into the detergent-resistant membrane fraction not only via prenylated C’-termini but additionally via reversible S-acylation at their N-termini [255].
In conclusion, the importance of sterol-rich membrane domains as sites where signalling cascades are originated to organise local actin remodelling [256, 257] is very much in accord with the finding that invadopodia, but also neurites, plant root hairs and pollen tubes, are lipid-raft enriched domains.
Phosphoinositide signaling and small GTPases at sites of invasion
An “Exocytic Signal” model suggesting that exocytosis and actin regulation are fully integrated events mediated by phosphoinositides (PtdIns)-based signaling has been recently proposed for polarized growth of fungal and metazoan cells [258]. PtdIns and their metabolism, which preferentially occurs at lipid rafts, participate also in invadopodia function, especially ECM degradation [259].
PtdIns(4,5)P2 is of special interest because it directly controls actin polymerization at the plasma membrane (reviewed in [260]). Both PtdIns(4,5)P2 and the kinase that generates it, type Iα PtdIns-4-phosphate 5-kinase, are enriched at invadopodia. Further, knockdown of the enzyme inhibited invadopodia formation and ECM degradation in a breast cancer cell line [261]. Class IA PtdIns3-kinase (PI3K), another lipid kinases that phosphorylate PtdIns, controls invadopodia formation in breast cancer cells, via its effectors 3-PtdIns–dependent protein kinase-1 (PDK1) and Akt [262]. Exocyst may be one of the relevant targets of PtdIns-based signalling, as suggested by the finding that PIP(2) binding to Exo70 participates in the control of cell motility, though it is not clear whether there is a direct role also in invasion into ECM [263].
Phosphoinositides are pivotal also for neurite formation and extension. In differentiating pyramidal neurons, activation of PI3K and generation of its product PtdIns(3,4,5)P3 occurs in a polarised manner and is associated with the specification and extension of the axon via the activation of Rap1B, Cdc42 and Rac1-dependent signalling [49]. Small GTPases provide a major signalling link between membrane lipids and the cytoskeleton in the developing forebrain [194]. Exposure of neurons to cholesterol-reducing drugs (see above) increased the rate of neurite outgrowth and branching through the inhibition of Rho isoprenylation and thus activity [248]. This is in accordance with the findings that in cultured neurons RhoA can have oposite biological effects from Rac1 and Cdc42, inhibiting rather than promoting neurite outgrowth [264]. Furthermore, phospholipase C 3 (PLC 3), a key neuronal enzyme catalyzing the hydrolysis of PI(4,5)P2 and leading to the generation of second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), promotes neurite outgrowth through downregulation of RhoA [265].
PtdIns participate in invasive growth also in plant cells, since the first characterized plant membrane phospholipid-regulating activity (PtdInsP-kinase) was reported as an effector of active Rop at the pollen tube tips [266]. Rapidly growing evidence indicates an important role for both PI3K and PI(4,5)P2 in pollen tube growth [267–269]. Specific Rab GTPases of the Rab11/A clade and all Rab8/E paralogues are currently the best known regulators of PtdInsP-kinases in Arabidopsis root hairs [270, 271]. These activities contribute to the establishment and function of the highly dynamic PIP2-rich plasmalemma domain at the growing tips of pollen tubes and root hairs (reviewed in [113]). Phospholipase D (PLD) and its product - phosphatidic acid (PA) is emerging as a regulator able to link F-actin cytoskeleton and membrane lipid dynamics in plants. While in animal cells F-actin polymerization is stimulated by binding of capping protein to PIP2, plant actin capping protein is inhibited by PA. At the same time in both cell types F-actin directly stimulates PLD activity, resulting in plant cells in positive feedback, locally amplifying both membrane and actin dynamics (reviewed in [272]).
Reactive oxygen species (ROS)
ROS as second messengers contributing to cell invasiveness
Reactive oxygen species (ROS), while best known for their function in pathological processes, are also essential intracellular second messengers in many signaling pathways, including those related to cell invasiveness. In cells, ROS are produced by several mechanisms, including the activity of NADPH oxidases (Nox), transmembrane proteins that catalyze NADPH-dependent formation of superoxide from oxygen via an electrogenic charge/electron transport (reviewed in [273]).
Both ROS and Nox proteins are essential for formation and function of invadopodia, inducing ECM degradation and cell invasiveness. Because ROS are very unstable, they must be generated locally. ROS production is targeted to invadopodia by Nox organizer proteins Tks4 (tyrosine kinase substrate with 4 SH3 domains) and Tks5 (tyrosine kinase substrate with 5 SH3 domains), two large scaffold proteins targeted to invadopodia by interaction with PI-3,4-P2. Tks proteins interact both with Nox activator proteins and Nox core enzymes, thereby inducing formation of an active Nox complex [274, 275]. Further mechanisms whereby ROS regulate invadopodia formation and function are not yet elucidated.
ROS are also required during neural development and function, including the proliferation of neuronal precursors, their differentiation into specific neuronal subtypes, as well as the survival and the plasticity of adult neurons. Cultured rat cortical neurons, as well as CNS areas rich in immature, migrating neurons, have high levels of ROS [276, 277].
Changes in ROS levels influenced the types of neurons produced in vitro, suggesting levels of ROS in neuronal progenitors control neuronal fate decisions. Intracellular synthesis of ROS in neuronal progenitors may be regulated by the cell fate determining factor Numb and its associated protein Nip1/Duoxa1 (Mammalian Numb-interacting Protein 1/Dual Oxidase Maturation Factor 1)[278]. ROS have been also shown to be essential for the NGF-induced differentiation of neuron-like, rat pheochromocytoma PC12 cells [279–281] via its receptor TrkA [282].
In tip-growing plant cells, polarized production of ROS depends on a high tip-focused free calcium ion (Ca2+) cytoplasmic gradient correlated with invasive growth of pollen tubes [283, 284]. The gradient is regulated by glutamate receptor-like calcium channels activated by D-Ser in transmitting tract of pistils [285]. A similar tip-focused Ca2+ gradient was described also in root hairs [70, 286, 287]. The cytoplasmic free calcium gradient connected to apical invasive growth seems to be regulated by active Rop GTPases via Ric proteins [100]. First indirect interaction of Rop GTPase, mediated via an Icr-like/Rip3 adaptor protein binding a specific kinesin, was reported recently by Mucha et al. [288]; for Icr1 see [228]). ROS-producing NADPH oxidases (Nox) are prominent candidate integral membrane proteins regulated directly by calcium and activated Rho GTPases also in plants, as demonstrated initially by forward genetic screens in Arabidopsis root hairs [103, 289] and later by pharmacological and antisense supression of NOX activity also in pollen tubes [290].
ROS signaling networks in invasive structures
The mechanisms of ROS effects in invadopodia formation and function are not well understood yet. ROS can oxidize redox-sensitive cysteins in catalytic sites of many proteins, including protein tyrosine phosphatases, generating thus sites with enhanced tyrosine kinase activity. Particularly, ROS can inactivate protein tyrosine phosphatase PTP-PEST that localize to invadopodia or LMW-PTP, resulting in enhancing protein tyrosine kinase activity, especially of Src kinase, that was shown to be essential for invadopodia function. Src kinase can act both upstream and downstream of this signaling. On one hand, ROS production enhances Src activity, on the other hand, Src phosphorylates Tks and NOX proteins to strengthen their interaction and promote enhanced Nox activity. Another mechanism of ROS effect in invadopodia can be regulation of MMPs secretion by fusion of MMPs-containing vesicles with plasma membrane (reviewed in [291, 292]).
The association of ROS signaling and RHO GTPases was described in both non-neuronal cells and in neurons. In invadopodia, Src can induce ROS generation by activating Rac1 GEF Vav2. Rac1 in turn activates Nox complexes to produce ROS [293]. In Aplysia neurons, ROS are likely to promote neurite outgrowth via inhibition of Rho. Interestingly, in PC12 cells Rac1 can also increase intracellular ROS levels by activating Nox complexes [294] or the cytosolic phospholipase A2/arachidonic acid/lipoxygenase-cascade [295]. Interference in Rac signalling by over-expression of a catalytically inactive mutant, RacN17, blocked the NGF-induced generation of ROS and morphological differentiation of PC12 cells [281]. STAT3 was also found to control NGF-induced ROS production during process outgrowth in PC12 cells suggesting broad involvement of ROS in multiple signaling pathways [296].
While plant NOX proteins are directly regulated by calcium, phosphorylation [290, 297, 298] and by binding to active Rop GTPases, positive regulatory feedback between NOX-ROS and ROS-activated calcium channels is proposed to operate at the growing tip contributing to the tip-high cytoplasmic free calcium gradient both in pollen tubes and root hairs [290, 298].
ROS could also have a direct impact on the cytoskeleton. Localized synthesis of ROS in Aplysia growth cones is required for F-actin assembly and dynamics. Short term exposure to a free radical scavenger, or inhibition of ROS sources such as NADPH oxidases and lipoxygenases, reduced the F-actin content in the peripheral domain of growth cones [299]. Further, prolonged treatment resulted in the disassembly of the actin cytoskeleton, causing severely impaired growth cone formation and outgrowth.
In plant cellsROS-mediated cell wall polysaccharide cleavage/relaxation or cross-linking may contribute to the regulation of cell expansion (reviewed in [300]). NOX-ROS might also modify local membrane lipid composition by lipid peroxidation and play important roles in membrane lipid raft organization.
Review and conclusions
Is there an ancestral toolbox for cell invasiveness?
