Abstract
Background
Assessing the ventilatory status of non-intubated infants in the Pediatric Intensive Care Unit (PICU) is a constant challenge. Methods to evaluate ventilation include arterial blood gas analysis (ABG), which is invasive and intermittent, and transcutaneous carbon dioxide monitoring (PtcCO2), which, while non-invasive, is also intermittent. A method that is non-invasive and continuous would be of great benefit in this population. We hypothesized that non-invasive capnometry via sidestream monitoring of exhaled carbon dioxide (CO2) would provide an acceptable measurement of ventilatory status when compared to ABG or PtcCO2.
Methods
Preliminary prospective study of infants less than one year of age admitted to the PICU in a large urban teaching hospital. Infants not intubated and not requiring non-invasive ventilation were eligible. A sidestream CO2 reading was obtained in a convenience sample of 39 patients. A simultaneous ABG was collected in those with an arterial catheter, and a PtcCO2 was obtained in those without.
Results
Correlation of sidestream CO2 with ABG was excellent (r2 = 0.907). Sidestream correlated less well with PtcCO2 (r2 = 0.649). Results were not significantly altered when weight and respiratory rate were added as independent variables. Bland-Altman analysis revealed a bias of -2.7 with a precision of ±6.5 when comparing sidestream CO2 to ABG, and a bias of -1.7 with a precision of ±9.9 when comparing sidestream CO2 to PtcCO2.
Conclusions
Performance of sidestream monitoring of exhaled CO2 is acceptable clinical trending to assess the effectiveness of ventilation in non-intubated infants in the PICU.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Respiratory monitoring of non-intubated children in the pediatric intensive care unit (PICU) is a constant challenge. Effectiveness of the patient’s respiratory effort can be monitored, to some extent, by visual observation of chest expansion, rate and depth of respirations, use of accessory muscles, and the quality and quantity of breath sounds. These subjective findings can be misleading, however, and objective means of following oxygenation and ventilation are needed in the majority of PICU patients. Pulse oximetry has become the standard of care for oxygenation monitoring in both the PICU and the general ward [1]. Monitoring of ventilation poses a more difficult challenge. Except in extreme cases, pulse oximetry will not reliably detect hypoventilation. Measurement of the partial pressure of carbon dioxide (PaCO2) through arterial blood gas analysis (ABG) is the gold standard for assessing ventilation. While a true reflection of ventilation, measurement of the PaCO2 is both invasive and intermittent, which greatly limits its use. Consequently, other measures of ventilatory monitoring have been established.
Transcutaneous carbon dioxide monitoring (PtcCO2) estimates the PaCO2 by warming the skin to induce hyperperfusion, enabling the electrochemical measurement of the partial pressure of oxygen and carbon dioxide [2]. Transcutaneous monitoring is considered a safe procedure, however tissue injury may occur at the measuring site, including blisters, burns, and skin tears. These complications are rare with current technology, and primarily occur when the PtcCO2 is left in place for long periods of time, so continuous monitoring is generally avoided. In patients with poor skin integrity or adhesive allergy, transcutaneous monitoring may be relatively contraindicated [3, 4]. Various clinical factors may increase the discrepancy between arterial and transcutaneous values of carbon dioxide, including hyperoxia (PaO2 > 100 torr), hypoperfusion, improper electrode placement or application, body wall edema, and the thickness of the patient’s skin or subcutaneous tissue [3–5].
Capnography is regularly used in operating rooms and intensive care units to monitor carbon dioxide clearance in tracheally intubated patients [6–8]. Exhaled carbon dioxide generally reflects PaCO2, but the correlation decreases predictably with increasing dead space ventilation [9]. In recent years, there has been widening use of oral and nasal capnometry for monitoring ventilation in non-intubated adults and children [10, 11]. Noninvasive capnography has been used in emergency departments, pediatric intensive care units, during polysomnography, during sedation, and during interfacility transport [10–18]. However, the ability of these devices to reliably capture exhaled CO2 from non-intubated infants with high respiratory rates and low tidal volumes is unknown [19]. We prospectively compared sidestream carbon dioxide (CO2), with PtcCO2 and/or PaCO2 in infants less than one year of age admitted to the PICU to determine if sidestream monitoring provides an acceptable measurement of the effectiveness of ventilation. Should the performance of this technology prove acceptable for clinical trending, it would provide the benefits of both non-invasive and continuous monitoring of ventilatory status.
