Abstract
Background
Migraine headache is one of the most common neurological disorders, but the pathophysiology contributing to migraine is poorly understood. Intracranial interleukin-6 (IL-6) levels have been shown to be elevated during migraine attacks, suggesting that this cytokine may facilitate pain signaling from the meninges and contribute to the development of headache.
Methods
Cutaneous allodynia was measured in rats following stimulation of the dura with IL-6 alone or in combination with the MEK inhibitor, U0126. The number of action potentials and latency to the first action potential peak in response to a ramp current stimulus as well as current threshold were measured in retrogradely-labeled dural afferents using patch-clamp electrophysiology. These recordings were performed in the presence of IL-6 alone or in combination with U0126. Association between ERK1 and Nav1.7 following IL-6 treatment was also measured by co-immunoprecipitation.
Results
Here we report that in awake animals, direct application of IL-6 to the dura produced dose-dependent facial and hindpaw allodynia. The MEK inhibitor U0126 blocked IL-6-induced allodynia indicating that IL-6 produced this behavioral effect through the MAP kinase pathway. In trigeminal neurons retrogradely labeled from the dura, IL-6 application decreased the current threshold for action potential firing. In response to a ramp current stimulus, cells treated with IL-6 showed an increase in the numbers of action potentials and a decrease in latency to the first spike, an effect consistent with phosphorylation of the sodium channel Nav1.7. Pretreatment with U0126 reversed hyperexcitability following IL-6 treatment. Moreover, co-immunoprecipitation experiments demonstrated an increased association between ERK1 and Nav1.7 following IL-6 treatment.
Conclusions
Our results indicate that IL-6 enhances the excitability of dural afferents likely via ERK-mediated modulation of Nav1.7 and these responses contribute to migraine-related pain behavior in vivo. These data provide a cellular mechanism by which IL-6 in the meninges causes sensitization of dural afferents therefore contributing to the pathogenesis of migraine headache.
Similar content being viewed by others
Background
Migraine is characterized as episodes of unilateral throbbing headache accompanied by a variety of symptoms, including aura, nausea, vomiting, photophobia and phonophobia [1]. Although the mechanisms contributing to migraine pathophysiology are not fully known, one hypothesis proposes that migraine is an inflammatory disease [2]. This idea is supported by the efficacy of non-steroidal anti-inflammatory drugs (NSAIDs) in migraine therapy as well as increased intracranial levels of inflammatory mediators during migraine attacks [2, 3]. Interleukin-6 (IL-6), which is one such mediator found to be elevated during migraine attacks [3, 4], is a cytokine with an established role in modulating various inflammatory pain conditions, including skin incision, carrageenan injection, burn-injury pain and pancreatitis-induced pain [5–9]. IL-6 levels are increased under inflammatory conditions and increases in IL-6 parallel pain intensity over time [10–12]. IL-6's pain promoting actions are thought to be mediated by a direct action on nociceptors because sensory neuron specific knockout of the IL-6 co-receptor reduces nociceptive sensitization [13]. Moreover, neutralizing IL-6 using a monoclonal antibody is effective in treating human rheumatoid arthritis [14]. Thus, accumulating evidence points to IL-6 as a contributing factor to many pain conditions possibly including migraine.
Activation and sensitization of meningeal nociceptors leads to afferent signaling that is thought to contribute to the headache that occurs during migraine. However, the contribution of IL-6 to this process and the mechanisms by which this may occur have not yet been explored. Following acute IL-6 application, trigeminal ganglion neurons display phosphorylation of ERK [15] indicating that these neurons respond to IL-6 through activation of the Mitogen-Activated Protein Kinase (MAPK) signaling pathway. Activation of the ERK1/2 MAPK pathway has been implicated in induction and maintenance of various pain conditions via transcriptional, translational or post-translational regulation [15–17]. Recent work has identified the voltage-gated sodium channel Nav1.7 as a novel downstream post-translational target for MAPK. Nav1.7 is a threshold sodium channel expressed on small and medium DRG neurons [18] and inhibition of ERK1/2 decreased neuronal excitability by inhibiting Nav1.7 phosphorylation and altering its gating properties [19].
Taken together, these studies led us to propose that increased levels of IL-6 in the meninges produces migraine-related pain behavior and this hypothesis was addressed using a preclinical model of headache. Further, we examined whether dural afferent excitability was increased following IL-6 exposure and whether this increased excitability is consistent with sodium channel phosphorylation.