Comparison of cell invasiveness mechanisms in lineages as diverse as plants and metazoans should provide hints towards reconstruction of a potential set of ancestral mechanisms and pathways that may have been at the root of the phenomenon of eukaryotic cell invasiveness. Indeed, many structural and signalling motifs appear to be common or conserved (see Table 1). The closely interconnected membrane trafficking processes and actin filament rearrangements, orchestrated by small GTPases and integrating multiple signalling inputs, in particular those utilizing (phospho)lipid-protein interactions and reactive oxygen species, appear to be a recurrent theme in evolutionally distant lineages and thus good candidates for possible ancestral mechanisms, possibly reaching back to the LECA. Regardless of what LECA looked like, it likely have had large cells and lived in an anisotropic environment, necessitating cellular polarity to navigate in gradients of physical and chemical inputs.. Invasivness of present eukaryotic cells then appears as a natural consequence of growth or locomotion of their polarized ancestors in semi-solid substrates such as e.g. sediments.
However, while homology of individual proteins can be readily assessed, the situation is much less clear in case of cellular processes where conserved components could have combined in varying manner to achieve convergent overall network topology (see Figure 2). Nevertheless, the Rho/Rac/Rop GTPases are apparently as old as the eukaryotes [301], and they a playing a comparable part in organisms as diverse as opisthokonts and plants. Thus they emerge as good candidates for a group of truly conserved ancestral master polarity regulators. While we cannot exclude the possibility that some of the components of the pathways leading from the small GTPases to their ultimate cytoskeletal or secretory effectors may have been recruited from a common cellular toolbox independently, i.e. in a convergent fashion, it is plausible that even these mechanisms are, at least to some extent, ancestral. This, however, does not exclude lineage-specific “implementation” of some regulatory steps, as can be illustrated e.g.in case of RHO-dependent control of actin nucleation [302]. Both small G proteins and actin do have distinguishable prokaryotic homologs. Albeit the relationship of the bacterial GTPases to distinct eukaryotic clades remains unresolved [301, 303], at least in some case a bacterial small GTPase was found to contribute to cell polarity control [304], opening up the intriguing possibility that some of the machinery involved in invasiveness might have predated LECA,
Striking similarity between cell invasiveness mechanisms in plants and metazoans, including pathological conditions such as invasive cancer, opens great possibilities for transfer of findings related to invasive cell growth from one biological system into the others. As an example, the role of exocyst and NADPH oxidases in invasive pollen tube growth was discovered earlier than the role of these signalling modules for cancer cell invasion. Such a transfer of findings, although cautious, could result in facilitation of the research focused on treatment of pathological conditions related to invasive cell growth, including, e.g., also the metastatic cancer and neuronal regeneration.
Reviewers comments
Reviewer's report 1: Arcady Mushegian, Kansas University Medical Center, United States of America
The manuscript by Vaškovi#ová et al., poses an interesting evolutionary and functional question, namely whether the mechanisms of cell invasive growth in plants and animals share homologous molecular components. The study is the review of the evidence, which gives an affirmative answer: the examples of cell invasion that are selected for the review are all dependent on the rearrangements of actin cytoskeleton, controlled by small GTPases of Rho, ARF and Rab families. Moreover, in both plants and animals the lipid (phosphoinositol) signalling is involved, as well as possibly membrane microdomains.
I have no objection against publication of this review as is, but the authors are invited to consider whether some of the following modifications would improve the manuscript.
First, the definition of invasive growth is, in my opinion, loose. Invasion surely implies growing through, or within, another living tissue; the authors suggest that much, pointing out the importance of the digestion of the intercellular matrix, for which the appropriate enzymes should be secreted. If this is a necessary part of the definition, then pollen tube growth qualifies, but the growth of root hairs seems not to: secretion of “lubricants” is surely not the same as secretion of active lytic enzymes, or is it - lubricants do not have even to be proteins? On the other hand, growth of haustoria of parasitic plants seem to qualify in every way.
Author response: We realize that we should have taken more care to define invasiveness at the first place, and we have now corrected this omission. In our opinion (and in agreement with Reviewer 2), the environment invaded by the cells does not need to be a living tissue - it is sufficient that it is semi-solid. In real-life situations, the environments are also usually rather complex (soil is a good example of this).
Secretion of both the digestive enzymes and other materials such as the root hair mucilage (i.e. polysaccharides, glycoproteins) or even small molecules critically depends on a local activity of the exocytotic pathway, and at least in this sense the inclusion of root hairs can be justified. From this point of view, we would not consider the haustoria a relevant model for cellular invasiveness, as they are multicellular structures.
Second, the (near) absence of fungi in the discussion is puzzling. Surely, if root hairs qualify, fungal hyphae should too; but even if not, the growth of parasitic fungi is invasive, and, at least in the case of Candida and some plant parasites even reasonably well studied genetically. In fact, the names of many genes involved in the process, notably Cdc42, are from the fungal systems!
Author response: We agree that fungi, in particular the budding yeast, have served as paradigmatic models for the study of cell polarity and cell invasion. We have now added an explanation of the reasoning behind our choice of models, as well as references to recent reviews on cell polarity and hyphal growth in fungi.
Third, if at least some components of the invadopodia are shared between plants and animals - and also fungi, if the authors will be willing to talk about them, too - then an inference is that these components were also present in LECA. Were they organized into a coherent system, and what that system might have been?
A putative answer presents itself, i.e., these components, including cortical/apical actin cytoskeleton, GTP regulators, and associated membrane remodeling components may have been involved in forming pseudopodia and other cytoplasmic protrusions. In fact, among such protrusions in any organisms, the best-studied one from genetic point of view may be S.cerevisiae bud, which is very much controlled by Cdc42 and other players discussed in the review. It seems to be worth discussing the present-day unicellular eukaryotes more explicitly, even though they are obviously not LECA.
Author response: We have also now expanded somewhat the concluding section on the “ancestral toolbox of cell invasiveness”, suggesting that the RHO clade GTPases emerge as possible candidates for truly conserved invasivity regulators. While we do not dare to postulate that LECA was capable of forming pseudopodia or similar protrusions, we do believe that it was polar, and that this alone may have provided a sufficient starting point for the evolution of invasiveness.
Reviewer’s report 2: Valerian Dolja, Oregon State University, United States of America
This review article discusses parallels and differences in the molecular mechanisms of the polarized cell elongation in animals and pants interpreted as cell invasiveness (I think that invasiveness is a better term than ‘inasivity’ which does not appear in Webster). Both the cell growth within the tissue (as in neurons, cancer cells, or pollen tubes), and into the surrounding substrate (as in root hairs) are considered under the common umbrella, and rightfully so. The mechanisms of invasive growth are interpreted in evolutionary terms; a much needed, rather refreshing, and enjoyable prospective in the field of cell biology. I found the article very engaging, well written and useful for the broad audience interested in the interplay of vesicle transport, cytoskeleton, lipid signaling, and ROS.
Author response: Thanks for suggesting the term “invasiveness” - we are now using it throughout.
There are two major comments I would like the authors to consider. The first has to do with relating commonalities/homologies in the tip growth mechanisms in animals and plants to LECA. The supergroups of plants and unikonts are indeed separated from each other as deeply as it goes among the eukaryotes, likely all the way to LECA. However, it does not mean that the plant and animal homologous proteins involved in invasive growth (e.g., small GTPases) have evolved to fulfill this role. Because LECA was certainly unicellular, it seems more likely that the small GTPases, and other components of polar growth machinery were recruited from available toolbox later, along with evolving multicellularity and tissue differentiation.
Author response: Although we agree that LECA lacked the features we associate with advanced multicellularity (i.e. cell diferentiation), we are not that sure that it was unicellular, as it may have lived in some form of colonies or consortia. Even modern day prokaryotes can form rather sophisticated multicellular structures, justifying even the application of the concept of a “body plan” (see Rieger et al., Commun Integr Biol. 2008 1(1): 78–8). However, we do not consider multicellularity essential for invasiveness - a need to navigate in a complex semi-solid environment may have been enough, and the heterogeneity of the environment naturally resulted in cell polarity (be it even “only” due to the gradient of environmental conditions). Small GTPases may have been recruited as “master regulators” of diverse pathways related to cell polarity already at this stage, while subsequent - more diverse - steps of the pathways may have been either also inherited or independently recruited. We are now discussing this hypothesis in the final section.
The second comment has to do with the missing discussion of significant roles played by the F-actin-associated motors, myosins, in the polarized/invasive cell growth. These roles were recently described in several models including root hairs and moss protonemal cells. It was found that elimination of the particular class XI myosins abolishes root hair growth or protonema elongation [1–5]. Furthermore, these myosins contribute to developmentally regulated organization of the F-actin bundles in the growing root hairs; in some multiple knockouts of myosin genes, the depolarization/branching defects (analogous to those seen when AtFH8 is up-regulated) were described [4]. Given that several plant myosins are expressed specifically in pollen [6], it seems likely that the myosins are indispensible for pollen tube growth as well. Although polar growth of the budding yeast cells cannot be considered invasive, it also relies on myosin V closely related to plant myosins XI [7]. Although the roles of myosins V in animal polar cell growth are less understood, myosin Va was found to pull ER into dendritic spines of the neurons [8]. Therefore, myosins emerge as important players in polar growth that contribute to the directed vesicle transport along the biosynthetic pathway [9, 10] and to organization of the tip growth area presumably via bundling or transporting F-actin [4, 5].
Author response: We thank the reviewer for pointing out the missing discussion on the role of myosins in invasive growth. We now included a subchapter “Myosins” in Cytoskeleton(s) part of our review, where the role of myosins is discussed.
Reviewer’s report 3: Purificacion Lopez-Garcia, Centre National de la Recherche Scientifique, France
This is an interesting and synthetic review on the molecular mechanisms and effectors responsible for cell invasive growth in animals and plants, where most data are available. The review highlights the similarities and homologies existing between both so that, at the end, the question of whether such mechanisms existed already in the last common eukaryotic ancestor (LECA) can be asked.