Methods
This study was approved by the Institutional Review Board at Children’s Memorial Hospital in Chicago, Illinois, now the Ann & Robert H. Lurie Children’s Hospital of Chicago. Infants admitted to the PICU who were age 1 year or less, without a tracheostomy or immediate need for invasive or non-invasive ventilation were eligible for enrollment. Additional information was recorded on each subject including age in months, weight in kilograms (kg), respiratory rate, and diagnosis. A convenience sample of subjects was enrolled from March 2007 – September 2008.
Sidestream sampling was performed on all subjects in the study. The sidestream cannula (Philips Microstream EtCo2 circuit, Smart Capnoline O2), a two prong nasal cannula with a CO2 detection port that hangs in front of the mouth, was placed on the subject and left in place until a steady state reading was obtained. The sampling rate is 50 ml/min and the sampling line is 100 cm long. Oxygen can be delivered through the nasal prongs, if desired, and was only used as indicated for patient care. The value of the exhaled CO2 was recorded (Microcap Microstream Oridion Machine) after a consistent reading had been present for 2 minutes. For those subjects without an arterial catheter in place, a PtcCO2 (Radiometer Copenhagen, Tina TCM 4) was obtained. The PtcCO2 machine was calibrated prior to placing the sidestream cannula on the subject. The PtcCO2 electrode was placed at the same time as the sidestream nasal cannula and the reading was recorded when no further increase was observed for 30-60 seconds. In those subjects who had an indwelling arterial catheter in place as part of their routine care, an arterial blood gas was drawn immediately after the sidestream reading was recorded. The majority of subjects had a single sidestream CO2 measurement and either a PtcCO2 or a PaCO2, but 4 subjects had both a PtcCO2 and a PaCO2.
The correlation between a single sidestream CO2 reading and the simultaneous PtcCO2 or PaCO2 was examined using Spearman’s rho. Since correlation is expected when two methods attempt to measure the same physiologic parameter, a Bland Altman analysis was also conducted [20] to analyze the differences between the sidestream reading and either the PtcCO2 or the PaCO2. Bias, the mean difference between values, and precision, the standard deviation (SD) of the bias, were calculated for PtcCO2 to sidestream and PaCO2 to sidestream differences.
The effect of respiratory rate and weight on the relationship between PtcCO2 or PaCO2 and sidestream CO2 was assessed by simple linear regression models for the unadjusted effect and multiple linear regression models for the adjusted effect. We examined both absolute values of sidestream CO2 and log-transformed sidestream due to the non-normal distribution of the sidestream values.
Results
Forty-three sample sets were obtained from 39 subjects for analysis. Please see Table 1 for full demographic data. In the PtcCO2 comparison group there were 29 subjects. Admission diagnoses included respiratory illness (n = 16), cardiothoracic surgery (n = 9), and other (n = 4). In the ABG comparison group there were 14 subjects. Admission diagnoses included respiratory illness (n = 1), cardiothoracic surgery (n = 12), and other (n = 1). The predominance of cardiothoracic surgery subjects in the ABG group reflects the practice patterns at our institution for placing and keeping arterial lines in non-intubated subjects (Table 1).
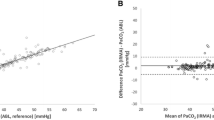
The correlation between sidestream CO2 and PaCO2 was excellent (r2 0.907, Figure 1A). Sidestream CO2 correlated less well with PtcCO2 values (r2 0.649, Figure 2A). The Bland-Altman analysis revealed good agreement between PaCO2 and sidestream CO2 (bias –2.7, precision ±6.5, Figure 1B). Agreement was less robust when comparing sidestream CO2 and PtcCO2 (bias –1.7, precision ±9.9, Figure 2B).
The influence of respiratory rate and weight on performance of sidestream CO2 monitoring is presented in Table 2. Sidestream performance was unaffected by either factor. Therefore, sidestream measurements were accurate across the range of size and respiratory rates found in the infants in this study.