Methods
Animals
Adult male Sprague Dawley rats (175-200 g) were maintained in a climate-controlled room on a 12 h light/dark cycle with food and water ad libitum. All procedures were performed in accordance with the policies and recommendations of the International Association for the Study of Pain, the National Institutes of Health guidelines for the handling and use of laboratory animals, and were approved by the Institutional Animal Care and Use Committee of the University of Arizona.
Surgical preparation
-
1.
Tracer injection
Dural afferents were identified as previously described [20, 21]. Seven days prior to sacrifice, animals were anesthetized with a combination of ketamine and xylazine (80 mg/kg and 12 mg/kg; Sigma-Aldrich). Two holes were made in the skull under a dissecting microscope to carefully expose but not damage the dura and fluorogold (5 μl/hole; 4% in SIF, synthetic interstitial fluid, pH 7.4, 320 mOsm) was applied onto the dura. A small piece of gelfoam was retained in the hole to increase the absorption of the dye and prevent spread of the tracer outside of the hole. Holes were covered with bone wax to prevent tracer spread. The incision was closed with sutures. Immediately postoperatively, animals received a single subcutaneous injection of gentamicin (8 mg/kg) to minimize infection. Undamaged dura at the injection sites was evaluated at the time the animals were sacrificed and only animals with intact dura and no signs of damage were used for further experiments.
-
2.
Dural cannulation
Dura cannulae were implanted as previously described [20, 21]. Animals were anesthetized with a combination of ketamine and xylazine (80 mg/kg and 12 mg/kg; Sigma-Aldrich). A 2 cm incision was made to expose the skull. A 1 mm hole was made in the skull (above the transverse sinus; 2 mm left of the sagittal suture and 2 mm anterior to the lambdoid suture) with a hand drill (DH-0 Pin Vise; Plastics One, Roanoke, VA) to carefully expose the dura. A guide cannula (22 GA, #C313G; Plastics One), designed to extend 0.5 mm from the pedestal to avoid irritation of the dural tissue, was inserted into the hole and sealed into place with glue. Two additional 1 mm holes were made in the parietal bones to receive stainless-steel screws (Small Parts), and dental acrylic was used to fix the cannula to the screws. A dummy cannula (#C313DC; Plastics One) was inserted to ensure patency of the guide cannula. Immediately postoperatively, animals received a single subcutaneous injection of gentamicin (8 mg/kg) to minimize infection. Rats were housed separately and allowed 6 to 8 days of recovery.
Cell culture
-
1.
Whole-cell patch clamp
Seven days following fluorogold application, trigeminal ganglia were removed, enzymatically treated, and mechanically dissociated as previously described [21]. Rats were anesthetized with isoflurane (Phoenix Pharmaceuticals) and sacrificed by decapitation. The trigeminal ganglion (TG) were removed and placed in ice-cold Hanks balanced-salt solution (divalent free). Ganglia were cut into small pieces and incubated for 25 mins in 20 U/ml Papain (Worthington) followed by 25 mins in 3 mg/ml Collagenase TypeII (Worthington). Ganglia were then triturated through fire-polished pasteur pipettes and plated on poly-D-lysine (Becton Dickinson) and laminin (Sigma)-coated plates. After several hours at room temperature to allow adhesion, cells were cultured in a room-temperature, humidified chamber in Liebovitz L-15 medium supplemented with 10% FBS, 10 mM glucose, 10 mM HEPES and 50 U/ml penicillin/streptomycin. Cells were used within 24 h post plating.
-
2.
Western Blotting
Rat trigeminal ganglia (TG) were excised aseptically and placed in Hank's Buffered Salt Solution (HBSS, Invitrogen) on ice. The ganglia were dissociated enzymatically with collagenase A (1 mg/ml, 25 min, Roche) and collagenase D (1 mg/ml, Roche) with papain (30 U/ml, Roche) for 20 min at 37°C. To eliminate debris, 70 μm (BD) cell strainers were used. The dissociated cells were resuspended in DMEM/F12 (Invitrogen) containing 1X pen-strep (Invitrogen), 1X GlutaMax, 3 μg/ml 5-FDU (5-Fluoro-2'-deoxyuridine) (Sigma), 7 μg/ml uridine (Sigma) and 10% fetal bovine serum (Hyclone). The cells were plated in 6-well plates (BD Falcon) and incubated at 37°C in a humidified 95% air/5%CO2 incubator. On day 5 the cells were washed in DMEM/F12 media for 15 mins followed by treatment.