I have few comments on the review part, which is very well documented and naturally oriented to the final question asked in the manuscript. Perhaps, it would have been important to mention data existing for other eukaryotic phyla whenever available. Animals and plants are divergent in the eukaryotic tree, but the possibility that they have shared a most recent common ancestor (for instance, to the exclusion of excavates) cannot be completely ruled out.
Author response: With the exception of fungi, which are, however, rather close to metazoans, surprisingly little is known about mechanisms of invasiveness in other major phyla (we did include this comment and a reference to recent - mostly cytological - Phytophtora work in the new version of the paper).
At any rate, the question of whether invasive cell growth mechanisms and effectors existed already in LECA is posed in a very cautious and reasoned (even shy) manner. The authors highlight the fact that even if individual proteins are homologous in animals and plants and, therefore, potentially ancestral, the processes themselves may have resulted from the combination of different effectors in the two eukaryotic lineages. Although not clearly stated, the authors seem to suggest by this that the processes for invasive growth were present in LECA but that different combinations of effectors occurred in different eukaryotic lineages. This would imply a last common ancestry for both, effectors and processes. However, it might also be that from a same pool of ancestral proteins, the same or different proteins were recruited independently for a similar function, invasive growth, in later eukaryotic evolution, i.e. evolved by convergence from a common pool of proteins. The two possibilities should be perhaps more clearly distinguished.
Author response: Since the question of distinguishing conserved invasiveness mechanisms from those recruited convergently from a common “toolbox” has been raised by all three reviewers, we attempted to respond to it by expanding the final discussion.
Finally, the authors did not evoke the logical next question: do these proteinsn involved in invasive growth have homologs in prokaryotes? Do they play a role in analogous processes (e.g. cell polarization and gliding in social bacteria)? Describing those processes in bacteria is likely out the scope of this manuscript, but a comment on these might underscore the evolutionary orientation of this review.
Author response: We do agree that prokaryotes present a fascinating area of research that would be well worth a separate paper; we did, at least, include a small note on possible prokaryotic homologs of some of the components in the expanded final discussion.
Please, do not use “kingdom” to refer to plants, animals and fungi. The 5-kingdom classification by Witthaker has no phylogenetic support.
Author response: While we did not use the term “kingdom” in the Whittaker sense, but rather in a looser sense of a “lineage”, we agree that it is better to get rid of this term completely, which we did.
Abbreviations
- ARF:
-
ADP-ribosylation factor
- Cobl:
-
Cordon-bleu
- DAG:
-
Diacylglycerol
- ECM:
-
Extracellular matrix
- GAPs:
-
GTPase-activating proteins
- GDI:
-
GDP dissociation inhibitor
- GDP/GTP:
-
Exchange factor
- GDP:
-
Guanosine diphosphate
- GTP:
-
Guanosine triphosphate
- Ig:
-
Immunoglobulin-like
- IGF-1:
-
Insulin growth factor -1
- IP3:
-
1,4,5-trisphosphate
- LECA:
-
Last common eukaryotic ancestor
- MACF1:
-
Microtubule-actin crosslinking factor 1
- NGF:
-
Neuronal growth factor
- Nox:
-
NADPH oxidases
- PA:
-
Phosphatidic acid
- Pak-1:
-
p21-activated kinase
- PDK1:
-
3-PtdIns–dependent protein kinase-1
- PI3K:
-
PtdIns3-kinase
- PLCd3:
-
Phospholipase Cd3
- PLD:
-
Phospholipase D
- PtdIns:
-
Phosphoinositides
- ROS:
-
Reactive oxygen species
- SPARC:
-
Secreted protein acidic and rich in cysteine
- TGN:
-
Trans golgi network.
References
Simpson AG, Roger AJ: The real ‘kingdoms’ of eukaryotes. Curr Biol. 2004, 14: R693-R696. 10.1016/j.cub.2004.08.038.
Arkowitz RA, Bassilana M: Polarized growth in fungi: symmetry breaking and hyphal formation. Semin Cell Dev Biol. 2011, 22: 806-815. 10.1016/j.semcdb.2011.08.010.
Dickinson JR: Filament formation in Saccharomyces cerevisiae–a review. Folia Microbiol (Praha). 2008, 53: 3-14. 10.1007/s12223-008-0001-6.
Howell AS, Lew DJ: Morphogenesis and the cell cycle. Genetics. 2012, 190: 51-77. 10.1534/genetics.111.128314.
Kebdani N, Pieuchot L, Deleury E, Panabieres F, Le Berre JY, Gourgues M: Cellular and molecular characterization of Phytophthora parasitica appressorium-mediated penetration. New Phytol. 2010, 185: 248-257. 10.1111/j.1469-8137.2009.03048.x.
Wang Y, Meng Y, Zhang M, Tong X, Wang Q, Sun Y, Quan J, Govers F, Shan W: Infection of Arabidopsis thaliana by Phytophthora parasitica and identification of variation in host specificity. Mol Plant Pathol. 2011, 12: 187-201. 10.1111/j.1364-3703.2010.00659.x.
Buccione R, Caldieri G, Ayala I: Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009, 28: 137-149. 10.1007/s10555-008-9176-1.
Caldieri G, Buccione R: Aiming for invadopodia: organizing polarized delivery at sites of invasion. Trends Cell Biol. 2010, 20: 64-70. 10.1016/j.tcb.2009.10.006.
Murphy DA, Courtneidge SA: The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011, 12: 413-426. 10.1038/nrm3141.
Poincloux R, Lizarraga F, Chavrier P: Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009, 122: 3015-3024. 10.1242/jcs.034561.
Kelly T, Mueller SC, Yeh Y, Chen WT: Invadopodia promote proteolysis of a wide variety of extracellular matrix proteins. J Cell Physiol. 1994, 158: 299-308. 10.1002/jcp.1041580212.
Chen WT: Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989, 251: 167-185. 10.1002/jez.1402510206.
Mueller SC, Chen WT: Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991, 99 (Pt 2): 213-225.
Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC: An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999, 18: 4440-4449. 10.1038/sj.onc.1202827.
Chen WT: Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme Protein. 1996, 49: 59-71.
Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, Chen WT: A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994, 54: 5702-5710.
Mueller SC, Yeh Y, Chen WT: Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J Cell Biol. 1992, 119: 1309-1325. 10.1083/jcb.119.5.1309.
Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, Chen WT: Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci U S A. 1997, 94: 7959-7964. 10.1073/pnas.94.15.7959.
Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R: Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006, 85: 1217-1231. 10.1016/j.ejcb.2006.08.003.
Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, Mueller SC: Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res. 2006, 312: 1240-1253. 10.1016/j.yexcr.2005.12.012.
Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T: Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005, 168: 441-452. 10.1083/jcb.200407076.
Linder S: The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007, 17: 107-117. 10.1016/j.tcb.2007.01.002.
Linder S, Aepfelbacher M: Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003, 13: 376-385. 10.1016/S0962-8924(03)00128-4.
Janostiak R, Tolde O, Bruhova Z, Novotny M, Hanks SK, Rosel D, Brabek J: Tyrosine phosphorylation within the SH3 domain regulates CAS subcellular localization, cell migration, and invasiveness. Mol Biol Cell. 2011, 22: 4256-4267. 10.1091/mbc.E11-03-0207.
Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, Anderson K, Machesky LM: The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010, 20: 339-345. 10.1016/j.cub.2009.12.035.
Schoumacher M, Goldman RD, Louvard D, Vignjevic DM: Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010, 189: 541-556. 10.1083/jcb.200909113.
Tolde O, Rosel D, Vesely P, Folk P, Brabek J: The structure of invadopodia in a complex 3D environment. Eur J Cell Biol. 2010, 89: 674-680. 10.1016/j.ejcb.2010.04.003.
Fishell G, Hanashima C: Pyramidal neurons grow up and change their mind. Neuron. 2008, 57: 333-338. 10.1016/j.neuron.2008.01.018.
Megias M, Emri Z, Freund TF, Gulyas AI: Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001, 102: 527-540. 10.1016/S0306-4522(00)00496-6.
Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR: Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002, 22: 3161-3173.
Valiente M, Marin O: Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010, 20: 68-78. 10.1016/j.conb.2009.12.003.
Zheng W, Yuan X: Guidance of cortical radial migration by gradient of diffusible factors. Cell Adh Migr. 2008, 2: 48-50. 10.4161/cam.2.1.6001.
Reiner O, Sapir T: Polarity regulation in migrating neurons in the cortex. Mol Neurobiol. 2009, 40: 1-14. 10.1007/s12035-009-8065-0.
Kawauchi T, Chihama K, Nabeshima Y, Hoshino M: Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006, 8: 17-26. 10.1038/ncb1338.
Letinic K, Sebastian R, Toomre D, Rakic P: Exocyst is involved in polarized cell migration and cerebral cortical development. Proc Natl Acad Sci U S A. 2009, 106: 11342-11347. 10.1073/pnas.0904244106.
Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH: Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004, 44: 263-277. 10.1016/j.neuron.2004.09.030.
Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG: Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004, 165: 709-721. 10.1083/jcb.200309025.
Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH: Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003, 114: 469-482. 10.1016/S0092-8674(03)00605-6.
Anton ES, Kreidberg JA, Rakic P: Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999, 22: 277-289. 10.1016/S0896-6273(00)81089-2.
Elias LA, Wang DD, Kriegstein AR: Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007, 448: 901-907. 10.1038/nature06063.
Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S: Emerging topics in reelin function. Eur J Neurosci. 2010, 31: 1511-1518.
Gongidi V, Ring C, Moody M, Brekken R, Sage EH, Rakic P, Anton ES: SPARC-like 1 regulates the terminal phase of radial glia-guided migration in the cerebral cortex. Neuron. 2004, 41: 57-69. 10.1016/S0896-6273(03)00818-3.
