Abstract
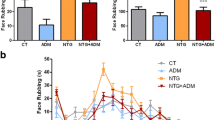
Acid-sensing ion channel 3 (ASIC3) is abundant in the trigeminal nervous system and is most sensitive to a slight pH decrease. Recent studies have indicated that ASIC3 in the peripheral trigeminal ganglia is likely involved in the pathogenesis of migraine pain. However, it is unclear whether this receptor plays a role in recurrent migraine, namely, migraine chronicity. Here, we aimed to investigate the role of ASIC3 in an animal model of recurrent migraine (RM). In this study, we established a rat model of RM through repeated administration of inflammatory soup (IS) onto the dura. Then, we tested the mechanical pain thresholds of the face and hindpaws by von Frey filaments. qRT-PCR, Western blot and immunofluorescence labelling were used to detect the expression and localization of ASIC3 in the trigeminal nucleus caudalis (TNC). The protein levels of calcitonin gene-related peptide (CGRP), its receptor component receptor activity modifying protein 1 (RAMP1) and c-Fos were analysed following treatment with the ASIC3 inhibitor APETx2 and activator 2-guanidine-4-methylquinazoline (GMQ). We found decreased pain thresholds after repeated dural inflammatory stimulation, which suggested the establishment of an RM model. Based on this model, we observed elevated expression of ASIC3 in the TNC group compared to that in the Sham group. ASIC3 was primarily expressed in neurons but not in astrocytes of the TNC. Moreover, APETx2 attenuated tactile allodynia and significantly decreased the expression of c-Fos, CGRP and RAMP1, while GMQ aggravated these effects compared to those observed in the IS + vehicle group. These findings indicate a critical role of ASIC3 channels in the pathophysiology of RM, and ASIC3 might represent a potential therapeutic target to prevent the progression of migraine.





Similar content being viewed by others
Abbreviations
- ASIC3:
-
Acid-sensing ion channel 3
- APETx2:
-
Anthopleura elegantissima
- CGRP:
-
Calcitonin gene-related peptide
- C2:
-
Cervical spinal 2
- CNS:
-
Central nervous system
- DMSO:
-
Dimethyl sulfoxide
- GMQ:
-
2-Guanidine-4-methylquinazoline
- IS:
-
Inflammatory soup
- PBS:
-
Phosphate-buffered solution
- PGE2:
-
Prostaglandin E2
- qRT-PCR:
-
Quantitative real-time reverse transcription-polymerase chain reaction
- RM:
-
Recurrent migraine
- RAMP1:
-
Receptor activity modifying protein 1
- SP:
-
Substance P
- TNC:
-
Trigeminal nucleus caudalis
- TRPV1:
-
Transient receptor potential vanilloid type 1
References
Bernstein C, Burstein R (2012) Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 8(2):89–99
Bigal ME, Serrano D, Reed M, Lipton RB (2008a) Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 71(8):559–566
Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, Serrano D, Lipton RB, AMPP Group (2008b) Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology 70:1525–1533
Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N (2005) Receptor activity-modifying protein (RAMP) isoform-specific regulation of adrenomedullin receptor trafficking by NHERF-1. J Biol Chem 280(25):23926–23935
Boyer N, Dallel R, Artola A, Monconduit L (2014) General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain 155(7):1196–1205
Burstein R, Cutrer M, Yarnitsky D (2000) The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 123(Pt 8):1703–1709
Cao Q, Wang W, Gu J, Jiang G, Wang K, Xu Z, Li J, Chen G, Wang X (2016) Elevated expression of acid-sensing ion channel 3 inhibits epilepsy via activation of interneurons. Mol Neurobiol 53(1):485–498
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Chen CH, Hsu YT, Chen CC, Huang RC (2009) Acid-sensing ion channels in neurons of the rat suprachiasmatic nucleus. J Physiol 587(Pt 8):1727–1737
Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW (2007) Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J Biol Chem 282(16):12260–12271
Deval E, Lingueglia E (2015) Acid-sensing ion channels and nociception in the peripheral and central nervous systems. Neuropharmacology 94:49–57
Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, Lingueglia E (2010) Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther 128(3):549–558
Deval E, Noël J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E (2011) Acid-sensing ion channels in postoperative pain. J Neurosci 31(16):6059–6066
Du J, Reznikov LR, Price MP, Zha XM, Lu Y, Moninger TO, Wemmie JA, Welsh MJ (2014) Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc Natl Acad Sci U S A 111(24):8961–8966
Durham PL, Masterson CG (2013) Two mechanisms involved in trigeminal CGRP release: implications for migraine treatment. Headache 53(1):67–80
Dussor G (2015) ASICs as therapeutic targets for migraine. Neuropharmacology 94:64–71
Haut SR, Bigal ME, Lipton RB (2006) Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol 5(2):148–157
Ho TW, Edvinsson L, Goadsby PJ (2010) CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6(10):573–582
Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA (2008) Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain 137(3):662–669
Jang MU, Park JW, Kho HS, Chung SC, Chung JW (2015) Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis 17(2):187–193
Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO (2010) Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol 161(4):950–960
Kreple CJ, Lu Y, Taugher RJ, Schwager-Gutman AL, Du J, Stump M, Wang Y, Ghobbeh A, Fan R, Cosme CV, Sowers LP, Welsh MJ, Radley JJ, LaLumiere RT, Wemmie JA (2014) Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat Neurosci 17(8):1083–1091
Lei Z, Sami Shaikh A, Zheng W, Yu X, Yu J, Li J (2016) Non-proton ligand-sensing domain of acid-sensing ion channel 3 is required for itch sensation. J Neurochem 139(6):1093–1101
Li YF, Wu LJ, Li Y, Xu L, Xu TL (2003) Mechanisms of H +, modulation of glycinergic response in rat sacral dorsal commissural neurons. J Physiol 552(Pt 1):73–87
Li WG, Yu Y, Zhang ZD, Cao H, Xu TL (2010) ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain 6(1):88
Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, Serrano D, Stewart WF, American Migraine Prevalence Prevention Advisory Group (2008) Cutaneous allodynia in the migraine population. Ann Neurol 63:148–158
Masashi I, Masahiko I, Ji Q, Tani T (2012) Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci 19:77
Melo-Carrillo A, Lopez-Avila A (2013) A chronic animal model of migraine induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia 33(13):1096–1105
Meng QY, Wang W, Chen XN, Xu TL, Zhou JN (2009) Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience 159(3):1126–1134
Mercado F, López IA, Acuna D, Vega R, Soto E (2006) Acid-sensing ionic channels in the rat vestibular endorgans and ganglia. J Neurophysiol 96(3):1615–1624
Miesenböck G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394(6689):192–195
Mitsikostas DD, Sanchez del Rio M (2001) Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res Brain Res Rev 35(1):20–35
Oshinsky ML, Gomonchareonsiri S (2007) Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 47(7):1026–1036
Riesco N, Cernudamorollón E, Pascual J (2017) Neuropeptides as a marker for chronic headache. Curr Pain Headache Rep 21(4):18
Ryu S, Liu B, Yao J, Fu Q, Qin F (2007) Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci 27(47):12797–12807
Salinas M, Lazdunski ME (2009) Structural elements for the generation of sustained currents by the acid pain sensor ASIC3. J Biol Chem 284(46):31851–31859
Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, Calabresi P (2007) Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 32(6):1384–1390
Seybold VS (2009) The role of peptides in central sensitization. Handb Exp Pharmacol 194:451–491
Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP (2007) ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain 129(1–2):102–112
Storer RJ, Supronsinchai W, Srikiatkhachorn A (2015) Animal models of chronic migraine. Curr Pain Headache Rep 19(1):467
Strassman AM, Raymond SA, Burstein R (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384(6609):560–564
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21(3):531–543
Traynelis SF, Cull-Candy SG (1990) Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 345(6273):347–350
Uddman R, Tajti J, Hou M, Sundler F, Edvinsson L (2002) Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia 22(2):112–116
Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA (2011) Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain 152(10):2348–2356
Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M (1997) Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272(34):20975–20978
Wu B, Wang S, Qin G, Xie J, Tan G, Zhou J, Chen L (2017) Protein kinase C γ contributes to central sensitization in a rat model of chronic migraine. J Mol Neurosci 63(2):131–141
Yagi J, Wenk HN, Naves LA, McCleskey EW (2006) Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99(5):501–509
Yan J, Wei X, Bischoff C, Edelmayer RM, Dussor G (2013) pH-evoked dural afferent signaling is mediated by ASIC3 and is sensitized by mast cell mediators. Headache 53(8):1250–1261
Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL (2010) A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68(1):61–72
Zhang Z, Dickerson IM, Russo AF (2006) Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology 147(4):1932–1940
Zhang Z, Winborn CS, Marquez de Prado B, Russo AF (2007) Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 27(10):2693–2703
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81671093 and 81500957) and the District Science and Technology Projects of Yuzhong Chongqing (No. 20160107).
Author information
Authors and Affiliations
Contributions
LXC and JYZ developed the experimental design. LXC, GCQ and WHY oversaw the experiments. SW, CYL and BXW performed the experiments. SW drafted the manuscript. LXC revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All experimental protocols were approved by the Ethics Committee of the Department of Medical Research (First Affiliated Hospital of Chongqing Medical University).
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wang, S., Wu, BX., Liu, CY. et al. Expression of ASIC3 in the Trigeminal Nucleus Caudalis Plays a Role in a Rat Model of Recurrent Migraine. J Mol Neurosci 66, 44–52 (2018). https://doi.org/10.1007/s12031-018-1113-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1113-3