Electrophysiology
Whole cell patch-clamp experiments were performed on isolated rat TG using a MultiClamp 700B (Axon Instruments) patch-clamp amplifier and pClamp 10 acquisition software (Axon Instruments). Recordings were sampled at 2 kHz and filtered at 1 kHz (Digidata 1322A, Axon Instruments). Pipettes (OD: 1.5 mm, ID: 0.86 mm, Sutter Instrument) were pulled using a P-97 puller (Sutter Instrument) and heat polished to 2.5-4 MΩ resistance using a microforge (MF-83, Narishige). Series resistance was typically < 7 MΩ and was compensated 60-80%. All recordings were performed at room temperature. A Nikon TE2000-S Microscope equipped with a mercury arc lamp (X-Cite® 120) was used to identify FG-labeled dural afferents. Data were analyzed using Clampfit 10 (Molecular Devices) and Origin 8 (OriginLab). Cell sizes were not significantly different among groups (Vehicle: 38.91 ± 2.214 pF vs IL-6: 39.62 ± 1.712 pF vs IL-6 + U0126: 41.98 ± 2.526 pF, p > 0.05). Pipette solution contained (in mM) 140 KCl, 11 EGTA, 2 MgCl2, 10 NaCl, 10 HEPES, 1 CaCl2 pH 7.3 (adjusted with N-methyl glucamine), and was ~ 320 mosM. External solution contained (in mM) 135 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 10 Glucose, 10 HEPES, pH 7.4 (adjusted with N-methyl glucamine), and was ~ 320 mosM.
Behavioral testing
Rats were acclimated to suspended Plexiglas chambers (30 cm long × 15 cm wide × 20 cm high) with a wire mesh bottom (1 cm2). Ten μl of vehicle or testing solution was injected through an injection cannula (28GA, #C313I; Plastics One) cut to fit the guide cannula. Withdrawal thresholds to probing the face and hind-paws were determined at 1-h intervals after administration. A behavioral response to calibrated von Frey filaments applied to the midline of the forehead, at the level of the eyes, was indicated by a sharp withdrawal of the head. Paw withdrawal (PW) thresholds were determined by applying von Frey filaments to the plantar aspect of the hind-paws, and a response was indicated by a withdrawal of the paw. The withdrawal thresholds were determined by the Dixon up-down method [22]. Maximum filament strengths were 8 and 15 g for the face and hind-paws, respectively.
Western blotting
Protein was extracted from cells in lysis buffer (50 mM Tris HCl, 1% Triton X-100, 150 mM NaCl, and 1 mM EDTA at pH 7.4) containing protease and phosphatase inhibitor mixtures (Sigma) with an ultrasonicator on ice, and cleared of cellular debris and nuclei by centrifugation at 14,000 RCF for 15 min at 4°C. Fifteen micrograms of protein per well were loaded and separated by standard 7.5% or 10% SDS-PAGE. Proteins were transferred to Immobilon-P membranes. Blots were incubated with primary antibody overnight at 4°C and detected the following day with appropriate secondary antibodies. Signal was detected by ECL on chemiluminescent films. Densitometric analyses were performed with Image J software (NIH).
Co-immunoprecipitation
After protein extraction, 70 μg protein was incubated with Nav1.7 antibody (NeuroMab) overnight at 4°C followed by 3 h incubation with 20 μl protein G-Sepharose beads. After washing 3 times with lysis buffer, the pelleted beads were resuspended in 1X Laemmli Sample Buffer containing 5% v/v β-mercaptoethanol and total ERK (tERK) bound to the precipitated beads was analyzed by western blotting.
Data analysis
All data are presented as means ± SEM unless otherwise noted. Statistical evaluation was performed by linear regression analysis, unpaired t-test, one-way analysis of variance (ANOVA) followed by post hoc Newman-Keuls test, or two-way ANOVA. For behavioral experiments, data were converted to area over the time-effect curve to allow for multiple comparisons.