Gupta A, Sanada K, Miyamoto DT, Rovelstad S, Nadarajah B, Pearlman AL, Brunstrom J, Tsai LH: Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat Neurosci. 2003, 6: 1284-1291. 10.1038/nn1151.
Rakic S, Yanagawa Y, Obata K, Faux C, Parnavelas JG, Nikolic M: Cortical interneurons require p35/Cdk5 for their migration and laminar organization. Cereb Cortex. 2009, 19: 1857-1869. 10.1093/cercor/bhn213.
Causeret F, Terao M, Jacobs T, Nishimura YV, Yanagawa Y, Obata K, Hoshino M, Nikolic M: The p21-activated kinase is required for neuronal migration in the cerebral cortex. Cereb Cortex. 2009, 19: 861-875.
Banker GA, Cowan WM: Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977, 126: 397-42. 10.1016/0006-8993(77)90594-7.
Craig AM, Banker G: Neuronal polarity. Annu Rev Neurosci. 1994, 17: 267-310. 10.1146/annurev.ne.17.030194.001411.
Dotti CG, Sullivan CA, Banker GA: The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988, 8: 1454-1468.
Arimura N, Kaibuchi K: Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007, 8: 194-205. 10.1038/nrn2056.
Bradke F, Dotti CG: Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol. 2000, 10: 574-581. 10.1016/S0959-4388(00)00124-0.
Barnes AP, Solecki D, Polleux F: New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr Opin Neurobiol. 2008, 18: 44-52. 10.1016/j.conb.2008.05.003.
de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG: Centrosome localization determines neuronal polarity. Nature. 2005, 436: 704-708. 10.1038/nature03811.
Asada N, Sanada K, Fukada Y: LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J Neurosci. 2007, 27: 11769-11775. 10.1523/JNEUROSCI.1938-07.2007.
Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR: Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004, 7: 136-144. 10.1038/nn1172.
Sapir T, Shmueli A, Levy T, Timm T, Elbaum M, Mandelkow EM, Reiner O: Antagonistic effects of doublecortin and MARK2/Par-1 in the developing cerebral cortex. J Neurosci. 2008, 28: 13008-13013. 10.1523/JNEUROSCI.2363-08.2008.
de Anda FC, Meletis K, Ge X, Rei D, Tsai LH: Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci. 2010, 30: 10391-10406. 10.1523/JNEUROSCI.0381-10.2010.
Chae K, Lord EM: Pollen tube growth and guidance: roles of small, secreted proteins. Ann Bot. 2011, 108: 627-636. 10.1093/aob/mcr015.
Lord EM: Adhesion and guidance in compatible pollination. J Exp Bot. 2003, 54: 47-54. 10.1093/jxb/erg015.
Tabuchi A, Li LC, Cosgrove DJ: Matrix solubilization and cell wall weakening by beta-expansin (group-1 allergen) from maize pollen. Plant J. 2011, 68: 546-559. 10.1111/j.1365-313X.2011.04705.x.
Szymanski DB, Cosgrove DJ: Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol. 2009, 19: R800-R811. 10.1016/j.cub.2009.07.056.
Winship LJ, Obermeyer G, Geitmann A, Hepler PK: Pollen tubes and the physical world. Trends Plant Sci. 2011, 16: 353-355. 10.1016/j.tplants.2011.03.010.
Carol RJ, Dolan L: Building a hair: tip growth in Arabidopsis thaliana root hairs. Philos Trans R Soc Lond B Biol Sci. 2002, 357: 815-821. 10.1098/rstb.2002.1092.
Schiefelbein J, Kwak SH, Wieckowski Y, Barron C, Bruex A: The gene regulatory network for root epidermal cell-type pattern formation in Arabidopsis. J Exp Bot. 2009, 60: 1515-1521. 10.1093/jxb/ern339.
Fischer U, Ikeda Y, Grebe M: Planar polarity of root hair positioning in Arabidopsis. Biochem Soc Trans. 2007, 35: 149-151. 10.1042/BST0350149.
Baluska F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua NH, Barlow PW, Volkmann D: Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol. 2000, 227: 618-632. 10.1006/dbio.2000.9908.
Vissenberg K, Fry SC, Verbelen JP: Root hair initiation is coupled to a highly localized increase of xyloglucan endotransglycosylase action in Arabidopsis roots. Plant Physiol. 2001, 127: 1125-1135. 10.1104/pp.010295.
Singh SK, Fischer U, Singh M, Grebe M, Marchant A: Insight into the early steps of root hair formation revealed by the procuste1 cellulose synthase mutant of Arabidopsis thaliana. BMC Plant Biol. 2008, 8: 57-10.1186/1471-2229-8-57.
Zarsky V, Fowler JE: Root hairs. ROP (Rho-related protein from plants) GTPases for spatial control of root hair morphogenesis. 2009, New York: Springer, 191-210.
Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S: Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci U S A. 2007, 104: 20996-21001. 10.1073/pnas.0708586104.
Monshausen GB, Messerli MA, Gilroy S: Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008, 147: 1690-1698. 10.1104/pp.108.123638.
Iwano M, Entani T, Shiba H, Kakita M, Nagai T, Mizuno H, Miyawaki A, Shoji T, Kubo K, Isogai A: Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 2009, 150: 1322-1334. 10.1104/pp.109.139329.
Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z: Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010, 143: 99-110. 10.1016/j.cell.2010.09.003.
Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, Vanneste S, Zhang J, Simon S, Covanova M: ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010, 143: 111-121. 10.1016/j.cell.2010.09.027.
Bourne HR, Sanders DA, McCormick F: The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990, 348: 125-132. 10.1038/348125a0.
Bourne HR, Sanders DA, McCormick F: The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991, 349: 117-127. 10.1038/349117a0.
Hall A: Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994, 10: 31-54. 10.1146/annurev.cb.10.110194.000335.
Hall A: Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005, 33: 891-895.
Harris SD: Cdc42/Rho GTPases in fungi: variations on a common theme. Mol Microbiol. 2011, 79: 1123-1127. 10.1111/j.1365-2958.2010.07525.x.
Kwon MJ, Arentshorst M, Roos ED, van den Hondel CA, Meyer V, Ram AF: Functional characterization of Rho GTPases in Aspergillus niger uncovers conserved and diverged roles of Rho proteins within filamentous fungi. Mol Microbiol. 2011, 79: 1151-1167. 10.1111/j.1365-2958.2010.07524.x.
Etienne-Manneville S: Cdc42–the centre of polarity. J Cell Sci. 2004, 117: 1291-1300. 10.1242/jcs.01115.
Heasman SJ, Ridley AJ: Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008, 9: 690-701. 10.1038/nrm2476.
Nobes CD, Hall A: Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995, 81: 53-62. 10.1016/0092-8674(95)90370-4.
Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M: Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003, 8: 1019-1027. 10.1111/j.1365-2443.2003.00695.x.
Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D’Souza-Schorey C, Chavrier P: The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008, 181: 985-998. 10.1083/jcb.200709076.
Luo L, Liao YJ, Jan LY, Jan YN: Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994, 8: 1787-1802. 10.1101/gad.8.15.1787.
Allen MJ, Shan X, Murphey RK: A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci. 2000, 16: 754-765. 10.1006/mcne.2000.0903.
Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L: Rac GTPases control axon growth, guidance and branching. Nature. 2002, 416: 442-447. 10.1038/416442a.
Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ: Rac function and regulation during Drosophila development. Nature. 2002, 416: 438-442. 10.1038/416438a.
Malartre M, Ayaz D, Amador FF, Martin-Bermudo MD: The guanine exchange factor vav controls axon growth and guidance during Drosophila development. J Neurosci. 2010, 30: 2257-2267. 10.1523/JNEUROSCI.1820-09.2010.
Miyamoto Y, Yamauchi J: Cellular signaling of Dock family proteins in neural function. Cell Signal. 2010, 22: 175-182. 10.1016/j.cellsig.2009.09.036.
Song JK, Giniger E: Noncanonical Notch function in motor axon guidance is mediated by Rac GTPase and the GEF1 domain of Trio. Dev Dyn. 2011, 240: 324-332. 10.1002/dvdy.22525.
Chen L, Liao G, Waclaw RR, Burns KA, Linquist D, Campbell K, Zheng Y, Kuan CY: Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J Neurosci. 2007, 27: 3884-3893. 10.1523/JNEUROSCI.3509-06.2007.
Kassai H, Terashima T, Fukaya M, Nakao K, Sakahara M, Watanabe M, Aiba A: Rac1 in cortical projection neurons is selectively required for midline crossing of commissural axonal formation. Eur J Neurosci. 2008, 28: 257-267. 10.1111/j.1460-9568.2008.06343.x.
Corbetta S, Gualdoni S, Ciceri G, Monari M, Zuccaro E, Tybulewicz VL, de Curtis I: Essential role of Rac1 and Rac3 GTPases in neuronal development. FASEB J. 2009, 23: 1347-1357. 10.1096/fj.08-121574.
Tahirovic S, Hellal F, Neukirchen D, Hindges R, Garvalov BK, Flynn KC, Stradal TE, Chrostek-Grashoff A, Brakebusch C, Bradke F: Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci. 2010, 30: 6930-6943. 10.1523/JNEUROSCI.5395-09.2010.
Chen L, Liao G, Yang L, Campbell K, Nakafuku M, Kuan CY, Zheng Y: Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci U S A. 2006, 103: 16520-16525. 10.1073/pnas.0603533103.
Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB: The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006, 9: 1099-1107. 10.1038/nn1744.
Yalovsky S, Bloch D, Sorek N, Kost B: Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol. 2008, 147: 1527-1543. 10.1104/pp.108.122150.
Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, Yalovsky S: A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 2010, 8: e1000282-10.1371/journal.pbio.1000282.
Nagawa S, Xu T, Yang Z: RHO GTPase in plants: Conservation and invention of regulators and effectors. Small GTPases. 2010, 1: 78-88. 10.4161/sgtp.1.2.14544.