Discussion
This study demonstrates that sidestream CO2 monitoring can provide an acceptable estimation of PaCO2 in infants with varying degrees of tachypnea. It is comparable to another commonly used non-invasive monitor, the transcutaneous CO2. To our knowledge, this is the only study comparing these different techniques in this age range. One retrospective study compared end tidal CO2 with venous CO2 and found them to be highly correlated [21]. This study included children age 5.5 months-20 years with a median age of 5.7 years. Additional studies in infants have examined the difference between sidestream CO2 and capillary CO2, or used exhaled mainstream CO2 to analyze the capnographic indices associated with bronchopulmonary dysplasia (BPD) [22, 23]. One of the studies was consistent with our findings, showing good correlation between sidestream CO2 and capillary CO2 in preterm infants without lung disease [22]. However, the correlation between sidestream CO2 and capillary CO2 was lost in infants with BPD. The gradient between the sidestream and capillary CO2 values in the BPD group was attributed to dead space ventilation and the ventilation-perfusion mismatch characteristic of this lung disease. None of these studies specifically evaluated the performance of sidestream CO2 in comparison to PtcCO2 or PaCO2 in infants.
In most cases, an estimated CO2 that is within 5 mm Hg of the PaCO2 would be considered acceptable for making clinical decisions when an arterial value is unavailable. In this study, the majority of measurements were within this range. Our results contrast with an adult study that did not show good correlation between sidestream CO2 and PaCO2 in non-intubated patients [24]. These adults were very tachypneic, and the authors speculated that this was a reflection of abnormal lung function, leading them to conclude that the usefulness of sidestream monitoring was limited in the presence of lung disease. As with all end-tidal CO2 monitoring, it is important to note that dead space ventilation will increase the discrepancy between exhaled CO2 and PaCO2 or PtcCO2[9]. This may explain the gradients seen between sidestream CO2 and PaCO2 in this adult study, and sidestream CO2 and capillary CO2 in the study of premature infants with BPD mentioned above. However, in our population, sidestream CO2 closely approximated PaCO2, and to a slightly lesser degree, PtcCO2, over a wide range of respiratory rates and degrees of distress in these infants, suggesting that sidestream monitoring of exhaled CO2 could be quite useful in this population.
Accurate sidestream monitoring is advantageous over ABG or PtcCO2 as it provides both a continuous and non-invasive means of monitoring ventilation. It is also much less labor intensive when compared to the alternate methods. Effective transcutaneous monitoring is dependent on both technical and patient factors. Repetitive ABG analysis requires the presence of an indwelling arterial catheter which can be difficult, painful, and time consuming to place.
The primary limitation to this preliminary study was its small sample size. Larger numbers and repeated measurements would enable further elucidation of patient or operator characteristics that impact the precision and reliability of these measurements. As the gold standard, PaCO2 should still be obtained when either PtcCO2 or sidestream readings seem to contradict clinical assessment.
Conclusion
Performance of sidestream monitoring of exhaled CO2 is acceptable for assessing the effectiveness of ventilation in non-intubated infants in the PICU. It should be considered when continuous monitoring of ventilation is desired to aid in the early detection of changes in clinical status.
Abbreviations
- ABG:
-
Arterial blood gas
- BPD:
-
Bronchopulmonary dysplasia
- CO2 :
-
Carbon dioxide
- kg:
-
Kilograms
- PaCO2 :
-
Arterial partial pressure of carbon dioxide
- PICU:
-
Pediatric intensive care unit
- PtcCO2 :
-
Transcutaneous carbon dioxide monitoring
- SD:
-
Standard deviation.
References
Fouzas S, Priftis KN, Anthracopoulos MB: Pulse oximetry in pediatric practice. Pediatrics. 2011, 128: 740-752. 10.1542/peds.2011-0271.
Monaco F, Nickerson BG, McQuitty JC: Continuous transcutaneous oxygen and carbon dioxide monitoring in the pediatric ICU. Crit Care Med. 1982, 10: 765-766. 10.1097/00003246-198211000-00014.
Restrepo RD, Hirst KR, Wittnebel L, Wettstein R: AARC clinical practice guideline: transcutaneous monitoring of carbon dioxide and oxygen. Respir Care. 2012, 2012: 1955-1962.
Tobias JD: Transcutaneous carbon dioxide monitoring in infants and children. Pediatr Anesth. 2009, 19: 434-444. 10.1111/j.1460-9592.2009.02930.x.
Tobias JD, Wilson WR, Meyer DJ: Transcutaneous monitoring of carbon dioxide tension after cardiothoracic surgery in infants and children. Anesth Analg. 1999, 88: 531-534.
Eipe N, Doherty DR: A review of pediatric capnography. J Clin Monit Comput. 2010, 24: 261-268. 10.1007/s10877-010-9243-3.
Tobias JD, Meyer DJ: Noninvasive monitoring of carbon dioxide during respiratory failure in toddlers and infants: end-tidal versus transcutaneous carbon dioxide. Anesth Analg. 1997, 85: 55-58.