Chemicals
Fluorogold was purchased from Fluorochrome, LLC. and dissolved in synthetic interstitial fluid (SIF) (pH 7.4, 310 mOsm) to 4%. U0126 was from Tocris Biosciences. Rat recombinant IL-6 was from R&D Systems. Stock U0126 (10 mM) was prepared in Dimethyl sulfoxide (DMSO) and added to the culture media and the recording chamber to produce a final concentration of 10 μM in 0.1% DMSO for patch experiment. For the behavioral experiments, stock U0126 solutions (100 mM in DMSO) were prepared and diluted in SIF to the final concentration of 1 mM. Vehicle control was SIF with 1% DMSO. Stock rIL-6 (10 μg/ml) was prepared in sterile 0.1% BSA in PBS and diluted to final concentrations of 50 ng/ml (patch) and 100 ng/ml (behavior), respectively.
Results
Cutaneous allodynia following IL-6 administration to the dura
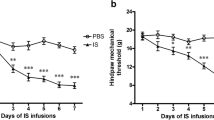
A preclinical in vivo migraine model was used to evaluate the effect of meningeal IL-6 application on mechanical withdrawal thresholds both to the face and hindpaws [23]. Mechanical allodynia following dural stimulation is used as a surrogate for migraine as the majority of migraine patients experience cutaneous allodynia during the headache phase and allodynia is more common in migraine compared to other types of headaches [24, 25]. Application of 1 ng IL-6 in SIF solution to the dura produced significant (p < 0.0001) time-dependent reductions in withdrawal thresholds to tactile stimuli applied to the face or the hind-paws compared with SIF application alone (Figure 1A). Maximal effects occurred 2 h after IL-6 application, and facial and hind-paw allodynia was present for at least 24 h (Figure 1A). IL-6 dose-dependently reduced the withdrawal thresholds compared with vehicle control as 1 ng IL-6 produced greater allodynia than that observed at 0.1 ng (Figure 1B). Co-application of the MEK inhibitor, U0126 (10 nmol) with 1 ng IL-6 prevented facial and hind-paw cutaneous allodynia (Figure 1C), indicating that IL-6 produces allodynia following dural application via activation of the MAP kinase (ERK) signaling pathway.
Application of IL-6 to the dura elicited cutaneous allodynia via activation of the MEK/ERK pathway. (A) Withdrawal thresholds to tactile stimuli applied to the face and the hind-paws were measured in rats before and immediately after dural application of 1 ng IL-6 (n = 16) or SIF (n = 17). For both facial and hind-paw responses, two-factor analysis of variance indicated that response thresholds of IL-6-treated rats were significantly (p < 0.0001) less than those of SIF-treated rats. (B) Rats received dural administration of SIF (white bar, n = 17), 0.1 ng IL-6 (gray bar, n = 22) or 1 ng IL-6 (black bar, n = 16). Withdrawal thresholds to tactile stimuli were measured for 5 h and data were converted to area over the time-effect curve. IL-6 dose-dependently decreased the withdrawal threshold both in the face and in the hind-paw. Significant (*p < 0.05, **p < 0.01) differences among means for each group were determined by analysis of variance followed by Newman-Keuls post hoc test. (C) Application of 1 ng IL-6 was given with vehicle (white bars, 1% DMSO, n = 12) or with U0126 (black bars, 1 nmol, n = 12). Withdrawal thresholds to tactile stimuli were measured for 5 h and data were converted to area over the time-effect curve and normalized as a percentage of the IL-6-treated group. Coapplication of U0126 significantly abolished behavioral signs of tactile allodynia of the face and hind-paw (*p < 0.05, **p < 0.01).
Activation of the ERK pathway mediates IL-6-induced hyperexcitability of dural afferents
Nav1.7 is known to generate currents in response to slow ramp depolarization due to its slow inactivation kinetics, hence a ramp stimulus protocol was used to preferentially elicit activity of Nav1.7 [26]. Although this protocol elicits activation of Nav1.7 it should be noted that other sodium channels such as Nav1.8 may also be recruited as Nav1.7 and Nav1.8 are thought to work together in generating repetitive firing in sensory neurons [27, 28]. Thus, this protocol likely produces firing via activation of multiple sodium channels but an increase in firing is nonetheless indicative of Nav1.7 sensitization.