Li H, Lin Y, Heath RM, Zhu MX, Yang Z: Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999, 11: 1731-1742.
Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K: Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 2001, 20: 2779-2788. 10.1093/emboj/20.11.2779.
Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L: A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005, 438: 1013-1016. 10.1038/nature04198.
Fischer U, Ikeda Y, Ljung K, Serralbo O, Singh M, Heidstra R, Palme K, Scheres B, Grebe M: Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr Biol. 2006, 16: 2143-2149. 10.1016/j.cub.2006.08.091.
Berken A, Thomas C, Wittinghofer A: A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 2005, 436: 1176-1180. 10.1038/nature03883.
Locke S, Fricke I, Mucha E, Humpert ML, Berken A: Interactions in the pollen-specific receptor-like kinases-containing signaling network. Eur J Cell Biol. 2010, 89: 917-923. 10.1016/j.ejcb.2010.08.002.
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH: Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004, 16: 1220-1234. 10.1105/tpc.020834.
Duan Q, Kita D, Li C, Cheung AY, Wu HM: FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci U S A. 2010, 107: 17821-17826. 10.1073/pnas.1005366107.
Zhou K, Wang Y, Gorski JL, Nomura N, Collard J, Bokoch GM: Guanine nucleotide exchange factors regulate specificity of downstream signaling from Rac and Cdc42. J Biol Chem. 1998, 273: 16782-16786. 10.1074/jbc.273.27.16782.
Olson MF: Guanine nucleotide exchange factors for the Rho GTPases: a role in human disease?. J Mol Med (Berl). 1996, 74: 563-571. 10.1007/s001090050060.
Pasteris NG, Cadle A, Logie LJ, Porteous ME, Schwartz CE, Stevenson RE, Glover TW, Wilroy RS, Gorski JL: Isolation and characterization of the faciogenital dysplasia (Aarskog-Scott syndrome) gene: a putative Rho/Rac guanine nucleotide exchange factor. Cell. 1994, 79: 669-678. 10.1016/0092-8674(94)90552-5.
Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggio S, Tete S, Polishchuk R, Castronovo V, Buccione R: Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 2009, 69: 747-752. 10.1158/0008-5472.CAN-08-1980.
Kost B: Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends Cell Biol. 2008, 18: 119-127. 10.1016/j.tcb.2008.01.003.
Donaldson JG, Jackson CL: ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011, 12: 362-375. 10.1038/nrm3117.
Nielsen ME, Feechan A, Bohlenius H, Ueda T, Thordal-Christensen H: Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc Natl Acad Sci U S A. 2012, 109: 11443-11448. 10.1073/pnas.1117596109.
Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C: An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 2001, 20: 4973-4986. 10.1093/emboj/20.17.4973.
Hashimoto S, Onodera Y, Hashimoto A, Tanaka M, Hamaguchi M, Yamada A, Sabe H: Requirement for Arf6 in breast cancer invasive activities. Proc Natl Acad Sci U S A. 2004, 101: 6647-6652. 10.1073/pnas.0401753101.
Tague SE, Muralidharan V, D’Souza-Schorey C: ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci U S A. 2004, 101: 9671-9676. 10.1073/pnas.0403531101.
Muralidharan-Chari V, Hoover H, Clancy J, Schweitzer J, Suckow MA, Schroeder V, Castellino FJ, Schorey JS, D’Souza-Schorey C: ADP-ribosylation factor 6 regulates tumorigenic and invasive properties in vivo. Cancer Res. 2009, 69: 2201-2209. 10.1158/0008-5472.CAN-08-1301.
Hernandez-Deviez DJ, Casanova JE, Wilson JM: Regulation of dendritic development by the ARF exchange factor ARNO. Nat Neurosci. 2002, 5: 623-624.
Albertinazzi C, Za L, Paris S, de Curtis I: ADP-ribosylation factor 6 and a functional PIX/p95-APP1 complex are required for Rac1B-mediated neurite outgrowth. Mol Biol Cell. 2003, 14: 1295-1307. 10.1091/mbc.E02-07-0406.
Hernandez-Deviez DJ, Roth MG, Casanova JE, Wilson JM: ARNO and ARF6 regulate axonal elongation and branching through downstream activation of phosphatidylinositol 4-phosphate 5-kinase alpha. Mol Biol Cell. 2004, 15: 111-120.
Moore CD, Thacker EE, Larimore J, Gaston D, Underwood A, Kearns B, Patterson SI, Jackson T, Chapleau C, Pozzo-Miller L: The neuronal Arf GAP centaurin alpha1 modulates dendritic differentiation. J Cell Sci. 2007, 120: 2683-2693. 10.1242/jcs.006346.
Song XF, Yang CY, Liu J, Yang WC: RPA, a class II ARFGAP protein, activates ARF1 and U5 and plays a role in root hair development in Arabidopsis. Plant Physiol. 2006, 141: 966-976. 10.1104/pp.106.077818.
Yoo CM, Wen J, Motes CM, Sparks JA, Blancaflor EB: A class I ADP-ribosylation factor GTPase-activating protein is critical for maintaining directional root hair growth in Arabidopsis. Plant Physiol. 2008, 147: 1659-1674. 10.1104/pp.108.119529.
Yoo CM, Quan L, Cannon AE, Wen J, Blancaflor EB: AGD1, a class 1 ARF-GAP, acts in common signaling pathways with phosphoinositide metabolism and the actin cytoskeleton in controlling Arabidopsis root hair polarity. Plant J. 2012, 69: 1064-1076. 10.1111/j.1365-313X.2011.04856.x.
Hutagalung AH, Novick PJ: Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011, 91: 119-149. 10.1152/physrev.00059.2009.
Stenmark H: Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009, 10: 513-525.
Woollard AA, Moore I: The functions of Rab GTPases in plant membrane traffic. Curr Opin Plant Biol. 2008, 11: 610-619. 10.1016/j.pbi.2008.09.010.
Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC: MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007, 26: 1499-1510. 10.1038/sj.emboj.7601606.
Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI: Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007, 13: 496-510. 10.1016/j.devcel.2007.08.012.
Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den BR, Lambert J, Van Belle S, Cocquyt V, Gespach C: Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst. 2010, 102: 866-880. 10.1093/jnci/djq153.
Peranen J: Rab8 GTPase as a regulator of cell shape. Cytoskeleton (Hoboken). 2011, 68: 527-539. 10.1002/cm.20529.
Wang L, Liang Z, Li G: Rab22 controls NGF signaling and neurite outgrowth in PC12 cells. Mol Biol Cell. 2011, 22: 3853-3860. 10.1091/mbc.E11-03-0277.
Mori Y, Matsui T, Furutani Y, Yoshihara Y, Fukuda M: Small GTPase Rab17 regulates dendritic morphogenesis and postsynaptic development of hippocampal neurons. J Biol Chem. 2012, 287: 8963-8973. 10.1074/jbc.M111.314385.
Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M: van den BW, van Dongen W, Richter S, Geldner N, Takano J et al.: Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010, 22: 1344-1357. 10.1105/tpc.109.072637.
Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E: The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell. 2004, 16: 1589-1603. 10.1105/tpc.021634.
de Graaf BH, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM: Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant Cell. 2005, 17: 2564-2579. 10.1105/tpc.105.033183.
Szumlanski AL, Nielsen E: The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell. 2009, 21: 526-544. 10.1105/tpc.108.060277.
Vignjevic D, Montagnac G: Reorganisation of the dendritic actin network during cancer cell migration and invasion. Semin Cancer Biol. 2008, 18: 12-22. 10.1016/j.semcancer.2007.08.001.
Jacobs T, Causeret F, Nishimura YV, Terao M, Norman A, Hoshino M, Nikolic M: Localized activation of p21-activated kinase controls neuronal polarity and morphology. J Neurosci. 2007, 27: 8604-8615. 10.1523/JNEUROSCI.0765-07.2007.
Geitmann A: How to shape a cylinder: pollen tube as a model system for the generation of complex cellular geometry. Sex Plant Reprod. 2010, 23: 63-71. 10.1007/s00497-009-0121-4.
Gossot O, Geitmann A: Pollen tube growth: coping with mechanical obstacles involves the cytoskeleton. Planta. 2007, 226: 405-416. 10.1007/s00425-007-0491-5.
Cheung AY, Niroomand S, Zou Y, Wu HM: A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc Natl Acad Sci U S A. 2010, 107: 16390-16395. 10.1073/pnas.1008527107.
Daher FB, Geitmann A: Actin depolymerizing factors ADF7 and ADF10 play distinct roles during pollen development and pollen tube growth. Plant Signal Behav. 2012, 7: 879-881.
Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK: Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta. 2005, 221: 95-104. 10.1007/s00425-004-1423-2.
Gibbon BC, Kovar DR, Staiger CJ: Latrunculin B has different effects on pollen germination and tube growth. Plant Cell. 1999, 11: 2349-2363.
Ketelaar T, de Ruijter NC, Emons AM: Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell. 2003, 15: 285-292. 10.1105/tpc.007039.
Chen T, Teng N, Wu X, Wang Y, Tang W, Samaj J, Baluska F, Lin J: Disruption of actin filaments by latrunculin B affects cell wall construction in Picea meyeri pollen tube by disturbing vesicle trafficking. Plant Cell Physiol. 2007, 48: 19-30.
Muallem S, Kwiatkowska K, Xu X, Yin HL: Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995, 128: 589-598. 10.1083/jcb.128.4.589.
Bradke F, Dotti CG: The role of local actin instability in axon formation. Science. 1999, 283: 1931-1934. 10.1126/science.283.5409.1931.
Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR: The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007, 292: C1690-C1700.
Valentijn KM, Gumkowski FD, Jamieson JD: The subapical actin cytoskeleton regulates secretion and membrane retrieval in pancreatic acinar cells. J Cell Sci. 1999, 112 (Pt 1): 81-96.