Sivarajan VB, Bohn D: Monitoring of standard hemodynamic parameters: heart rate, systemic blood pressure, atrial pressure, pulse oximetry, and end-tidal CO2. Pediatr Crit Care Med. 2011, 12: S2-S11.
McSwain SD, Hamel DS, Smith PB, Gentile MA, Srinivasan S, Meliones JN, Cheifetz IM: End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care. 2010, 55: 288-293.
Bhende M: Capnography in the pediatric emergency department. Pediatr Emerg Care. 1999, 15: 64-69. 10.1097/00006565-199902000-00019.
Yosefy C, Hay E, Nasri Y, Magen E, Reisin L: End tidal carbon dioxide as a predictor of the arterial PCO2 in the emergency department setting. Emerg Med J. 2004, 21: 557-559. 10.1136/emj.2003.005819.
Kirk VG, Batuyong ED, Bohn SG: Transcutaneous carbon dioxide monitoring and capnography during pediatric polysomnography. Sleep. 2006, 29: 1601-1608.
Sullivan KJ, Kissoon N, Goodwin SR: End-tidal carbon dioxide monitoring in pediatric emergencies. Pediatr Emerg Care. 2005, 21: 327-332. 10.1097/01.pec.0000159064.24820.bd.
Langhan ML, Chen L: Current utilization of continuous end-tidal carbon dioxide monitoring in pediatric emergency departments. Pediatr Emerg Care. 2008, 24: 211-213. 10.1097/PEC.0b013e31816a8d31.
Langhan M: Continuous end-tidal carbon dioxide monitoring in pediatric intensive care units. J Crit Care. 2009, 24: 227-230. 10.1016/j.jcrc.2008.04.004.
Price DD, Wilson SR, Fee ME: Sidestream end-tidal carbon dioxide monitoring during helicopter transport. Air Med J. 2007, 26: 55-59. 10.1016/j.amj.2006.10.004.
Krauss B, Hess DR: Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007, 50: 172-181. 10.1016/j.annemergmed.2006.10.016.
Lightdale JR, Goldmann DA, Feldman HA, Newburg AR, DiNardo JA, Fox VL: Microstream capnography improves patient monitoring during moderate sedation: a randomized, controlled trial. Pediatrics. 2006, 117: e1170-e1178. 10.1542/peds.2005-1709.
Nobel JJ: Carbon dioxide monitors, exhaled gas. Pediatr Emerg Care. 1996, 12: 239-240. 10.1097/00006565-199606000-00024.
Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986, 1 (8476): 307-310.
Moses JM, Alexander JL, Agus MS: The correlation and level of agreement between end-tidal and blood gas pCO2 in children with respiratory distress: a retrospective analysis. BMC Pediatr. 2009, 9: 20-10.1186/1471-2431-9-20.
Lopez E, Mathlouthi J, Lescure S, Krauss B, Jarreau PH, Moriette G: Capnography in spontaneously breathing preterm infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2011, 46: 896-902. 10.1002/ppul.21445.
Fouzas S, Häcki C, Latzin P, Proietti E, Schulzke S, Frey U, Delgado-Eckert E: Volumetric capnography in infants with bronchopulmonary dysplasia. J Pediatr. 2014, 164: 283-288. 10.1016/j.jpeds.2013.09.034.
Jabre P, Jacob L, Auger H, Jaulin C, Monribot M, Aurore A, Margenet A, Marty J, Combes X: Capnography monitoring in nonintubated patients with respiratory distress. Am J Emerg Med. 2009, 27: 1056-1059. 10.1016/j.ajem.2008.08.017.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2431/14/163/prepub
Acknowledgements
No funding source was used for the study design, data collection, analysis, interpretation, in the writing of the manuscript or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BMC recruited subjects and collected data for the study, drafted the initial manuscript, and approved the final manuscript as submitted. RC developed initial study design, recruited subjects and collected data for the study, and approved the final manuscript as submitted. DMG helped with initial conceptualization of the study, performed the statistical analysis, and approved the final manuscript as submitted. RAR developed final study design, recruited subjects and collected data for the study, revised the manuscript, and approved the final manuscript as submitted.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Coates, B.M., Chaize, R., Goodman, D.M. et al. Performance of capnometry in non-intubated infants in the pediatric intensive care unit. BMC Pediatr 14, 163 (2014). https://doi.org/10.1186/1471-2431-14-163
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2431-14-163