Retrogradely-labeled cells in vitro were selected for patch clamp experiments. Slow ramp currents from 0.1 to 0.7 nA with Δ = 0.2 nA were injected over 1 s (Figure 2A) to mimic slow depolarization. If cells fired in response to this protocol no further testing is done. If they did not fire with this protocol, a second protocol was run where the final ramp amplitude is 2 nA in 1 s. If cells fire in response to this protocol they were included in the data analysis as they technically responded to a ramp current injection but they are given 0 spikes for 0.1, 0.3, 0.5, and 0.7 nA since they did not fire in response to any of the slower ramps. If they did not fire in response to the 2 nA ramp they were excluded from analysis as they were determined to be cells that likely would not fire in response to a ramp. Dural afferents acutely treated with 50 ng/ml IL-6 for 15 min showed a significant increase in the number of spikes and a decrease in the latency to the first AP spike (Figure 2A and 2B), consistent with increased Nav1.7 activity. Pretreatment with 10 μM U0126 for 10 min significantly reversed the IL-6-induced increase in excitability (Figure 2A and 2B) indicating that, similar to IL-6 induced allodynia, these changes are due to activation of ERK signaling.
IL-6 promoted ERK-dependent hyperexcitability of dural afferents in response to ramp current stimuli. (A) Action potentials were elicited by 1 s ramp current injection ranging from 0.1 to 0.7 nA in 0.2 nA increments from resting membrane potential. Dural afferents treated with IL-6 showed increased numbers of action potentials and shorter time-to-first action potential (AP) peak compared with vehicle-treated dural afferents. IL-6-induced hyperexcitability was blocked by pretreatment with 10 μM U0126. (B) Difference in the mean numbers of action potentials among groups was analyzed by comparing the slopes and intercepts generated from linear regression. Comparison among several groups for time-to-first spike was performed by two-factor analysis of variance. Dural afferents treated with 50 ng/ml IL-6 (red square, n = 16) showed a significant (p < 0.05) increase in number of action potentials and a significant decrease in time-to-first peak compared with vehicle-treated dural afferents (black circle, n = 12). Pretreatment with 10 μM U0126 (blue triangle, n = 13) for 10 mins significantly reversed IL-6-induced hyperexcitability.
Current clamp configuration was used to determine the current threshold, i.e. the minimum current required to initiate an action potential in the same group of dural afferents. Action potentials were elicited by injecting rectangular current steps (25 ms, Δ = 10 pA). The current threshold was significantly decreased for dural afferents acutely pretreated with IL-6 for 15 mins (217.4 ± 17.98 nA, n = 31) compared with dural afferents treated with vehicle (319.3 ± 25.14 nA, n = 30, **p < 0.01 vs vehicle) (Figure 3A and 3B). Although there was no depolarization of the resting membrane potential following IL-6 treatment, pretreatment with the MEK inhibitor U0126 significantly hyperpolarized resting membrane potentials (-67.24 ± 1.474 mV) compared with vehicle (-63.45 ± 0.7208, p < 0.05) or IL-6 treated neurons (-62.40 ± 0.7737 mV, p < 0.05). This finding is consistent with previous studies of Nav1.7 where U0126 treatment hyperpolarized resting membrane potentials [19]. Pretreatment with the MEK inhibitor U0126 for 10 mins reversed the IL-6-induced changes in current threshold (374.6 ± 52.45 nA, n = 13, ## p < 0.01 vs IL-6 alone), again indicating that IL-6 acts through the MAP kinase pathway.
IL-6 treatment significantly increased the dural afferent excitability, which was blocked by pretreatment with U0216. (A) Current threshold was measured in dural afferents treated with vehicle (white bar, n = 30), 50 ng/ml IL-6 (black bar, n = 32) or 50 ng/ml IL-6 + 10 μM U0126 (gray bar, n = 13). Significant (**p < 0.01) differences among means for each group were determined by analysis of variance followed by Newman-Keuls post hoc test. Current threshold was significantly lowered after IL-6 treatment (**p < 0.01). Pretreatment with U0126 for 10 mins significantly reversed the hyperexcitability induced by IL-6 (## p < 0.01). (B) Action potentials were elicited by 25 ms step current injection from resting membrane potential. Horizontal line and arrow indicate 0 and -70 mV membrane potential, respectively. Current threshold for action potentials in the representative dural afferents treated with vehicle, 50 ng/ml IL-6 or 50 ng/ml IL-6 + 10 μM U0126.