Goley ED, Welch MD: The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006, 7: 713-726. 10.1038/nrm2026.
Stradal TE, Scita G: Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol. 2006, 18: 4-10. 10.1016/j.ceb.2005.12.003.
Lorenz M, Yamaguchi H, Wang Y, Singer RH, Condeelis J: Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Curr Biol. 2004, 14: 697-703. 10.1016/j.cub.2004.04.008.
Cudmore S, Cossart P, Griffiths G, Way M: Actin-based motility of vaccinia virus. Nature. 1995, 378: 636-638. 10.1038/378636a0.
Gouin E, Welch MD, Cossart P: Actin-based motility of intracellular pathogens. Curr Opin Microbiol. 2005, 8: 35-45. 10.1016/j.mib.2004.12.013.
Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM: Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004, 43: 81-94. 10.1016/j.neuron.2004.05.015.
Mongiu AK, Weitzke EL, Chaga OY, Borisy GG: Kinetic-structural analysis of neuronal growth cone veil motility. J Cell Sci. 2007, 120: 1113-1125. 10.1242/jcs.03384.
Korobova F, Svitkina T: Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010, 21: 165-176. 10.1091/mbc.E09-07-0596.
Pommereit D, Wouters FS: An NGF-induced Exo70-TC10 complex locally antagonises Cdc42-mediated activation of N-WASP to modulate neurite outgrowth. J Cell Sci. 2007, 120: 2694-2705. 10.1242/jcs.03475.
Szymanski DB: Breaking the WAVE complex: the point of Arabidopsis trichomes. Curr Opin Plant Biol. 2005, 8: 103-112. 10.1016/j.pbi.2004.11.004.
Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB: A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc Natl Acad Sci U S A. 2008, 105: 4044-4049. 10.1073/pnas.0710294105.
Deeks MJ, Hussey PJ, Davies B: Formins: intermediates in signal-transduction cascades that affect cytoskeletal reorganization. Trends Plant Sci. 2002, 7: 492-498. 10.1016/S1360-1385(02)02341-5.
Cvrckova F, Novotny M, Pickova D, Zarsky V: Formin homology 2 domains occur in multiple contexts in angiosperms. BMC Genomics. 2004, 5: 44-10.1186/1471-2164-5-44.
Grunt M, Zarsky V, Cvrckova F: Roots of angiosperm formins: the evolutionary history of plant FH2 domain-containing proteins. BMC Evol Biol. 2008, 8: 115-10.1186/1471-2148-8-115.
Lizarraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P: Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009, 69: 2792-2800. 10.1158/0008-5472.CAN-08-3709.
Ridley AJ: Life at the leading edge. Cell. 2011, 145: 1012-1022. 10.1016/j.cell.2011.06.010.
Matusek T, Gombos R, Szecsenyi A, Sanchez-Soriano N, Czibula A, Pataki C, Gedai A, Prokop A, Rasko I, Mihaly J: Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008, 28: 13310-13319. 10.1523/JNEUROSCI.2727-08.2008.
Cheung AY, Wu HM: Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell. 2004, 16: 257-269. 10.1105/tpc.016550.
Ye J, Zheng Y, Yan A, Chen N, Wang Z, Huang S, Yang Z: Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell. 2009, 21: 3868-3884. 10.1105/tpc.109.068700.
Yi K, Guo C, Chen D, Zhao B, Yang B, Ren H: Cloning and functional characterization of a formin-like protein (AtFH8) from Arabidopsis. Plant Physiol. 2005, 138: 1071-1082. 10.1104/pp.104.055665.
Deeks MJ, Cvrckova F, Machesky LM, Mikitova V, Ketelaar T, Zarsky V, Davies B, Hussey PJ: Arabidopsis group Ie formins localize to specific cell membrane domains, interact with actin-binding proteins and cause defects in cell expansion upon aberrant expression. New Phytol. 2005, 168: 529-540. 10.1111/j.1469-8137.2005.01582.x.
Goode BL, Eck MJ: Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007, 76: 593-627. 10.1146/annurev.biochem.75.103004.142647.
Vidali L, van Gisbergen PA, Guerin C, Franco P, Li M, Burkart GM, Augustine RC, Blanchoin L, Bezanilla M: Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc Natl Acad Sci U S A. 2009, 106: 13341-13346.
Firat-Karalar EN, Welch MD: New mechanisms and functions of actin nucleation. Curr Opin Cell Biol. 2011, 23: 4-13. 10.1016/j.ceb.2010.10.007.
Ahuja R, Pinyol R, Reichenbach N, Custer L, Klingensmith J, Kessels MM, Qualmann B: Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell. 2007, 131: 337-350. 10.1016/j.cell.2007.08.030.
Vidali L, Burkart GM, Augustine RC, Kerdavid E, Tuzel E, Bezanilla M: Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell. 2010, 22: 1868-1882. 10.1105/tpc.109.073288.
Peremyslov VV, Prokhnevsky AI, Dolja VV: Class XI myosins are required for development, cell expansion, and F-Actin organization in Arabidopsis. Plant Cell. 2010, 22: 1883-1897. 10.1105/tpc.110.076315.
Prokhnevsky AI, Peremyslov VV, Dolja VV: Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc Natl Acad Sci U S A. 2008, 105: 19744-19749. 10.1073/pnas.0810730105.
Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV: Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol. 2008, 146: 1109-1116. 10.1104/pp.107.113654.
Ojangu EL, Jarve K, Paves H, Truve E: Arabidopsis thaliana myosin XIK is involved in root hair as well as trichome morphogenesis on stems and leaves. Protoplasma. 2007, 230: 193-202. 10.1007/s00709-006-0233-8.
Peremyslov VV, Mockler TC, Filichkin SA, Fox SE, Jaiswal P, Makarova KS, Koonin EV, Dolja VV: Expression, splicing, and evolution of the myosin gene family in plants. Plant Physiol. 2011, 155: 1191-1204. 10.1104/pp.110.170720.
Wagner W, Brenowitz SD, Hammer JA: Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat Cell Biol. 2011, 13: 40-48. 10.1038/ncb2132.
McMichael BK, Cheney RE, Lee BS: Myosin X regulates sealing zone patterning in osteoclasts through linkage of podosomes and microtubules. J Biol Chem. 2010, 285: 9506-9515. 10.1074/jbc.M109.017269.
Peremyslov VV, Klocko AL, Fowler JE, Dolja VV: Arabidopsis Myosin XI-K Localizes to the Motile Endomembrane Vesicles Associated with F-actin. Front Plant Sci. 2012, 3: 184-
Schott DH, Collins RN, Bretscher A: Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol. 2002, 156: 35-39. 10.1083/jcb.200110086.
Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J: Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009, 186: 571-587. 10.1083/jcb.200812176.
Kronenberg G, Gertz K, Baldinger T, Kirste I, Eckart S, Yildirim F, Ji S, Heuser I, Schrock H, Hortnagl H: Impact of actin filament stabilization on adult hippocampal and olfactory bulb neurogenesis. J Neurosci. 2010, 30: 3419-3431. 10.1523/JNEUROSCI.4231-09.2010.
Ren H, Xiang Y: The function of actin-binding proteins in pollen tube growth. Protoplasma. 2007, 230: 171-182. 10.1007/s00709-006-0231-x.
Strohmaier AR, Porwol T, Acker H, Spiess E: Three-dimensional organization of microtubules in tumor cells studied by confocal laser scanning microscopy and computer-assisted deconvolution and image reconstruction. Cells Tissues Organs. 2000, 167: 1-8. 10.1159/000016760.
Kikuchi K, Takahashi K: W. Cancer Sci. 2008, 99: 2252-2259. 10.1111/j.1349-7006.2008.00927.x.
Govek EE, Hatten ME, Van Aelst L: The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011, 71: 528-553. 10.1002/dneu.20850.
Pak CW, Flynn KC, Bamburg JR: Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci. 2008, 9: 136-147. 10.1038/nrn2236.
Stiess M, Bradke F: Neuronal polarization: the cytoskeleton leads the way. Dev Neurobiol. 2011, 71: 430-444. 10.1002/dneu.20849.
Gallo G: The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol. 2011, 71: 201-220. 10.1002/dneu.20852.
Goryunov D, He CZ, Lin CS, Leung CL, Liem RK: Nervous-tissue-specific elimination of microtubule-actin crosslinking factor 1a results in multiple developmental defects in the mouse brain. Mol Cell Neurosci. 2010, 44: 1-14. 10.1016/j.mcn.2010.01.010.
Leung CL, Sun D, Zheng M, Knowles DR, Liem RK: Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J Cell Biol. 1999, 147: 1275-1286. 10.1083/jcb.147.6.1275.
Wu X, Kodama A, Fuchs E: ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008, 135: 137-148. 10.1016/j.cell.2008.07.045.
Kodama A, Karakesisoglou I, Wong E, Vaezi A, Fuchs E: ACF7: an essential integrator of microtubule dynamics. Cell. 2003, 115: 343-354. 10.1016/S0092-8674(03)00813-4.
Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL: Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010, 140: 74-87. 10.1016/j.cell.2009.12.011.
Sieberer BJ, Ketelaar T, Esseling JJ, Emons AM: Microtubules guide root hair tip growth. New Phytol. 2005, 167: 711-719. 10.1111/j.1469-8137.2005.01506.x.
Bao Y, Kost B, Chua NH: Reduced expression of alpha-tubulin genes in Arabidopsis thaliana specifically affects root growth and morphology, root hair development and root gravitropism. Plant J. 2001, 28: 145-157. 10.1046/j.1365-313X.2001.01142.x.
Potocky M, Elias M, Profotova B, Novotna Z, Valentova O, Zarsky V: Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta. 2003, 217: 122-130.
Bibikova TN, Blancaflor EB, Gilroy S: Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999, 17: 657-665. 10.1046/j.1365-313X.1999.00415.x.
Cai G, Faleri C, Del Casino C, Emons AM, Cresti M: Distribution of callose synthase, cellulose synthase, and sucrose synthase in tobacco pollen tube is controlled in dissimilar ways by actin filaments and microtubules. Plant Physiol. 2011, 155: 1169-1190. 10.1104/pp.110.171371.
Wu G, Gu Y, Li S, Yang Z: A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets. Plant Cell. 2001, 13: 2841-2856.
Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z: Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005, 120: 687-700. 10.1016/j.cell.2004.12.026.
Novick P, Medkova M, Dong G, Hutagalung A, Reinisch K, Grosshans B: Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans. 2006, 34: 683-686. 10.1042/BST0340683.
Wu H, Rossi G, Brennwald P: The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008, 18: 397-404. 10.1016/j.tcb.2008.06.007.
Koumandou VL, Dacks JB, Coulson RM, Field MC: Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol Biol. 2007, 7: 29-10.1186/1471-2148-7-29.
Synek L, Schlager N, Elias M, Quentin M, Hauser MT, Zarsky V: AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J. 2006, 48: 54-72. 10.1111/j.1365-313X.2006.02854.x.
Zarsky V, Cvrckova F, Potocky M, Hala M: Exocytosis and cell polarity in plants - exocyst and recycling domains. New Phytol. 2009, 183: 255-272. 10.1111/j.1469-8137.2009.02880.x.
Cvrckova F, Grunt M, Bezvoda R, Hala M, Kulich I, Rawat A, Zarsky V: Evolution of the land plant exocyst complexes. Front Plant Sci. 2012, 3: 159-
Liu J, Yue P, Artym VV, Mueller SC, Guo W: The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell. 2009, 20: 3763-3771. 10.1091/mbc.E08-09-0967.
Lalli G: RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J Cell Sci. 2009, 122: 1499-1506. 10.1242/jcs.044339.
Lalli G, Hall A: Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol. 2005, 171: 857-869. 10.1083/jcb.200507061.
Cole RA, Synek L, Zarsky V, Fowler JE: SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant Physiol. 2005, 138: 2005-2018. 10.1104/pp.105.062273.
Hala M, Cole R, Synek L, Drdova E, Pecenkova T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE: An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell. 2008, 20: 1330-1345. 10.1105/tpc.108.059105.
Geitmann A, Dumais J: Not-so-tip-growth. Plant Signal Behav. 2009, 4: 136-138. 10.4161/psb.4.2.7633.
Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W: Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol. 2006, 8: 1383-1388. 10.1038/ncb1505.
Wang S, Liu Y, Adamson CL, Valdez G, Guo W, Hsu SC: The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J Biol Chem. 2004, 279: 35958-35966. 10.1074/jbc.M313778200.
Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K: IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005, 118: 2085-2092. 10.1242/jcs.02379.
Jadeski L, Mataraza JM, Jeong HW, Li Z, Sacks DB: IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial cells. J Biol Chem. 2008, 283: 1008-1017. 10.1074/jbc.M708466200.
Dupraz S, Grassi D, Bernis ME, Sosa L, Bisbal M, Gastaldi L, Jausoro I, Caceres A, Pfenninger KH, Quiroga S: The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci. 2009, 29: 13292-13301. 10.1523/JNEUROSCI.3907-09.2009.
Sosa L, Dupraz S, Laurino L, Bollati F, Bisbal M, Caceres A, Pfenninger KH, Quiroga S: IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat Neurosci. 2006, 9: 993-995. 10.1038/nn1742.
Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S: A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007, 17: 947-952. 10.1016/j.cub.2007.04.038.
Li S, Gu Y, Yan A, Lord E, Yang ZB: RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol Plant. 2008, 1: 1021-1035. 10.1093/mp/ssn051.
Das A, Guo W: Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol. 2011, 21: 383-386. 10.1016/j.tcb.2011.03.006.
Caldieri G, Giacchetti G, Beznoussenko G, Attanasio F, Ayala I, Buccione R: Invadopodia biogenesis is regulated by caveolin-mediated modulation of membrane cholesterol levels. J Cell Mol Med. 2009, 13: 1728-1740. 10.1111/j.1582-4934.2008.00568.x.
Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, Fukami K: Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 2009, 69: 8594-8602. 10.1158/0008-5472.CAN-09-2305.
Harel R, Futerman AH: Inhibition of sphingolipid synthesis affects axonal outgrowth in cultured hippocampal neurons. J Biol Chem. 1993, 268: 14476-14481.
Schwarz A, Rapaport E, Hirschberg K, Futerman AH: A regulatory role for sphingolipids in neuronal growth. Inhibition of sphingolipid synthesis and degradation have opposite effects on axonal branching. J Biol Chem. 1995, 270: 10990-10998. 10.1074/jbc.270.18.10990.
Pfrieger FW: Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes?. Bioessays. 2003, 25: 72-78. 10.1002/bies.10195.
Spor TC, Dezonne RS, Rehen SK, Gomes FC: Astrocytes treated by lysophosphatidic acid induce axonal outgrowth of cortical progenitors through extracellular matrix protein and epidermal growth factor signaling pathway. J Neurochem. 2011, 119: 113-123. 10.1111/j.1471-4159.2011.07421.x.
Ovecka M, Berson T, Beck M, Derksen J, Samaj J, Baluska F, Lichtscheidl IK: Structural sterols are involved in both the initiation and tip growth of root hairs in Arabidopsis thaliana. Plant Cell. 2010, 22: 2999-3019. 10.1105/tpc.109.069880.
Liu P, Li RL, Zhang L, Wang QL, Niehaus K, Baluska F, Samaj J, Lin JX: Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 2009, 60: 303-313. 10.1111/j.1365-313X.2009.03955.x.
Boutte Y, Grebe M: Cellular processes relying on sterol function in plants. Curr Opin Plant Biol. 2009, 12: 705-713. 10.1016/j.pbi.2009.09.013.
Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA: Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol. 2007, 9: 1381-1391. 10.1038/ncb1657.
Hanzal-Bayer MF, Hancock JF: Lipid rafts and membrane traffic. FEBS Lett. 2007, 581: 2098-2104. 10.1016/j.febslet.2007.03.019.
Leitinger B, Hogg N: The involvement of lipid rafts in the regulation of integrin function. J Cell Sci. 2002, 115: 963-972.
Mazzone M, Baldassarre M, Beznoussenko G, Giacchetti G, Cao J, Zucker S, Luini A, Buccione R: Intracellular processing and activation of membrane type 1 matrix metalloprotease depends on its partitioning into lipid domains. J Cell Sci. 2004, 117: 6275-6287. 10.1242/jcs.01563.
Otsuki M, Itoh T, Takenawa T: Neural Wiskott-Aldrich syndrome protein is recruited to rafts and associates with endophilin A in response to epidermal growth factor. J Biol Chem. 2003, 278: 6461-6469. 10.1074/jbc.M207433200.
Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010, 327: 46-50. 10.1126/science.1174621.
Ko M, Zou K, Minagawa H, Yu W, Gong JS, Yanagisawa K, Michikawa M: Cholesterol-mediated neurite outgrowth is differently regulated between cortical and hippocampal neurons. J Biol Chem. 2005, 280: 42759-42765. 10.1074/jbc.M509164200.
Meriane M, Tcherkezian J, Webber CA, Danek EI, Triki I, McFarlane S, Bloch-Gallego E, Lamarche-Vane N: Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J Cell Biol. 2004, 167: 687-698. 10.1083/jcb.200405053.
Pooler AM, Xi SC, Wurtman RJ: The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor pravastatin enhances neurite outgrowth in hippocampal neurons. J Neurochem. 2006, 97: 716-723. 10.1111/j.1471-4159.2006.03763.x.
Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M: Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J Cell Biol. 2002, 157: 521-532. 10.1083/jcb.200109059.
Shapiro L, Love J, Colman DR: Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007, 30: 451-474. 10.1146/annurev.neuro.29.051605.113034.
Gupta N, Wollscheid B, Watts JD, Scheer B, Aebersold R, DeFranco AL: Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol. 2006, 7: 625-633.
Xu X, Warrington AE, Wright BR, Bieber AJ, Van KV, Pease LR, Rodriguez M: A human IgM signals axon outgrowth: coupling lipid raft to microtubules. J Neurochem. 2011, 119: 100-112. 10.1111/j.1471-4159.2011.07416.x.
Morel J, Claverol S, Mongrand S, Furt F, Fromentin J, Bessoule JJ, Blein JP, Simon-Plas F: Proteomics of plant detergent-resistant membranes. Mol Cell Proteomics. 2006, 5: 1396-1411. 10.1074/mcp.M600044-MCP200.
Simon-Plas F, Perraki A, Bayer E, Gerbeau-Pissot P, Mongrand S: An update on plant membrane rafts. Curr Opin Plant Biol. 2011, 14: 642-649. 10.1016/j.pbi.2011.08.003.
Sorek N, Segev O, Gutman O, Bar E, Richter S, Poraty L, Hirsch JA, Henis YI, Lewinsohn E, Jurgens G: An S-acylation switch of conserved G domain cysteines is required for polarity signaling by ROP GTPases. Curr Biol. 2010, 20: 914-920. 10.1016/j.cub.2010.03.057.
del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA: Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004, 303: 839-842. 10.1126/science.1092571.
Golub T, Caroni P: PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol. 2005, 169: 151-165. 10.1083/jcb.200407058.
Yakir-Tamang L, Gerst JE: Phosphoinositides, exocytosis and polarity in yeast: all about actin?. Trends Cell Biol. 2009, 19: 677-684. 10.1016/j.tcb.2009.09.004.