IL-6 treatment promotes direct association between ERK and Nav1.7
To further explore whether IL-6-induced hyperexcitability of dural afferents was mediated through modulation of Nav1.7, we used a co-immunoprecipitation assay to determine direct associations between ERK and Nav1.7. In cells treated with IL-6 for 15 min, a significantly increased (*p < 0.05) amount of tERK was co-immunoprecipited with Nav1.7 compared to vehicle treatment (Figure 4A and 4B) although there was no change in the total level of Nav1.7 (data not shown). Pretreatment with the MEK inhibitor U0126 for 10 mins significantly reversed (*p < 0.05) the IL-6-induced increase in association between Nav1.7 and ERK. No signal was seen with cell lysates without primary antibody incubation (Figure 4A, Neg). In contrast to the observation that both tERK1 and tERK2 were detected in whole cell lysates (Figure 4A, Input), only tERK1 was detected in co-IP analysis (Figure 4A), consistent with a previous study showing that ERK1, but not ERK2, phosphorylated the L1 loop of Nav1.7 [19]. These data provide further evidence that IL-6-activated signaling pathways can regulate neuronal excitability through direct modulation of Nav1.7.
IL-6 enhanced direct association between ERK1 and Nav1.7. Western blot and quantification for tERK following 15 mins treatment of TG cultures with vehicle, IL-6 (50 ng/ml) or IL-6 (50 ng/ml) + U0126 (10 μM) (A) Interaction between ERK and Nav1.7 was examined by immunoprecipitation (IP) with antibody against Nav1.7 followed by immunoblotting (IB) for tERK. Omission of primary antibody was used as negative control and whole cell lysates were loaded as positive control. (B) ERK1 was quantified and values were expressed as percentage of vehicle. Significant (*p < 0.05) differences among means for each group were determined by analysis of variance followed by Newman-Keuls post hoc test.
Discussion
Understanding the endogenous processes that promote the activation and sensitization of meningeal nociceptors is important in explaining the mechanisms underlying migraine headache. The present findings provide direct evidence that IL-6 is important for sensitization of dural afferents by increasing neural excitability through modulation of Nav1.7. We also demonstrate that meningeal IL-6 application can produce migraine-like behavior through activation of the ERK pathway, supporting a role for IL-6 in migraine pathophysiology.
These studies demonstrate that direct meningeal application of exogenous IL-6 caused migraine-like behaviors in rats. However, the source of endogenous IL-6 during a migraine attack is not clear. Several lines of evidence have indicated that neurogenic inflammation underlies migraine headache pathogenesis with the involvement of at least 2 types of immune cells, dural mast cells and meningeal macrophages [29, 30]. The meninges are densely populated with mast cells, which reside in close proximity to afferent endings mostly within the dura compared to other meningeal layers [31–33]. A variety of well-known migraine precipitants, including stress (via the release of corticotrophin releasing hormone CRH or factor CRF) and CGRP trigger mast cell degranulation and the subsequent release of their inflammatory mediators [29]. Relevant to the studies described here are reports that human mast cells can release IL-6 following stimulation [34, 35]. In addition to mast cells, IL-6 released from dural macrophages may also contribute to stress-induced neurogenic inflammation [30]. Regardless of the source, IL-6 has the ability to sensitize nociceptors through actions on TRPV1 and ERK-mediated signaling to translation machinery [15, 36]. The experiments described here demonstrated that IL-6 application was able to sensitize identified dural afferents, and suggested additional mechanisms of IL-6 induced sensitization through phosphorylation of sodium channels.
Human genetic studies have demonstrated an important role for the sodium channel Nav1.7 in pain [37]. Gain-of-function mutations of Nav1.7 are directly linked with several extreme pain conditions in humans such as erythromelalgia and paroxysmal extreme pain disorder, whereas loss-of-function mutation of Nav1.7 is associated with congenital insensitivity to pain [37]. Although the gain-of-function mutations do not lead to headache and the location specific nature of the spontaneous pain in these disorders is poorly understood, these conditions highlight the importance of this channel in nociceptive signaling and suggest that sensitization of Nav1.7 may contribute to enhanced pain signaling from many areas including the head. Due to its distinctive slow development of closed-state inactivation, Nav1.7 is able to generate current in response to sub-threshold depolarization, thus playing an important role in amplifying excitatory inputs and modulating neuronal excitability [26]. Additionally, inhibition of Nav1.7 is known to decrease neuronal excitability [38, 39]. Preclinical work has also indicated an important role for Nav1.7 in mediating inflammatory pain as supported by the evidence that formalin-induced mechanical allodynia and thermal hyperalgesia are abrogated in Nav1.7 knockout mice [40]. Moreover, mRNA and protein levels of Nav1.7 increase following carrageenan injection, which parallel the increase in TTX-S currents [41]. Hence, preclinical and clinical studies have created a compelling rationale for targeting Nav1.7 in inflammatory pain. The present work indicates that IL-6 application increases the number of spikes and decreases the latency to the first AP in response to ramp stimuli protocols, which are consistent with hyperexcitability induced by Nav1.7 phosphorylation [19]. This IL-6-induced hyperexcitability is mediated through ERK signaling, which is similar to prior work showing that inhibition of ERK1/2 decreases excitability in DRG neurons [19]. Additionally, and consistent with the previous study showing that pERK1, but not pERK2 phosphorylated the L1 loop of Nav1.7 [19], increased association between ERK1 and Nav1.7 was detected following IL-6 treatment, indicating that IL-6-activated signaling pathways are capable of modulating Nav1.7 directly. While we cannot rule out the possibility that modulation of other channels contributes to electrophysiological effects following IL-6 treatment, the findings reported here support the hypothesis that IL-6 produces sodium channel-dependent hyperexcitability and migraine-related behavior through activation of the ERK pathway.