Pike LJ, Miller JM: Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998, 273: 22298-22304. 10.1074/jbc.273.35.22298.
Caroni P: New EMBO members’ review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001, 20: 4332-4336. 10.1093/emboj/20.16.4332.
Yamaguchi H, Yoshida S, Muroi E, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K: Phosphatidylinositol 4,5-bisphosphate and PIP5-kinase Ialpha are required for invadopodia formation in human breast cancer cells. Cancer Sci. 2010, 101: 1632-1638. 10.1111/j.1349-7006.2010.01574.x.
Yamaguchi H, Yoshida S, Muroi E, Yoshida N, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K: Phosphoinositide 3-kinase signaling pathway mediated by p110alpha regulates invadopodia formation. J Cell Biol. 2011, 193: 1275-1288. 10.1083/jcb.201009126.
Thapa N, Sun Y, Schramp M, Choi S, Ling K, Anderson RA: Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev Cell. 2012, 22: 116-130. 10.1016/j.devcel.2011.10.030.
Koh CG: Rho GTPases and their regulators in neuronal functions and development. Neurosignals. 2006, 15: 228-237. 10.1159/000101527.
Kouchi Z, Igarashi T, Shibayama N, Inanobe S, Sakurai K, Yamaguchi H, Fukuda T, Yanagi S, Nakamura Y, Fukami K: Phospholipase Cdelta3 regulates RhoA/Rho kinase signaling and neurite outgrowth. J Biol Chem. 2011, 286: 8459-8471. 10.1074/jbc.M110.171223.
Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH: Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999, 145: 317-330. 10.1083/jcb.145.2.317.
Ischebeck T, Vu LH, Jin X, Stenzel I, Lofke C, Heilmann I: Functional cooperativity of enzymes of phosphoinositide conversion according to synergistic effects on pectin secretion in tobacco pollen tubes. Mol Plant. 2010, 3: 870-881. 10.1093/mp/ssq031.
Ischebeck T, Stenzel I, Hempel F, Jin X, Mosblech A, Heilmann I: Phosphatidylinositol-4,5-bisphosphate influences Nt-Rac5-mediated cell expansion in pollen tubes of Nicotiana tabacum. Plant J. 2011, 65: 453-468. 10.1111/j.1365-313X.2010.04435.x.
Xu N, Gao XQ, Zhao XY, Zhu DZ, Zhou LZ, Zhang XS: Arabidopsis AtVPS15 is essential for pollen development and germination through modulating phosphatidylinositol 3-phosphate formation. Plant Mol Biol. 2011, 77: 251-260. 10.1007/s11103-011-9806-9.
Preuss ML, Schmitz AJ, Thole JM, Bonner HK, Otegui MS, Nielsen E: A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 2006, 172: 991-998. 10.1083/jcb.200508116.
Camacho L, Smertenko AP, Perez-Gomez J, Hussey PJ, Moore I: Arabidopsis Rab-E GTPases exhibit a novel interaction with a plasma-membrane phosphatidylinositol-4-phosphate 5-kinase. J Cell Sci. 2009, 122: 4383-4392. 10.1242/jcs.053488.
Pleskot R, Pejchar P, Bezvoda R, Lichtscheidl IK, Wolters-Arts M, Marc J, Zarsky V, Potocky M: Turnover of Phosphatidic Acid through Distinct Signaling Pathways Affects Multiple Aspects of Pollen Tube Growth in Tobacco. Front Plant Sci. 2012, 3: 54-
Bedard K, Krause KH: The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007, 87: 245-313. 10.1152/physrev.00044.2005.
Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA: Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009, 2: ra53-10.1126/scisignal.2000368.
Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM: Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009, 2: ra54-10.1126/scisignal.2000370.
Tsatmali M, Walcott EC, Crossin KL: Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005, 1040: 137-150. 10.1016/j.brainres.2005.01.087.
Tsatmali M, Walcott EC, Makarenkova H, Crossin KL: Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol Cell Neurosci. 2006, 33: 345-357. 10.1016/j.mcn.2006.08.005.
Kennedy KA, Ostrakhovitch EA, Sandiford SD, Dayarathna T, Xie X, Waese EY, Chang WY, Feng Q, Skerjanc IS, Stanford WL: Mammalian numb-interacting protein 1/dual oxidase maturation factor 1 directs neuronal fate in stem cells. J Biol Chem. 2010, 285: 17974-17985. 10.1074/jbc.M109.084616.
Katoh S, Mitsui Y, Kitani K, Suzuki T: Hyperoxia induces the differentiated neuronal phenotype of PC12 cells by producing reactive oxygen species. Biochem Biophys Res Commun. 1997, 241: 347-351. 10.1006/bbrc.1997.7514.
Katoh S, Mitsui Y, Kitani K, Suzuki T: Hyperoxia induces the neuronal differentiated phenotype of PC12 cells via a sustained activity of mitogen-activated protein kinase induced by Bcl-2. Biochem J. 1999, 338 (Pt 2): 465-470.
Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T: Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000, 275: 13175-13178. 10.1074/jbc.275.18.13175.
Kamata H, Oka S, Shibukawa Y, Kakuta J, Hirata H: Redox regulation of nerve growth factor-induced neuronal differentiation of PC12 cells through modulation of the nerve growth factor receptor, TrkA. Arch Biochem Biophys. 2005, 434: 16-25. 10.1016/j.abb.2004.07.036.
Camacho L, Malho R: Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J Exp Bot. 2003, 54: 83-92. 10.1093/jxb/erg043.
Coelho PC, Malho R: Correlative Analysis of [Ca](C) and Apical Secretion during Pollen Tube Growth and Reorientation. Plant Signal Behav. 2006, 1: 152-157. 10.4161/psb.1.3.2999.
Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijo JA: Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science. 2011, 332: 434-437. 10.1126/science.1201101.
Bibikova TN, Zhigilei A, Gilroy S: Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 1997, 203: 495-505. 10.1007/s004250050219.
Wymer CL, Bibikova TN, Gilroy S: Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 1997, 12: 427-439. 10.1046/j.1365-313X.1997.12020427.x.
Mucha E, Hoefle C, Huckelhoven R, Berken A: RIP3 and AtKinesin-. Eur J Cell Biol. 2010, 89: 906-916. 10.1016/j.ejcb.2010.08.003.
Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD: Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003, 422: 442-446. 10.1038/nature01485.
Potocky M, Jones MA, Bezvoda R, Smirnoff N, Zarsky V: Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007, 174: 742-751. 10.1111/j.1469-8137.2007.02042.x.
Weaver AM: Regulation of cancer invasion by reactive oxygen species and Tks family scaffold proteins. Sci Signal. 2009, 2: e56-
Giannoni E, Taddei ML, Chiarugi P: Src redox regulation: again in the front line. Free Radic Biol Med. 2010, 49: 516-527. 10.1016/j.freeradbiomed.2010.04.025.
Gianni D, Bohl B, Courtneidge SA, Bokoch GM: The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008, 19: 2984-2994. 10.1091/mbc.E08-02-0138.
Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW: Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991, 353: 668-670. 10.1038/353668a0.
Woo CH, Lee ZW, Kim BC, Ha KS, Kim JH: Involvement of cytosolic phospholipase A2, and the subsequent release of arachidonic acid, in signalling by rac for the generation of intracellular reactive oxygen species in rat-2 fibroblasts. Biochem J. 2000, 348 (Pt 3): 525-530.
Zhou L, Too HP: Mitochondrial localized STAT3 is involved in NGF induced neurite outgrowth. PLoS One. 2011, 6: e21680-10.1371/journal.pone.0021680.
Sagi M, Fluhr R: Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141: 336-340. 10.1104/pp.106.078089.
Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L: Local positive feedback regulation determines cell shape in root hair cells. Science. 2008, 319: 1241-1244. 10.1126/science.1152505.
Munnamalai V, Suter DM: Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. J Neurochem. 2009, 108: 644-661. 10.1111/j.1471-4159.2008.05787.x.
Swanson S, Gilroy S: ROS in plant development. Physiol Plant. 2010, 138: 384-392. 10.1111/j.1399-3054.2009.01313.x.
Elias M, Klimes V: Rho GTPases: deciphering the evolutionary history of a complex protein family. Methods Mol Biol. 2012, 827: 13-34. 10.1007/978-1-61779-442-1_2.
Cvrckova F, Rivero F, Bavlnka B: Evolutionarily conserved modules in actin nucleation: lessons from Dictyostelium discoideum and plants. Review article. Protoplasma. 2004, 224: 15-31.
Rivero F, Cvrckova F: Origins and evolution of the actin cytoskeleton. Adv Exp Med Biol. 2007, 607: 97-110. 10.1007/978-0-387-74021-8_8.
Zhang Y, Franco M, Ducret A, Mignot T: A bacterial Ras-like small GTP-binding protein and its cognate GAP establish a dynamic spatial polarity axis to control directed motility. PLoS Biol. 2010, 8: e1000430-10.1371/journal.pbio.1000430.
Acknowledgements
The authors were supported by grants from the Kellner Family Foundation Principal Investigator Grant (to JB, DR), the Ministry of Education of the Czech Republic (MSM0021620858 to VŽ, FC, JB), Grant Agency of the Czech Republic (P305/11/1629 to VŽ, P305/10/0433 to FC), and the Grant Agency of Charles University Grant 629312 (to DR). We thank the reviewers and Njainday Pulo Jobe for helpfull comments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors participated in writing and editing various portions of the manuscript. The final editing was done by KV, JB, DR and FC. All authors read and approved the final manuscript.
Katarína Vaškovičová, Viktor Žárský contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vaškovičová, K., Žárský, V., Rösel, D. et al. Invasive cells in animals and plants: searching for LECA machineries in later eukaryotic life. Biol Direct 8, 8 (2013). https://doi.org/10.1186/1745-6150-8-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1745-6150-8-8