Conclusions
This study provides direct evidence that IL-6 can sensitize dural afferents in a manner consistent with sodium channel phosphorylation and that it produces prolonged migraine-related pain behavior through activation of the ERK pathway. Although there is currently no direct link between Nav1.7 and migraine, these findings suggest that an IL-6/Nav1.7 signaling axis can be an important mediator of headache pain and that drugs targeting IL-6 signaling may have efficacy in the treatment of migraine headache.
References
Goadsby PJ, Lipton RB, Ferrari MD: Migraine-current understanding and treatment. N Engl J Med 2002, 346: 257–270. 10.1056/NEJMra010917
Waeber C, Moskowitz MA: Migraine as an inflammatory disorder. Neurology 2005, 64: S9-S15.
Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, Floridi A, Calabresi P: Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006, 46: 200–207. 10.1111/j.1526-4610.2006.00337.x
Fidan I, Yuksel S, Ymir T, Irkec C, Aksakal FN: The importance of cytokines, chemokines and nitric oxide in pathophysiology of migraine. J Neuroimmunol 2006, 171: 184–188. 10.1016/j.jneuroim.2005.10.005
Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, Yeomans DC: Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain 2007, 3: 28. 10.1186/1744-8069-3-28
De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ: The role of interleukin-6 in nociception and pain. Anesth Analg 2003, 96: 1096–1103. table of contents
Vardanyan M, Melemedjian OK, Price TJ, Ossipov MH, Lai J, Roberts E, Boos TL, Deschamps JR, Jacobson AE, Rice KC, Porreca F: Reversal of pancreatitis-induced pain by an orally available, small molecule interleukin-6 receptor antagonist. Pain 2010, 151: 257–265. 10.1016/j.pain.2010.05.022
Xu XJ, Hao JX, Andell-Jonsson S, Poli V, Bartfai T, Wiesenfeld-Hallin Z: Nociceptive responses in interleukin-6-deficient mice to peripheral inflammation and peripheral nerve section. Cytokine 1997, 9: 1028–1033. 10.1006/cyto.1997.0243
Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD: Proinflammatory cytokines mediating burn-injury pain. Pain 2008, 135: 98–107. 10.1016/j.pain.2007.05.012
Kurek JB, Austin L, Cheema SS, Bartlett PF, Murphy M: Up-regulation of leukaemia inhibitory factor and interleukin-6 in transected sciatic nerve and muscle following denervation. Neuromuscul Disord 1996, 6: 105–114. 10.1016/0960-8966(95)00029-1
Bao L, Zhu Y, Elhassan AM, Wu Q, Xiao B, Zhu J, Lindgren JU: Adjuvant-induced arthritis: IL-1 beta, IL-6 and TNF-alpha are up-regulated in the spinal cord. Neuroreport 2001, 12: 3905–3908. 10.1097/00001756-200112210-00010
Arruda JL, Colburn RW, Rickman AJ, Rutkowski MD, DeLeo JA: Increase of interleukin-6 mRNA in the spinal cord following peripheral nerve injury in the rat: potential role of IL-6 in neuropathic pain. Brain Res Mol Brain Res 1998, 62: 228–235.
Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, et al.: A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci 2009, 29: 13473–13483. 10.1523/JNEUROSCI.1822-09.2009
Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J: Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009, 68: 1580–1584. 10.1136/ard.2008.092866
Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ: IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci 2010, 30: 15113–15123. 10.1523/JNEUROSCI.3947-10.2010
Ji RR, Gereau RWt, Malcangio M, Strichartz GR: MAP kinase and pain. Brain Res Rev 2009, 60: 135–148. 10.1016/j.brainresrev.2008.12.011
Karim F, Wang CC, Gereau RWt: Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci 2001, 21: 3771–3779.
Rush AM, Cummins TR, Waxman SG: Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 2007, 579: 1–14. 10.1113/jphysiol.2006.121483
Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD: ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci 2010, 30: 1637–1647. 10.1523/JNEUROSCI.4872-09.2010
Wei X, Edelmayer RM, Yan J, Dussor G: Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 2011, 31: 1595–1600. 10.1177/0333102411427600
Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G: Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain 2011, 152: 106–113. 10.1016/j.pain.2010.09.036
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL: Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994, 53: 55–63. 10.1016/0165-0270(94)90144-9
Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, et al.: Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 2009, 65: 184–193. 10.1002/ana.21537
Burstein R, Cutrer MF, Yarnitsky D: The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 2000,123(Pt 8):1703–1709.
Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, Lipton RB: Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology 2008, 70: 1525–1533. 10.1212/01.wnl.0000310645.31020.b1
Cummins TR, Howe JR, Waxman SG: Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J Neurosci 1998, 18: 9607–9619.
Rush AM, Dib-Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG: A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc Natl Acad Sci USA 2006, 103: 8245–8250. 10.1073/pnas.0602813103
Choi JS, Waxman SG: Physiological interactions between Nav1.7 and Nav1.8 sodium channels: a computer simulation study. J Neurophysiol 2011, 106: 3173–3184. 10.1152/jn.00100.2011
Levy D: Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep 2009, 13: 237–240. 10.1007/s11916-009-0040-y
Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, Letourneau R, Theoharides TC, Waeber C, Moskowitz MA: Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain 2001, 124: 2490–2502. 10.1093/brain/124.12.2490
Dimlich RV, Keller JT, Strauss TA, Fritts MJ: Linear arrays of homogeneous mast cells in the dura mater of the rat. J Neurocytol 1991, 20: 485–503. 10.1007/BF01252276
Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC: Morphological and functional demonstration of rat dura mater mast cell-neuron interactions in vitro and in vivo. Brain Res 1999, 849: 1–15. 10.1016/S0006-8993(99)01855-7
Strassman AM, Weissner W, Williams M, Ali S, Levy D: Axon diameters and intradural trajectories of the dural innervation in the rat. J Comp Neurol 2004, 473: 364–376. 10.1002/cne.20106
Kruger-Krasagakes S, Moller A, Kolde G, Lippert U, Weber M, Henz BM: Production of interleukin-6 by human mast cells and basophilic cells. J Invest Dermatol 1996, 106: 75–79. 10.1111/1523-1747.ep12327815
Grabbe J, Welker P, Moller A, Dippel E, Ashman LK, Czarnetzki BM: Comparative cytokine release from human monocytes, monocyte-derived immature mast cells, and a human mast cell line (HMC-1). J Invest Dermatol 1994, 103: 504–508. 10.1111/1523-1747.ep12395649
Obreja O, Biasio W, Andratsch M, Lips KS, Rathee PK, Ludwig A, Rose-John S, Kress M: Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain 2005, 128: 1634–1641. 10.1093/brain/awh490
Dib-Hajj SD, Cummins TR, Black JA, Waxman SG: From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci 2007, 30: 555–563. 10.1016/j.tins.2007.08.004
Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, Kaczorowski GJ, Garcia ML, Koltzenburg M, Priest BT: ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol 2008, 74: 1476–1484. 10.1124/mol.108.047670
Estacion M, Waxman SG, Dib-Hajj SD: Effects of ranolazine on wild-type and mutant hNav1.7 channels and on DRG neuron excitability. Mol Pain 2010, 6: 35. 10.1186/1744-8069-6-35
Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN: Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA 2004, 101: 12706–12711. 10.1073/pnas.0404915101
Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG: Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108: 237–247. 10.1016/j.pain.2003.12.035
Acknowledgements
This work was supported by funds from The American Pain Society (GD), The National Headache Foundation (GD), The Rita Allen Foundation (TJP), NIH grant NS065926 (TJP) and NS072204 (GD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JY, OKM, TJP and GD conceived of the study and designed experiments, JY and OKM performed experiments. JY and OKM analyzed data. JY and GD wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yan, J., Melemedjian, O.K., Price, T.J. et al. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol Pain 8, 6 (2012). https://doi.org/10.1186/1744-8069-8-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-8-6